Optical Investigation of Eu3+ Doped Bi12GeO20 (BGO) Crystals
Abstract
1. Introduction
2. Materials and Methods
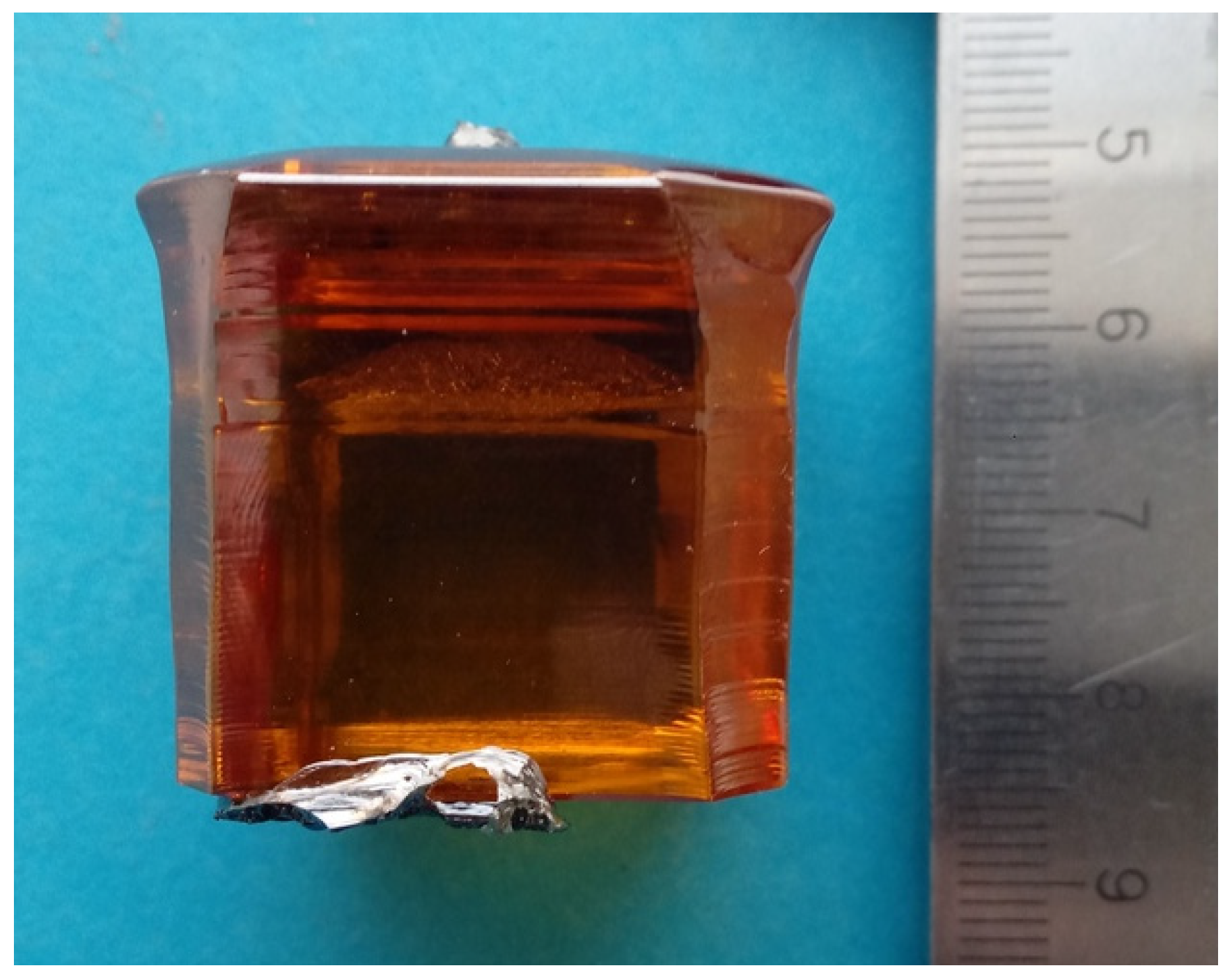
2.1. Crystal Growth
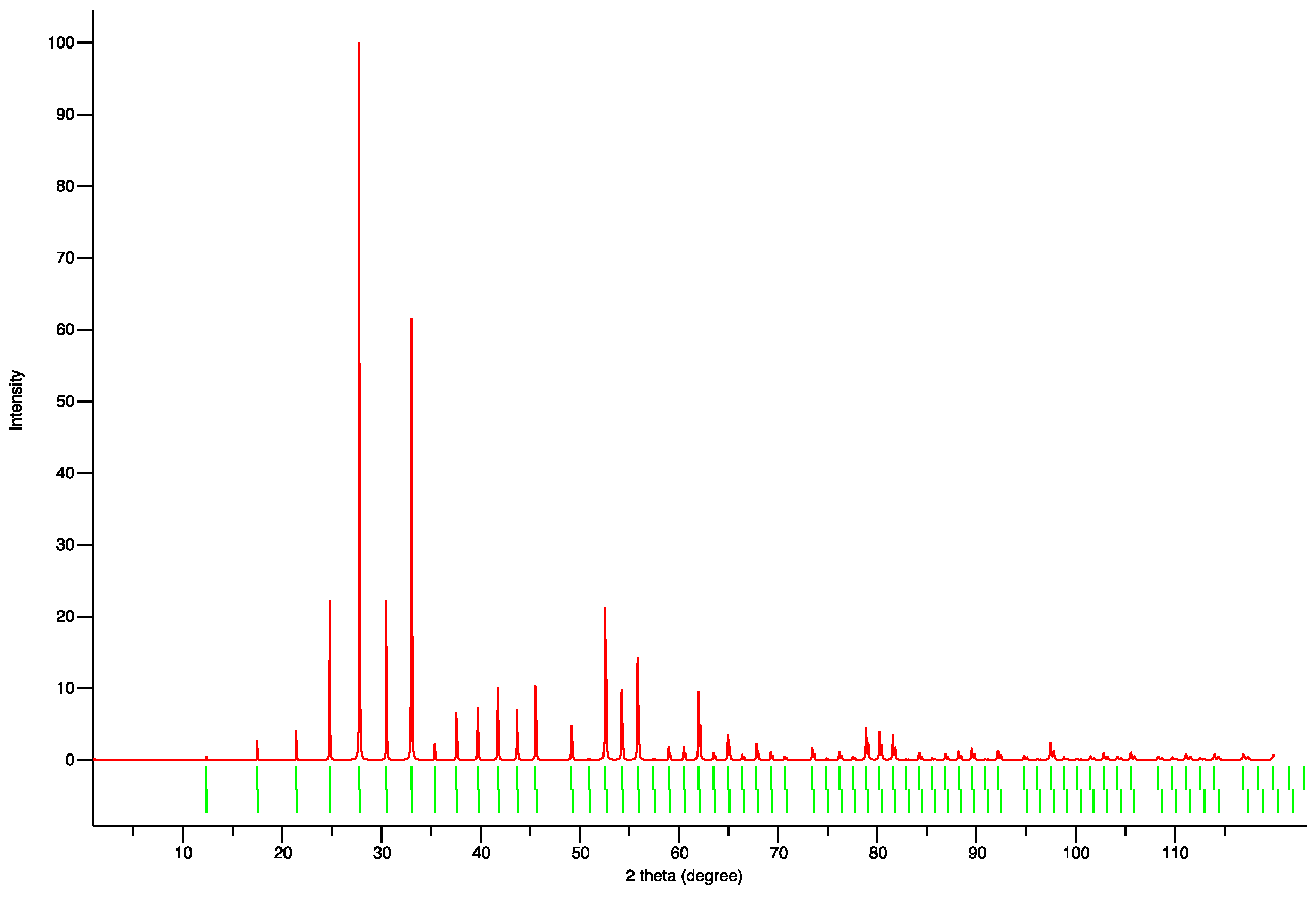
2.2. Samples Characterization
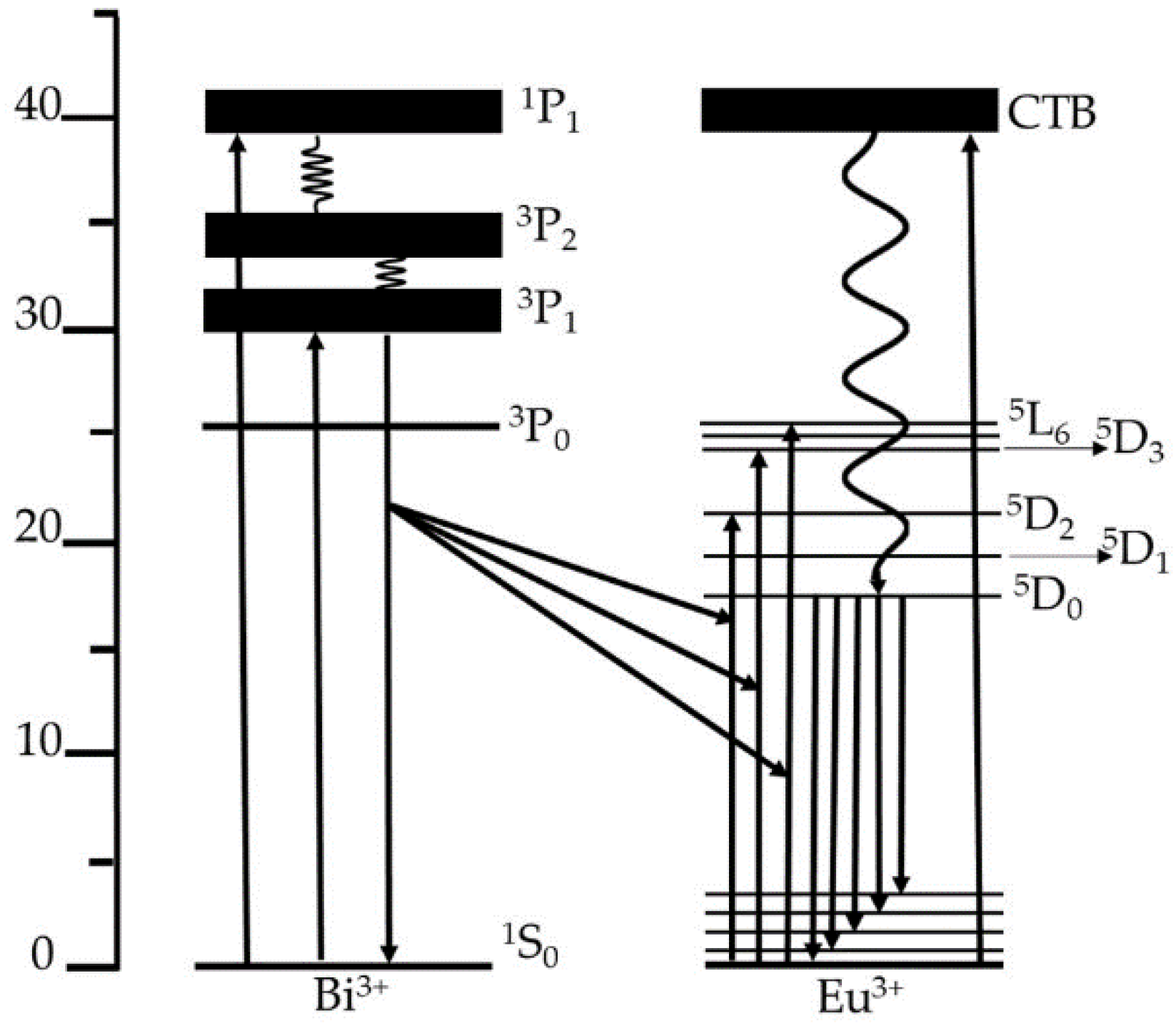
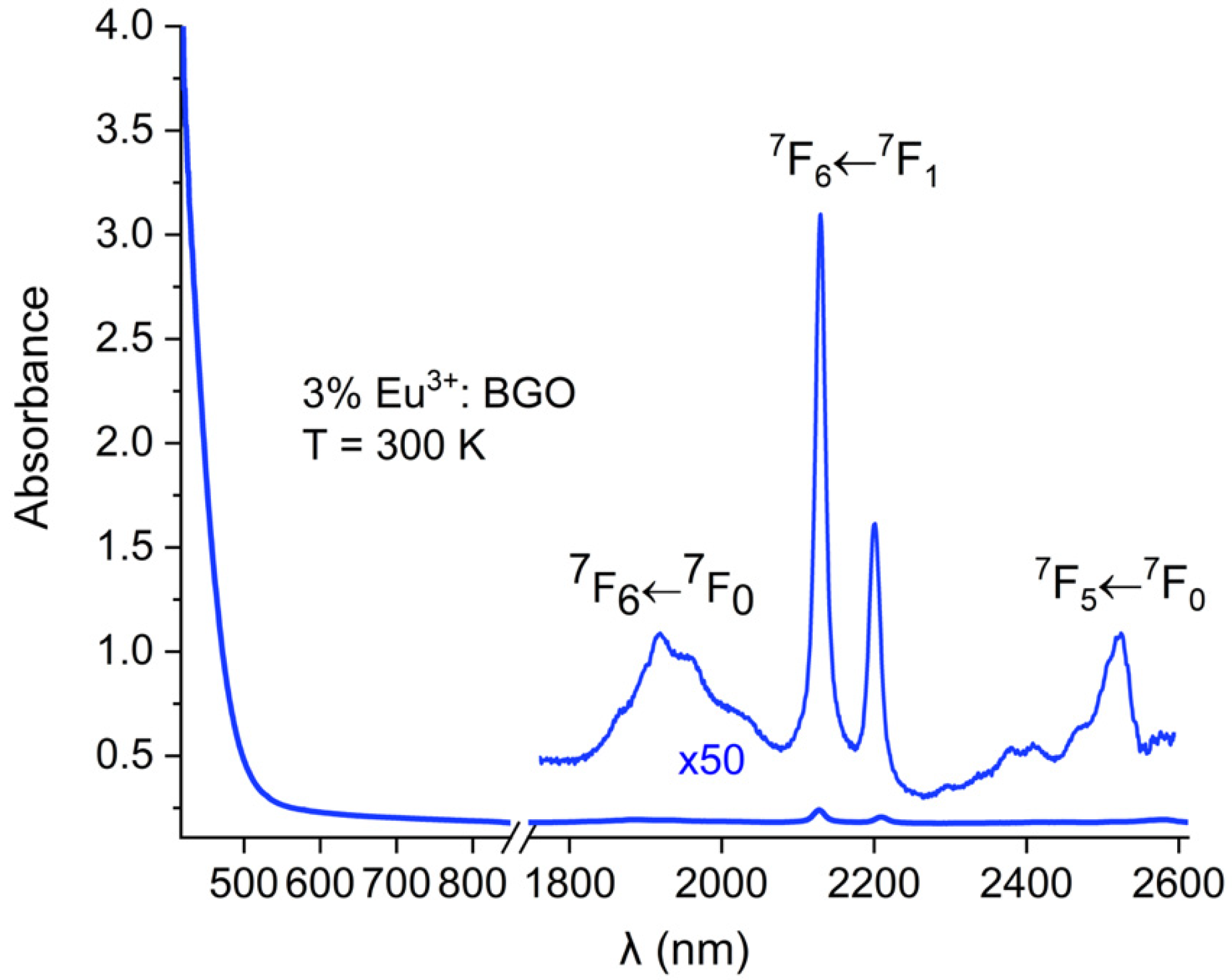
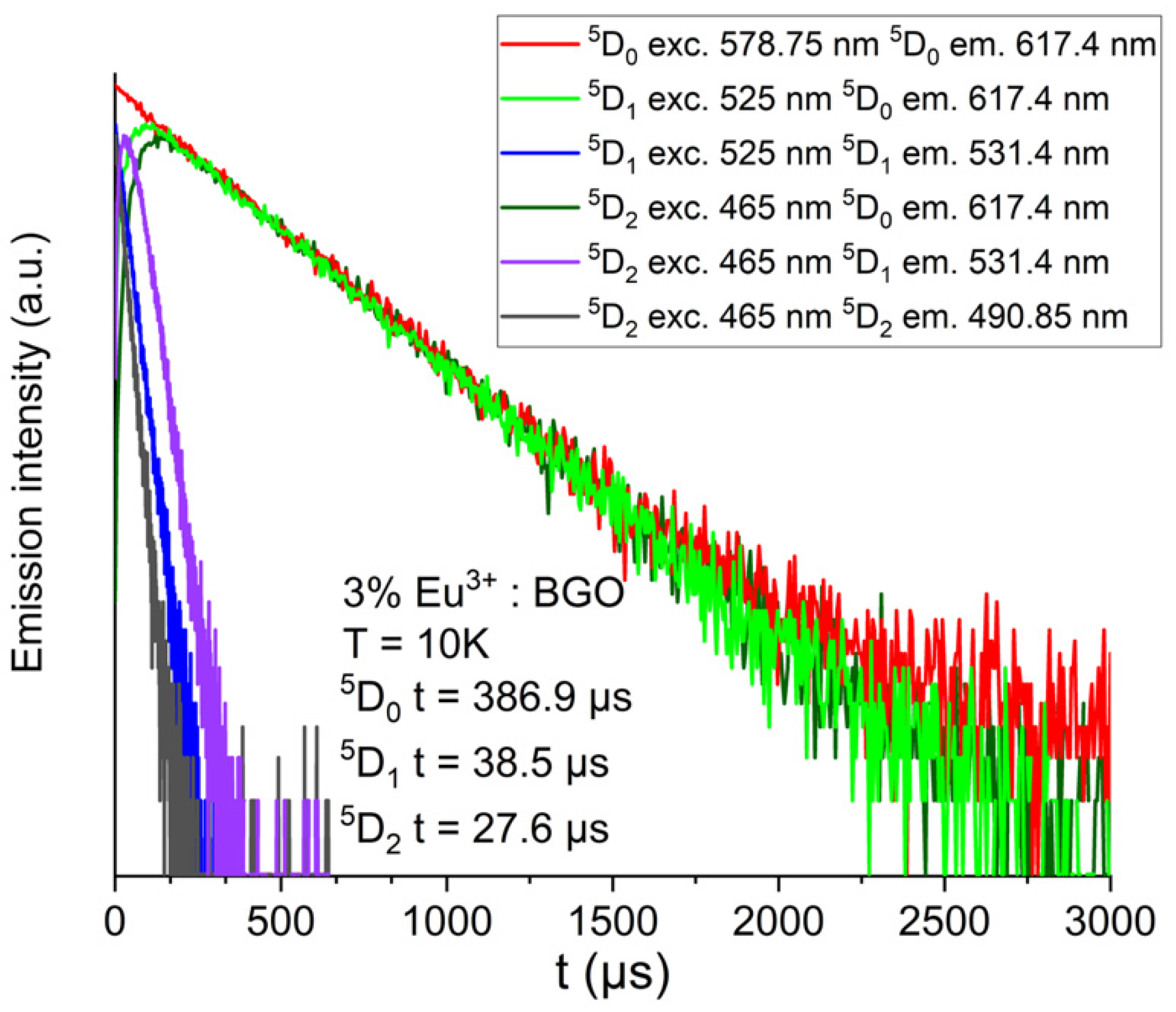
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Malinovskiy, V.K.; Gudaev, O.A.; Gusev, V.A.; Demenko, S.I. Photoinduced Phenomena in Sillenite Crystals; Nauka: Novosibirsk, Russia, 1990. [Google Scholar]
- Abrahams, S.C.; Jamieson, P.B.; Bernstein, J.L. Crystal Structure of Piezoelectric Bismuth Germanium Oxide Bi12GeO20. Chem. Phys. 1967, 44, 4034–4041. [Google Scholar] [CrossRef]
- Petricevic, S.J.; Mihailovic, P.; Radunovic, J. A miniature pockels cell with novel electrode geometry. Sensors 2009, 9, 5298–5307. [Google Scholar] [CrossRef] [PubMed]
- Bridoux, E.; Rouvaen, J.M.; Coussot, C.; Dieulesaint, E. Rayleigh-Wave Propagation on Bi12GeO20. Appl. Phys. Lett. 1971, 19, 523–524. [Google Scholar] [CrossRef]
- Wang, W.C.; Meng, K.S. Surface-wave correlation and time scaling in a structure of Bi12GeO20-Si-LiNbO3. Appl. Phys. Lett. 1975, 27, 375–377. [Google Scholar] [CrossRef]
- Huignard, J.P.; Micheron, F. High-sensitivity read-write volume holographic storage in BSO and BGO crystals. Appl. Phys. Lett. 1976, 29, 591–593. [Google Scholar] [CrossRef]
- Peltier, M.; Micheron, F. Volume hologram recording and charge transfer process in Bi12SiO20 and Bi12GeO20. J. Appl. Phys. 1977, 48, 3683–3690. [Google Scholar] [CrossRef]
- Douglas, G.G.; Zitter, R.N. Transport Processes of Photoinduced Carriers in Bismuth Germanium Oxide (Bi12GeO20). J. Appl. Phys. 1968, 39, 2133–2135. [Google Scholar] [CrossRef]
- Briat, B.; Borowiec, M.T.; Rjeily, H.B.; Ramaz, F.; Hamri, A.; Szymczak, H. Combined optical/mcd/odmr investigations of photochromism in doubly-doped Bi12 GeO20. Radiat. Eff. Defects Solids 2002, 157, 989–993. [Google Scholar] [CrossRef]
- Wood, A.W.; Hunt, C.A.; Martin, J.J. The low-temperature photochromic and photorefractive response of bismuth germanium oxide doped with molybdenum. J. Appl. Phys. 2007, 101, 1–7. [Google Scholar] [CrossRef]
- Stepanova, I.V.; Gorashchenko, N.G.; Subbotin, K.A.; Smirnov, V.A. Determination of the charge state of chromium in Cr:Bi12GeO20 single crystals by spectral luminescence methods. Opt. Spectrosc. English Transl. Opt. i Spektrosk. 2009, 107, 335–338. [Google Scholar] [CrossRef]
- Lu, J.; Dai, Y.; Zhu, Y.; Huang, B. Density functional characterization of pure and alkaline earth metal-doped Bi12GeO20, Bi12SiO20, and Bi12TiO20 photocatalysts. ChemCatChem 2011, 3, 378–385. [Google Scholar] [CrossRef]
- Marinova, V.; Lin, S.H.; Hsu, K.Y. Photorefractive Properties Enhancement of Doped Bismuth Sillenite Crystals. Opt. Mem. Neural Netw. Inf. Opt. 2011, 20, 7–22. [Google Scholar] [CrossRef]
- Marinova, V.; Liu, R.C.; Lin, S.H.; Hsu, K.Y. Real-time holography in ruthenium-doped bismuth sillenite crystals at 1064 nm. Opt. Lett. 2011, 36, 1981–1983. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Hehlen, M.P.; Brik, M.G.; Krämer, K.W. 50th anniversary of the Judd-Ofelt theory: An experimentalist’s view of the formalism and its application. J. Lumin. 2013, 136, 221–239. [Google Scholar] [CrossRef]
- Shlegel, V.N.; Borovlev, Y.A.; Grigoriev, D.N.; Grigorieva, V.D.; Danevich, F.A.; Ivannikova, N.V.; Postupaeva, A.G.; Vasiliev, Y.V. Recent progress in oxide scintillation crystals development by low-thermal gradient Czochralski technique for particle physics experiments. J. Instrum. 2017, 12. [Google Scholar] [CrossRef]
- Shlegel, V.N.; Pantsurkin, D.S. Growth of Bi12GeO20 and Bi12SiO20 crystals by the low-thermal gradient Czochralski technique. Crystallogr. Rep. 2011, 56, 339–344. [Google Scholar] [CrossRef]
- Oberschmid, R. Absorption Centers of Bi12GeO20 and Bi12SiO20 Crystals. Phys. Status Solidi 1985, 89, 263–270. [Google Scholar] [CrossRef]
- Boutinaud, P. Revisiting the spectroscopy of the Bi3+ ion in oxide compounds. Inorg. Chem. 2013, 52, 6028–6038. [Google Scholar] [CrossRef]
- Timmermans, C.W.M.; Blasse, G. The Luminescence of Some Oxidic Bismuth and Lead Compounds. J. Solid State Chem. 1984, 52, 222–232. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. Study of energy transfer from Sb3+, Bi3+, Ce3+ to Sm3+, Eu3+, Tb3+, Dy3+. J. Chem. Phys. 1967, 47, 1920–1926. [Google Scholar] [CrossRef]
- Blasse, G. The ultraviolet absorption bands of Bi3+ and Eu3+ in oxides. J. Solid State Chem. 1972, 4, 52–54. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Judd, B.R. Optical absorption intensities of rare-earth ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Carnall, W.T.; Crosswhite, H.; Crosswhite, H.M. Energy level structure and transition probabilities in the spectra of the trivalent lanthanides in LaF3. Energy 1978. [Google Scholar]
- Kolesnikov, I.E.; Povolotskiy, A.V.; Mamonova, D.V.; Kolesnikov, E.Y.; Kurochkin, A.V.; Lähderanta, E.; Mikhailov, M.D. Asymmetry ratio as a parameter of Eu3+ local environment in phosphors. J. Rare Earths 2018, 36, 474–481. [Google Scholar] [CrossRef]
- Li, N.; Xue, Y.; Wang, D.; Liu, B.; Guo, C.; Song, Q.; Xu, X.; Liu, J.; Li, D.; Xu, J.; et al. Spectroscopic properties of Eu:Bi4Ge3O12 single crystal grown by the micro-pulling-down method. J. Lumin. 2019, 208, 208–212. [Google Scholar] [CrossRef]
- Loiko, P.A.; Dashkevich, V.I.; Bagaev, S.N.; Orlovich, V.A.; Mateos, X.; Serres, J.M.; Vilejshikova, E.V.; Yasukevich, A.S.; Yumashev, K.V.; Kuleshov, N.V.; et al. Judd-Ofelt analysis of spectroscopic properties of Eu3+:KLu(WO4)2 crystal. J. Lumin. 2015, 168, 102–108. [Google Scholar] [CrossRef]
- Loiko, P.A.; Dashkevich, V.I.; Bagaev, S.N.; Orlovich, V.A.; Yasukevich, A.S.; Yumashev, K.V.; Kuleshov, N.V.; Dunina, E.B.; Kornienko, A.A.; Vatnik, S.M.; et al. Spectroscopic and photoluminescence characterization of Eu3+-doped monoclinic KY(WO4)2 crystal. J. Lumin. 2014, 153, 221–226. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, B.; Hua, R.; Yu, N.; Liu, B.; Sun, J.; Cheng, L.; Zhong, H.; Li, X.; Zhang, J.; et al. Self-assembled 3D flower-shaped NaY(WO4)2:Eu3+ microarchitectures: Microwave-assisted hydrothermal synthesis, growth mechanism and luminescent properties. CrystEngComm 2012, 14, 1760–1769. [Google Scholar] [CrossRef]
- Kaczkan, M.; Turczyński, S.; Malinowski, M. Spectroscopic properties and Judd-Ofelt analysis of Eu3+ in Y4Al2O9 crystals. J. Lumin. 2018, 196, 111–115. [Google Scholar] [CrossRef]
- Liu, Y.; Luo, W.; Li, R.; Liu, G.; Antonio, M.R.; Chen, X. Optical spectroscopy of Eu3+ doped ZnO nanocrystals. J. Phys. Chem. C 2008, 112, 686–694. [Google Scholar] [CrossRef]
- Liu, L.; Chen, X. Energy levels, fluorescence lifetime and Judd-Ofelt parameters of Eu3+ in Gd2O3 nanocrystals. Nanotechnology 2007, 18, 255704. [Google Scholar] [CrossRef]
- Chen, X.Y.; Liu, G.K. The standard and anomalous crystal-field spectra of Eu3+. J. Solid State Chem. 2005, 178, 419–428. [Google Scholar] [CrossRef]
- Chu, Y.; Zhang, Q.; Li, Y.; Liu, Z.; Xu, J.; Zeng, H.; Wang, H. Hydrothermal synthesis of Bi4Ge3O12: Eu3+ phosphors with high thermal stability and enhanced photoluminescence property. J. Alloys Compd. 2017, 693, 308–314. [Google Scholar] [CrossRef]
- Smentek, L.; Kȩdziorski, A. F ←→ f electric dipole transitions; old problems in a new light. J. Alloys Compd. 2009, 488, 586–590. [Google Scholar] [CrossRef]
- Souza, A.S.; Oliveira, Y.A.R.; Couto dos Santos, M.A. Enhanced approach to the Eu3+ ion 5D0→7F0 transition intensity. Opt. Mater. (Amst.) 2013, 35, 1633–1635. [Google Scholar] [CrossRef]
- Burdick, G.W.; Downer, M.C.; Sardar, D.K. A new contribution to spin-forbidden rare earth optical transition intensities: Analysis of all trivalent lanthanides. J. Chem. Phys. 1989, 91, 1511–1520. [Google Scholar] [CrossRef]
- Tanaka, M.; Nishimura, G.; Kushida, T. Contribution of J mixing to the 5D0 − 7F0 transition of Eu3+ ions in several host matrices. Phys. Rev. B 1994, 49, 16917–16925. [Google Scholar] [CrossRef]
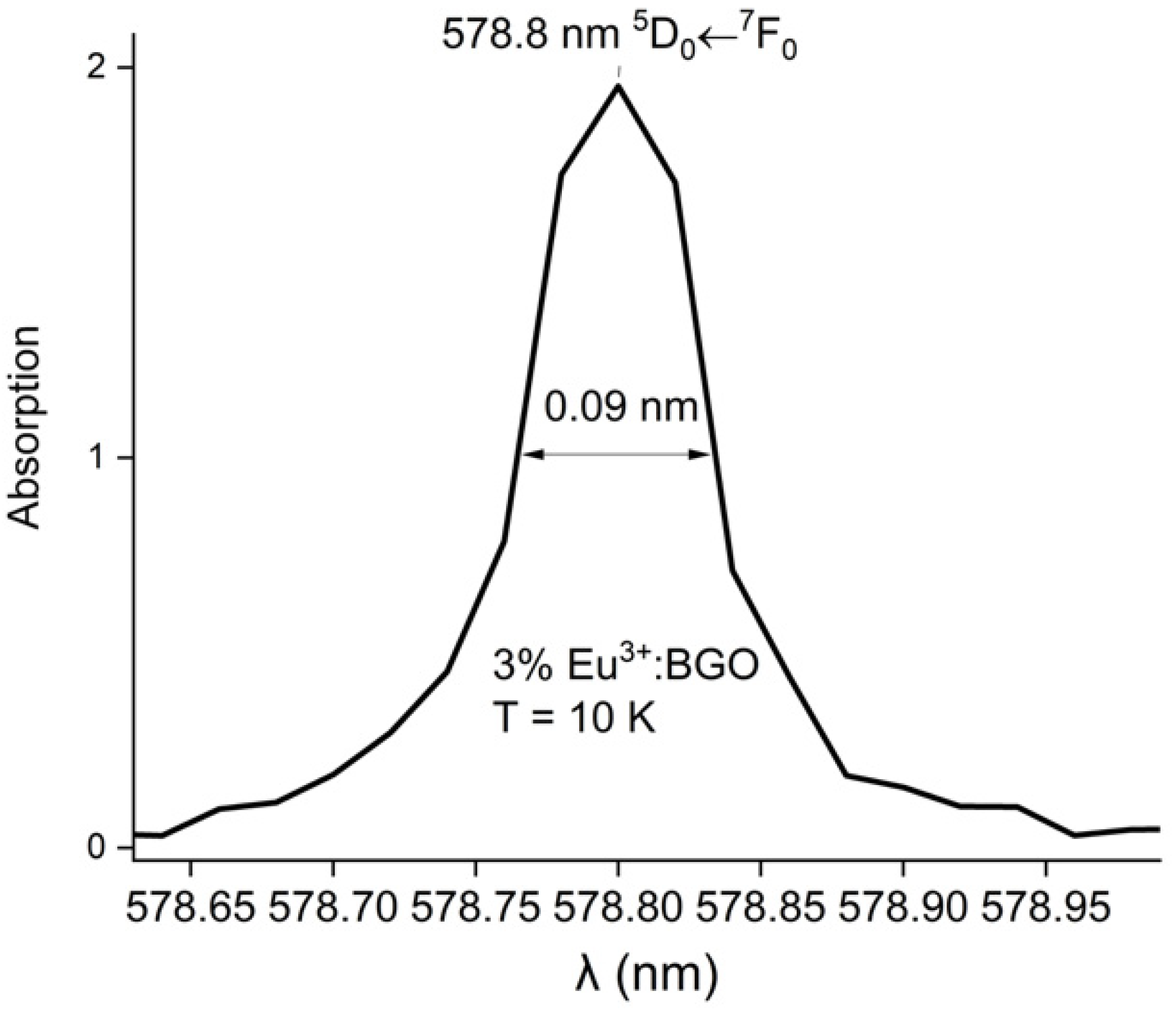
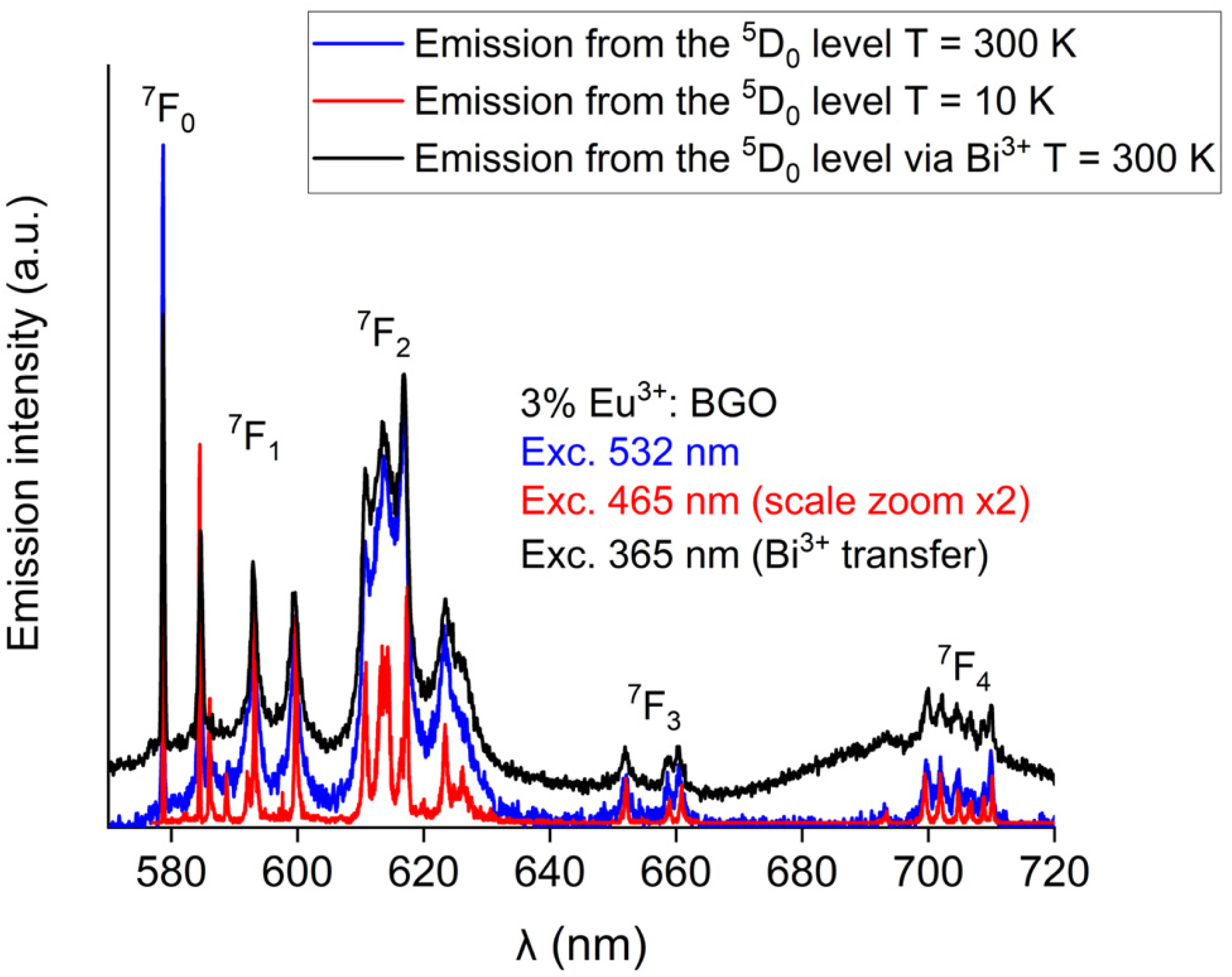
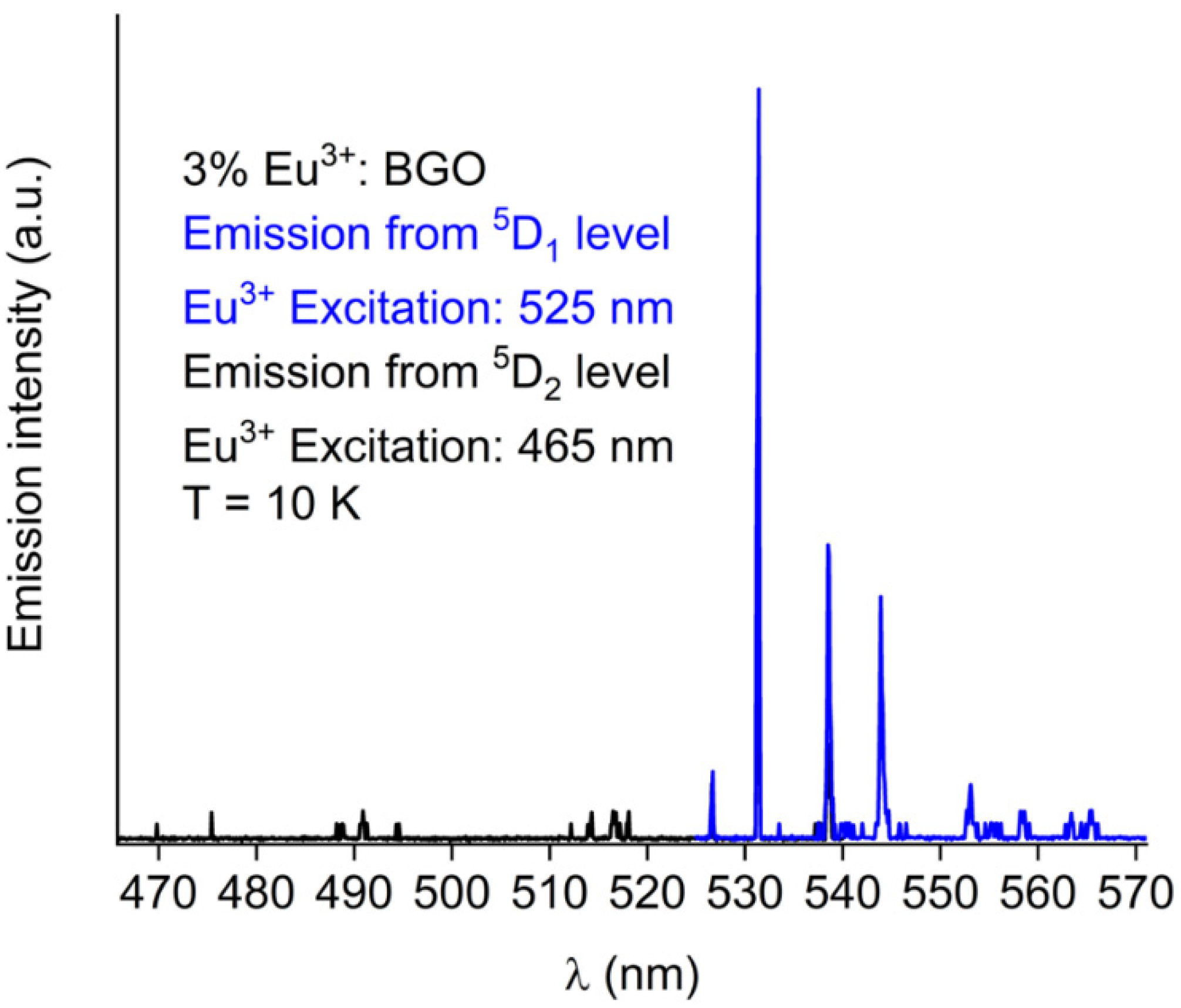
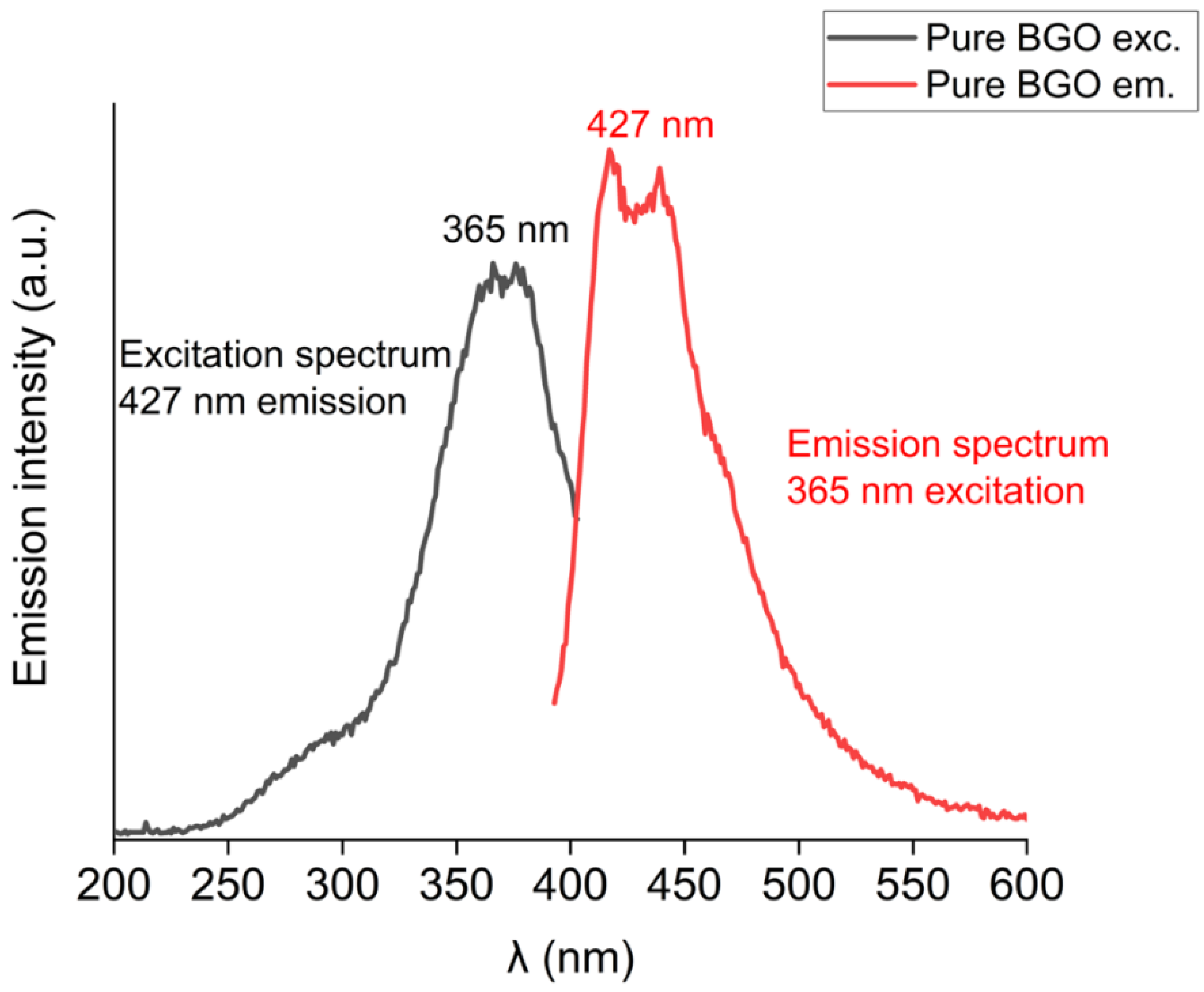


| Eu3+ Concentration (mol %) | R Value |
|---|---|
| 3 | 2.493 |
| 1 | 2.347 |
| 0.3 | 1.820 |
| Transition | λ (nm) | Acalc(s−1) | βcalc |
|---|---|---|---|
| 5D0→7F0 | 578 | 0 | 0 |
| 5D0→7F1 | 584 | 249.258 | 0.2557 |
| 5D0→7F2 | 614 | 652.459 | 0.6694 |
| 5D0→7F3 | 660 | 0 | 0 |
| 5D0→7F4 | 705 | 72.955 | 0.0748 |
| 5D0→7F5 | - | - | - |
| 5D0→7F6 | - | - | - |
| Σ | 974.672 | 1 | |
| Ω2 = 3.12224 × 10−20 cm2 | |||
| Ω4 = 0.77412 × 10−20 cm2 | |||
| τcalc = 1025.9 μs | |||
| Compound | Ω2 (10−20 cm2) | Ω4 (10−20 cm2) | Ω6 (10−20 cm2) | Reference |
|---|---|---|---|---|
| Bi12GeO20 | 3.12 | 0.77 | - | This work |
| Bi4Ge3O12 | 4.39 | 2.70 | 0.64 | [28] |
| KLu(WO4)2 | 20.76 | 5.23 | 7.96 | [29] |
| KY(WO4)2 | 36.70 | 11.50 | 3.40 | [30] |
| NaY(WO4)2 | 8.61 | 1.12 | 0.47 | [31] |
| Y4Al2O9 | 5.65 | 2.62 | 2.63 | [32] |
| LaF3 | 1.19 | 1.16 | 0.39 | [33] |
| YAlO3 | 2.66 | 6.33 | 0.80 | [33] |
| ZnO | 9.59 | 8.11 | 0.25 | [33] |
| Y2O3 | 9.86 | 2.23 | 0.32 | [33] |
| Gd2O3 | 12.39 | 2.02 | 0.19 | [34] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, M.; Ramazanova, T.F.; Grigoryeva, V.D.; Shlegel, V.N.; Kaczkan, M.; Fetliński, B.; Malinowski, M. Optical Investigation of Eu3+ Doped Bi12GeO20 (BGO) Crystals. Crystals 2020, 10, 285. https://doi.org/10.3390/cryst10040285
Kowalczyk M, Ramazanova TF, Grigoryeva VD, Shlegel VN, Kaczkan M, Fetliński B, Malinowski M. Optical Investigation of Eu3+ Doped Bi12GeO20 (BGO) Crystals. Crystals. 2020; 10(4):285. https://doi.org/10.3390/cryst10040285
Chicago/Turabian StyleKowalczyk, M., T.F. Ramazanova, V.D. Grigoryeva, V.N. Shlegel, M. Kaczkan, B. Fetliński, and M. Malinowski. 2020. "Optical Investigation of Eu3+ Doped Bi12GeO20 (BGO) Crystals" Crystals 10, no. 4: 285. https://doi.org/10.3390/cryst10040285
APA StyleKowalczyk, M., Ramazanova, T. F., Grigoryeva, V. D., Shlegel, V. N., Kaczkan, M., Fetliński, B., & Malinowski, M. (2020). Optical Investigation of Eu3+ Doped Bi12GeO20 (BGO) Crystals. Crystals, 10(4), 285. https://doi.org/10.3390/cryst10040285






