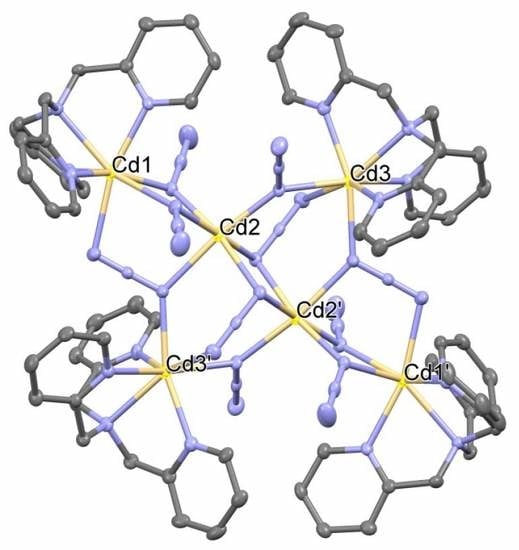
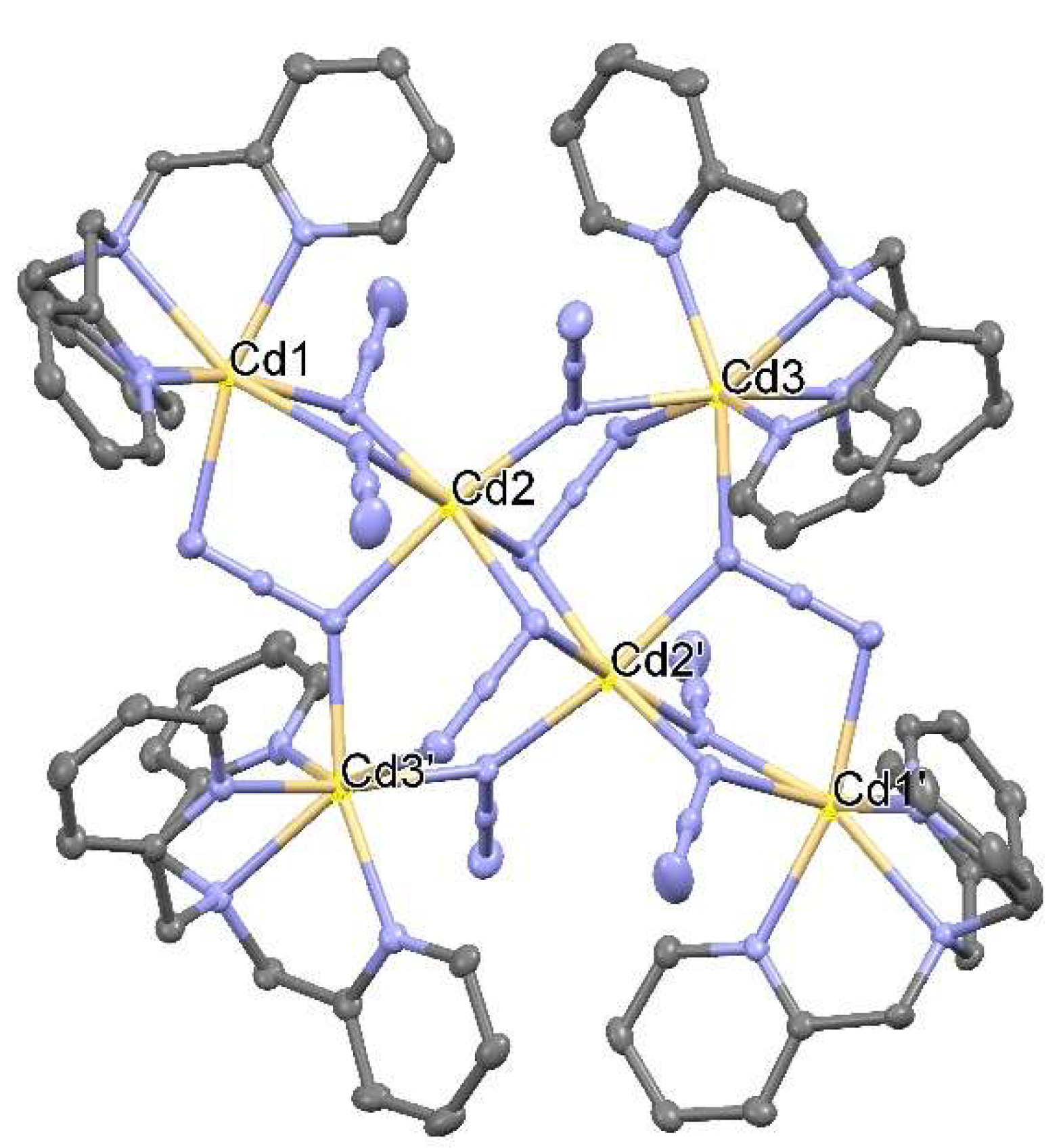
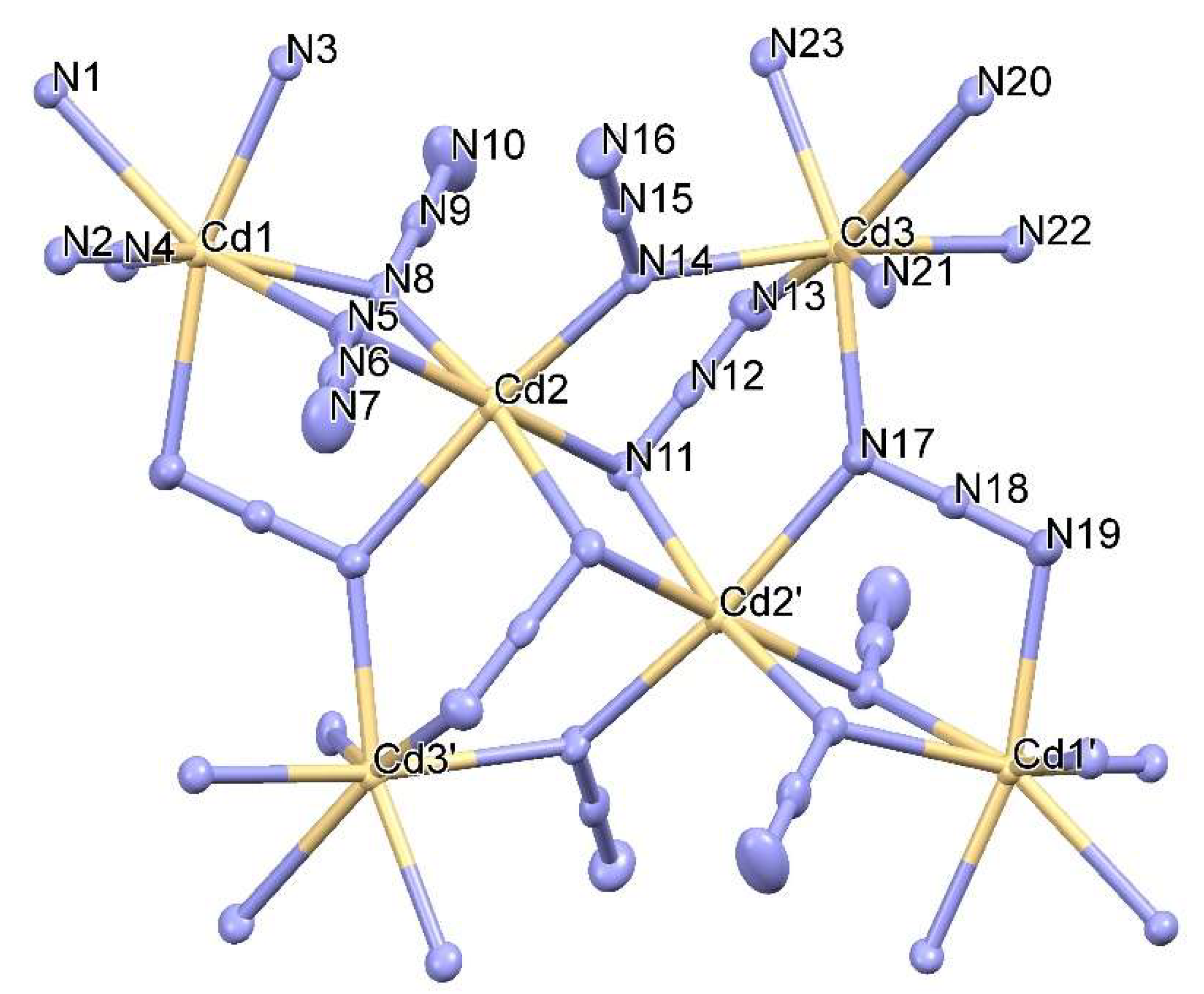
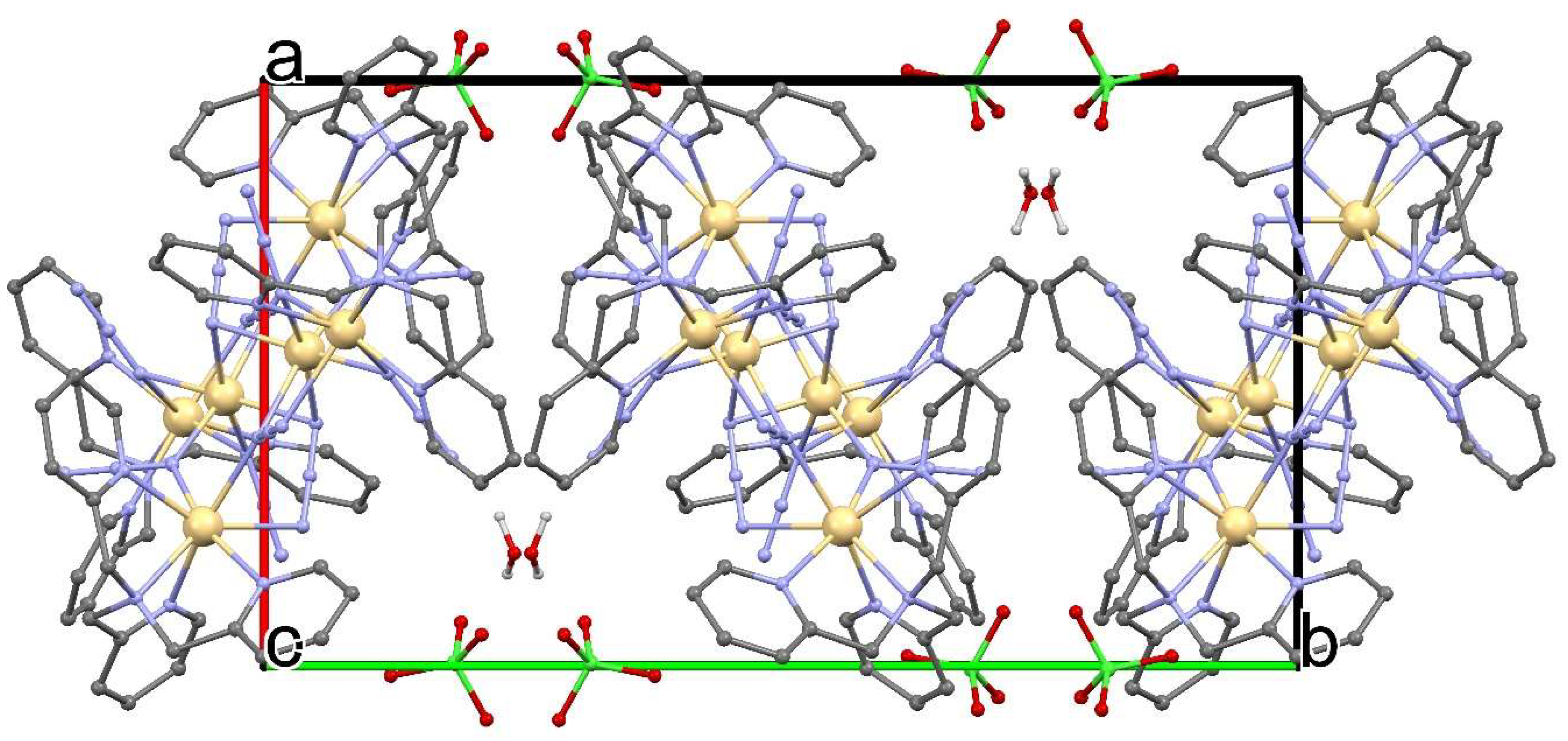
Hexnuclear Cadmium(II) Cluster Constructed from Tris(2-methylpyridyl)amine (TPA) and Azides
Abstract
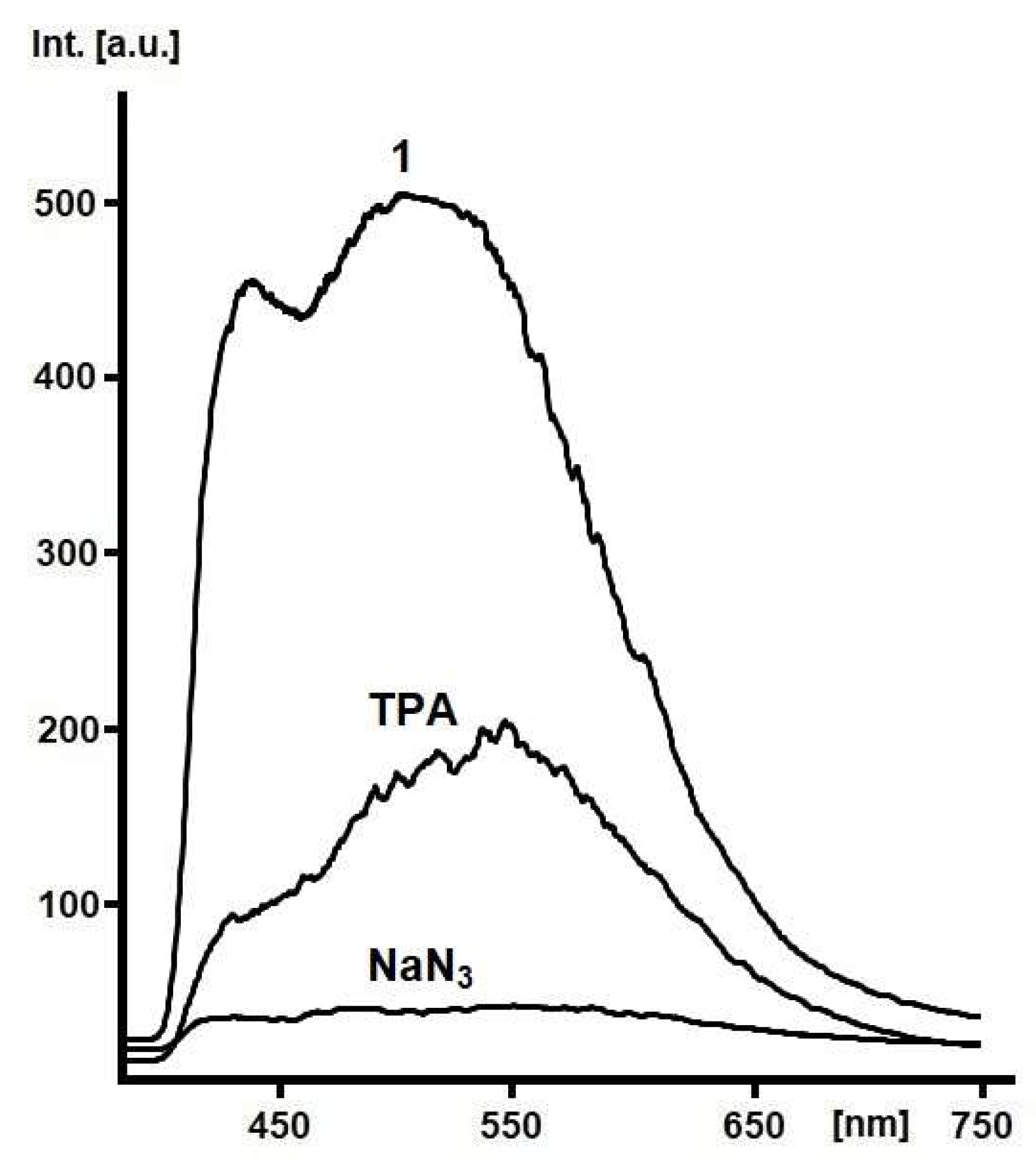
1. Introduction
2. Synthesis of [Cd6(TPA)4(μ3-1,1,3-N3)4(μ2-1,1-N3)6](ClO4)2·2H2O (1)
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Das, D.; Jana, S.; Ghosh, A. Modulation of nuclearity by Zn(II) and Cd(II) in their Complexes with a polytopic Mannich base ligand: A turn-on luminescence sensor for Zn(II) and detection of nitroaromatic explosives by Zn(II) complexes. Cryst. Growth Des. 2018, 18, 2335–2348. [Google Scholar] [CrossRef]
- Bai, S.-Q.; Fang, C.-J.; He, Z.; Gao, E.-Q.; Yan, C.-H.; Hor, T.S.A. Azide-bridged Cd(ii) 1D coordination polymer with Cd13 nano-crown-like cluster. CrystEngComm 2013, 15, 650–653. [Google Scholar] [CrossRef]
- Bai, S.-Q.; Gao, E.-Q.; He, Z.; Fang, C.-J.; Yan, C.H. Four one-dimensional cadmium(II) polymers: One chairlike chain containing four azido bridging modes and three double end-on azido-bridged uniform chains. CrystEngComm 2004, 6, 606–611. [Google Scholar] [CrossRef]
- Riddel, I.A.; Hristova, Y.R.; Clegg, J.K.; Wood, C.S.; Breiner, B.; Nitschke, J.K. Five discrete multinuclear metal-organic assemblies from one ligand: Deciphering the effects of different templates. J. Am. Chem. Soc. 2013, 135, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Massoud, S.S.; Louka, F.R.; Obaid, Y.K.; Vicente, R.; Ribas, J.; Fischer, R.C.; Mautner, F.A. Metal ions directing the geometry and nuclearity of azido-metal(ii) complexes derived from bis(2-(3,5-dimethyl-1H-pyrazol-1-yl)ethyl)amine. Dalton Trans. 2013, 42, 3968–3978. [Google Scholar] [CrossRef]
- Mautner, F.A.; Louka, F.R.; Hofer, J.; Spell, M.; Lefèvre, A.; Guilbeau, A.E.; Massoud, S.S. One-dimensional cadmium polymers with alternative di(EO/EE) and di(EO/EO/EO/EE) bridged azide bonding modes. Cryst. Growth Des. 2013, 13, 4518–4525. [Google Scholar] [CrossRef]
- Mautner, F.A.; Fischer, R.C.; Reichmann, K.; Gullett, E.; Ashkar, K.; Massoud, S.S. Synthesis and characterization of 1D and 2D cadmium(ii)-2,2′-bipyridine-N,N′-dioxide coordination polymers bridged by pseudohalides. J. Mol. Struct. 2019, 1175, 797–803. [Google Scholar] [CrossRef]
- Massoud, S.S.; Henary, M.M.; Maxwell, L.; Martín, A.; Ruiz, E.; Vicente, R.; Fischer, R.C.; Mautner, F.A. Structure, magnetic properties and DFT calculations of azido-copper(ii) complexes with different azido-bonding, nuclearity and dimensionality. New J. Chem. 2018, 42, 2627–2639. [Google Scholar] [CrossRef]
- Escuer, A.; Esteban, J.; Perlepes, S.P.; Stamatatos, T.C. The bridging azido ligand as a central “player” in high-nuclearity 3d-metal cluster chemistry. Coord. Chem. Rev. 2014, 275, 87–129. [Google Scholar] [CrossRef]
- Zheng, L.-L.; Leng, J.-D.; Liu, W.-T.; Zhang, W.-X.; Lu, J.-X.; Tong, M.-L. Cu2+-Mediated nucleophilic addition of different nucleophiles to dicyanamide—Synthesis, structures, and magnetic properties of a family of mononuclear, trinuclear, hexanuclear, and polymeric copper(II) complexes. Eur. J. Inorg. Chem. 2008, 2008, 4616–4624. [Google Scholar] [CrossRef]
- Aguirre-Díaz, L.M.; Reinares-Fisac, D.; Iglesias, M.; Gutiérrez-Puebla, E.; Gándara, F.; Snejko, N.; Monge, M.Á. Group 13th metal-organic frameworks and their role in heterogeneous catalysis. Coord. Chem. Rev. 2017, 335, 1–27. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Tao, Y.; Yu, Q.; Bu, X.-H.; Sakamoto, H.; Kitagawa, S. Selective gas adsorption and unique structural topology of a highly stable guest-free zeolite-type MOF material with N-rich chiral open channels. Chem. Eur. J. 2008, 14, 2771–2776. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.-H.; Tong, M.-L.; Chen, X.-M. Metal-organic molecular architectures with 2,2′-bipyridyl-like and carboxylate ligands. Coord. Chem. Rev. 2005, 249, 545–565. [Google Scholar] [CrossRef]
- Li, X.; Wu, B.L.; Niu, C.Y.; Niu, Y.Y.; Zhang, H.Y. Syntheses of Metal−2-(Pyridin-4-yl)-1H-imidazole-4,5-dicarboxylate networks with topological diversity: Gas adsorption, thermal stability and fluorescent emission properties. Cryst. Growth Des. 2009, 9, 3423–3431. [Google Scholar] [CrossRef]
- Brzostek, K.S.; Terlecki, M.; Sokołowski, K.; Lewinski, J. Chemical fixation and conversion of CO2 into cyclic and cage-type metal carbonates. Coord. Chem. Rev. 2017, 334, 199–231. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal–organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Yue, Q.; Gao, E.-Q. Azide and carboxylate as simultaneous coupler for magnetic coordination polymers. Coord Chem. Rev. 2019, 382, 1–31. [Google Scholar] [CrossRef]
- He, Y.; Li, B.; O’Keeffe, M.; Chen, B. Multifunctional metal–organic frameworks constructed from meta-benzenedicarboxylate units. Chem. Soc. Rev. 2014, 43, 5618–5656. [Google Scholar] [CrossRef]
- Patra, R.; Titia, H.M.; Goldberg, I. Coordination polymers of flexible polycarboxylic acids with metal ions. V. polymeric frameworks of 5-(3,5-dicarboxybenzyloxy)-3-pyridine carboxylic acid with Cd(ii), Cu(ii), Co(ii), Mn(ii) and Ni(ii) ions; synthesis, structure, and magnetic properties. CrystEngComm 2013, 15, 2863–2872. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Zhang, J.-P.; Lin, Y.-Y.; Chen, X.-M. Syntheses, structures, and photoluminescence of three coordination polymers of cadmium dicarboxylates. Cryst. Growth Des. 2006, 6, 1684–1689. [Google Scholar] [CrossRef]
- Schulz, A.; Villinger, A. Binary polyazides of cadmium and mercury. Chem. Eur. J. 2015, 21, 3649–3663. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, D.; Chowdhury, H.; Banerjee, S.; Saha, N.C.; Ghosh, B.K. Syntheses, crystal structures and luminescence behaviors of four neutral penta-/hexacoordinate cadmium(II) compounds containing a tridentate Schiff base: Variation in coordination numbers, nuclearities and dimensionalities by changing halides/pseudohalides. Inorg. Chim. Acta 2019, 485, 86–97. [Google Scholar] [CrossRef]
- Bai, H.; Liu, Y.; Liu, Z.; Yu, T.; Liu, Z.; Yang, Y.; Zhu, Y. Twelve cadmium (ii) coordination frameworks with asymmetric pyridinyl triazole carboxylate: Syntheses, structures, and fluorescence properties. Cryst. Growth Des. 2019, 19, 3785–3806. [Google Scholar]
- Afkhami, F.A.; Khandar, A.A.; Mahmoudi, G.; Amini, M.; Molins, E.; Garczarek, P.; Lipkowski, J.; White, J.M.; Kirillov, A.M. New cadmium(II) and zinc(II) coordination polymers derived from a pyridine-hydrazone block: Self-assembly generation, structural and topological features, and theoretical analysis. Inorg. Chim. Acta 2017, 458, 68–76. [Google Scholar] [CrossRef]
- Wan, J.; Cai, S.-L.; Zhang, K.; Li, C.-J.; Feng, Y.; Fan, J.; Zheng, S.-R.; Zhang, W.-G. Anion- and temperature-dependent assembly, crystal structures and luminescence properties of six new Cd(ii) coordination polymers based on 2,3,5,6-tetrakis(2-pyridyl)pyrazine. CrystEngComm 2016, 18, 5164–5176. [Google Scholar] [CrossRef]
- Khandar, A.A.; Afkhami, F.A.; Hosseini-Yazdi, S.A.; White, J.M.; Kassel, S.; Dougherty, W.G.; Lipkowski, J.; Van Derveer, D.; Giester, G.; Costantino, F. Anion influence in the structural diversity of cadmium coordination polymers constructed from a pyridine based Schiff base ligand. Inorg. Chim. Acta 2015, 427, 87–96. [Google Scholar] [CrossRef]
- He, Y.-C.; Guo, J.; Zhang, H.-M.; Ma, J.-F.; Liu, Y.-Y. Tuning the void volume in a series of isomorphic porous metal–organic frameworks by varying the solvent size and length of organic ligands. CrystEngComm 2014, 16, 5450–5457. [Google Scholar] [CrossRef]
- He, X.; Zhang, J.; Wu, X.-Y.; Lu, C.-Z. Syntheses, crystal structures and properties of a series of 3D cadmium coordination polymers with different topologies. Inorg. Chim. Acta 2010, 363, 1727–1734. [Google Scholar] [CrossRef]
- Goher, M.A.S.; Mautner, F.A.; Abu-Youssef, M.A.M.; Hafez, A.K.; Badr, A.M.-A. Synthesis and crystal structure of three new 2D polymeric cadmium(ii) complexes of some pyridine derivatives with different cadmium(ii)–azide topologies. J. Chem. Soc. Dalton Trans. 2002, 17, 3309–3312. [Google Scholar] [CrossRef]
- Zhang, N.; Song, L.-S.; Liu, X.-C.; Dong, W.-L.; Niu, Y.-Y.; Zhang, Z.-H. Three novel metal supramolecular polymers directed by 1,2-bis(pyridinium)ethane cation: Syntheses and crystal structures. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2012, 42, 965–971. [Google Scholar] [CrossRef]
- Yang, E.-C.; Shi, X.-J.; Liu, Z.-Y.; Zhao, X.-J. A cadmium(II)- and a nickel(II)-polymer with azide and 1,3-bis(4-pyridyl)propane ligands showing neutral pentanuclear cluster-based 3D MOF and self-interpenetrated 2D undulated layer. Inorg. Chem. Commun. 2010, 13, 733–736. [Google Scholar] [CrossRef]
- Song, P.-C.; Song, W.-C.; Tao, Y.; Hu, T.-L.; Zeng, Y.-F. Cadmium coordination polymers based on biimidazole and bibenzimidazole: Syntheses, crystal structures and fluorescent properties. Solid State Sci. 2010, 12, 1357–1363. [Google Scholar] [CrossRef]
- Cui, P.; Chen, Z.; Gao, D.; Zhao, B.; Shi, W.; Cheng, P. Syntheses, structures, and photoluminescence of a series of three-dimensional Cd (II) frameworks with a flexible ligand, 1, 5-bis (5-tetrazolo)-3-oxapentane. Cryst. Growth Des 2010, 10, 4370–4378. [Google Scholar] [CrossRef]
- Basak, S.; Sen, S.; Marschner, C.; Baumgartner, J.; Batten, S.R.; Turner, D.R.; Mitra, S. Synthesis, crystal structures and fluorescence properties of two new di- and polynuclear Cd(II) complexes with N2O donor set of a tridentate Schiff base ligand. Polyhedron 2008, 27, 1193–1200. [Google Scholar] [CrossRef]
- Nawrot, I.; Machura, B.; Kruszynski, R. Exploration of Cd(II)/pseudohalide/di-2-pyridylketone chemistry–rationalsynthesis, structural analysis and photoluminescence. CrystEngComm 2016, 18, 2650–2663. [Google Scholar] [CrossRef]
- Machura, B.; Nawrot, I.; Michalik, K. Synthesis, spectroscopic characterization and X-ray studies of two novel double open cubane-like cadmium(II) complexes. Polyhedron 2012, 31, 548–557. [Google Scholar] [CrossRef]
- Guo, L.-Y.; Su, H.-F.; Kurmoo, M.; Tung, C.-H.; Sun, D.; Zheng, L.-S. Core–shell {Mn7⊂(Mn,Cd)12} assembled from core {Mn7} disc. J. Am. Chem. Soc. 2017, 139, 14033–14066. [Google Scholar] [CrossRef]
- Soudani, S.; Mi, J.-X.; Lefebvre, F.; Jelsch, C.; Nasr, C.B. Synthesis and physico-chemical studies of a novel layered structure with a heptanuclear Cd complex: (C9N4H28)Cd7(H2O)2Cl18·nH2O (n = 5.89). J. Mol. Struct. 2015, 1084, 46–54. [Google Scholar] [CrossRef]
- Majumdar, D.; Biswas, J.K.; Mondal, M.; Babu, M.S.S.; Das, S.; Metre, R.K.; Kumar, S.S.S.; Bankura, K.; Mishra, D. Cd(II) pseudohalide complexes with N,N′-bis(3-ethoxysalicylidenimino)-1,3-diaminopropane: Crystal structures, hirshfeld surface, antibacterial and anti-biofilm properties. Chem. Sel. 2018, 3, 2912–2925. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mautner, F.A.; Fischer, R.C.; Williams, B.R.; Massoud, S.S.; Salem, N.M.H. Hexnuclear Cadmium(II) Cluster Constructed from Tris(2-methylpyridyl)amine (TPA) and Azides. Crystals 2020, 10, 317. https://doi.org/10.3390/cryst10040317
Mautner FA, Fischer RC, Williams BR, Massoud SS, Salem NMH. Hexnuclear Cadmium(II) Cluster Constructed from Tris(2-methylpyridyl)amine (TPA) and Azides. Crystals. 2020; 10(4):317. https://doi.org/10.3390/cryst10040317
Chicago/Turabian StyleMautner, Franz A., Roland C. Fischer, Bailey R. Williams, Salah S. Massoud, and Nahed M. H. Salem. 2020. "Hexnuclear Cadmium(II) Cluster Constructed from Tris(2-methylpyridyl)amine (TPA) and Azides" Crystals 10, no. 4: 317. https://doi.org/10.3390/cryst10040317
APA StyleMautner, F. A., Fischer, R. C., Williams, B. R., Massoud, S. S., & Salem, N. M. H. (2020). Hexnuclear Cadmium(II) Cluster Constructed from Tris(2-methylpyridyl)amine (TPA) and Azides. Crystals, 10(4), 317. https://doi.org/10.3390/cryst10040317