Syntheses, Crystal Structure and Magnetic Properties of Tl9RETe6 (RE = Ce, Sm, Gd)
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
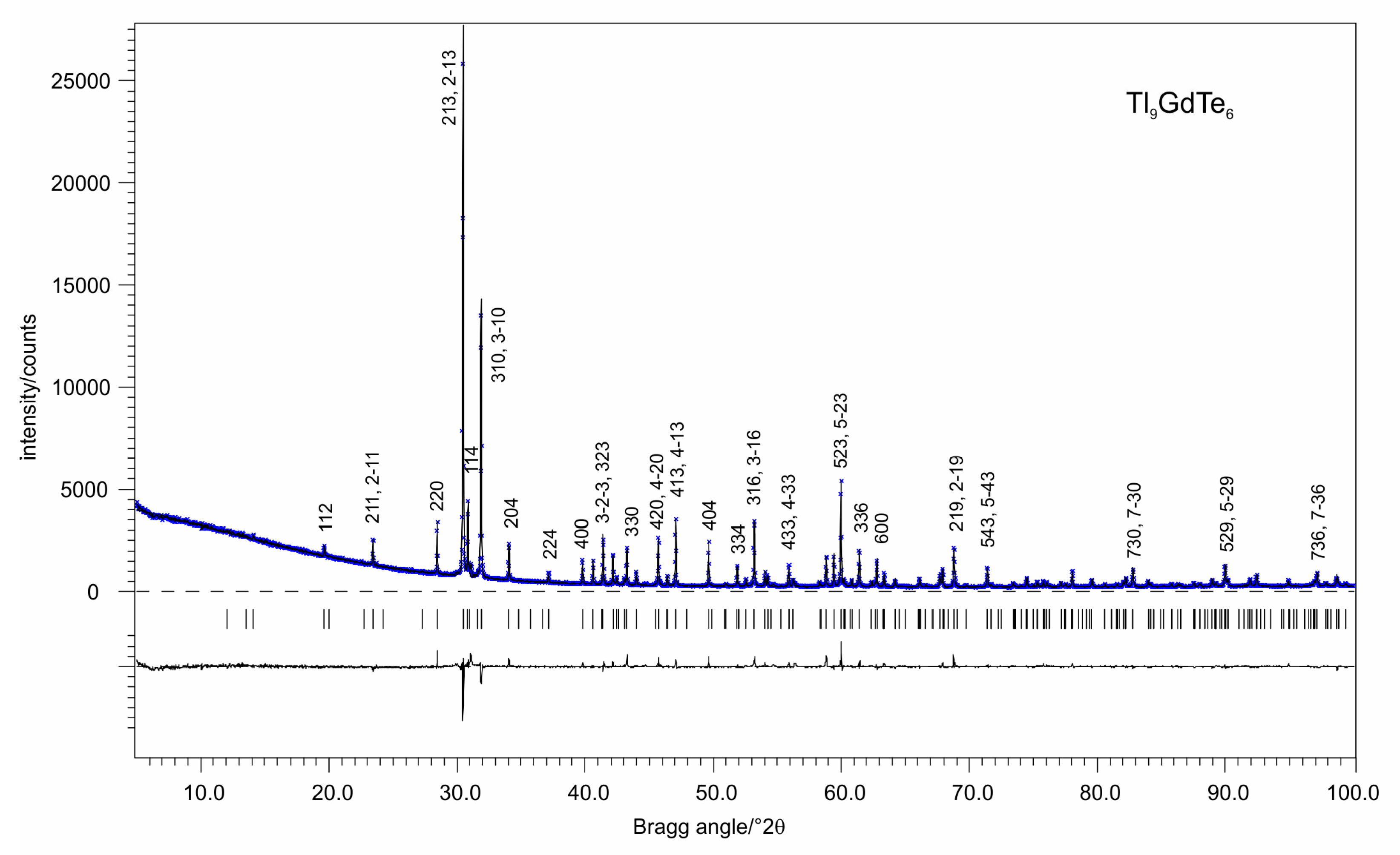
2.2. X-ray Powder Diffraction
2.3. Energy Dispersive X-ray Spectroscopy (EDX)
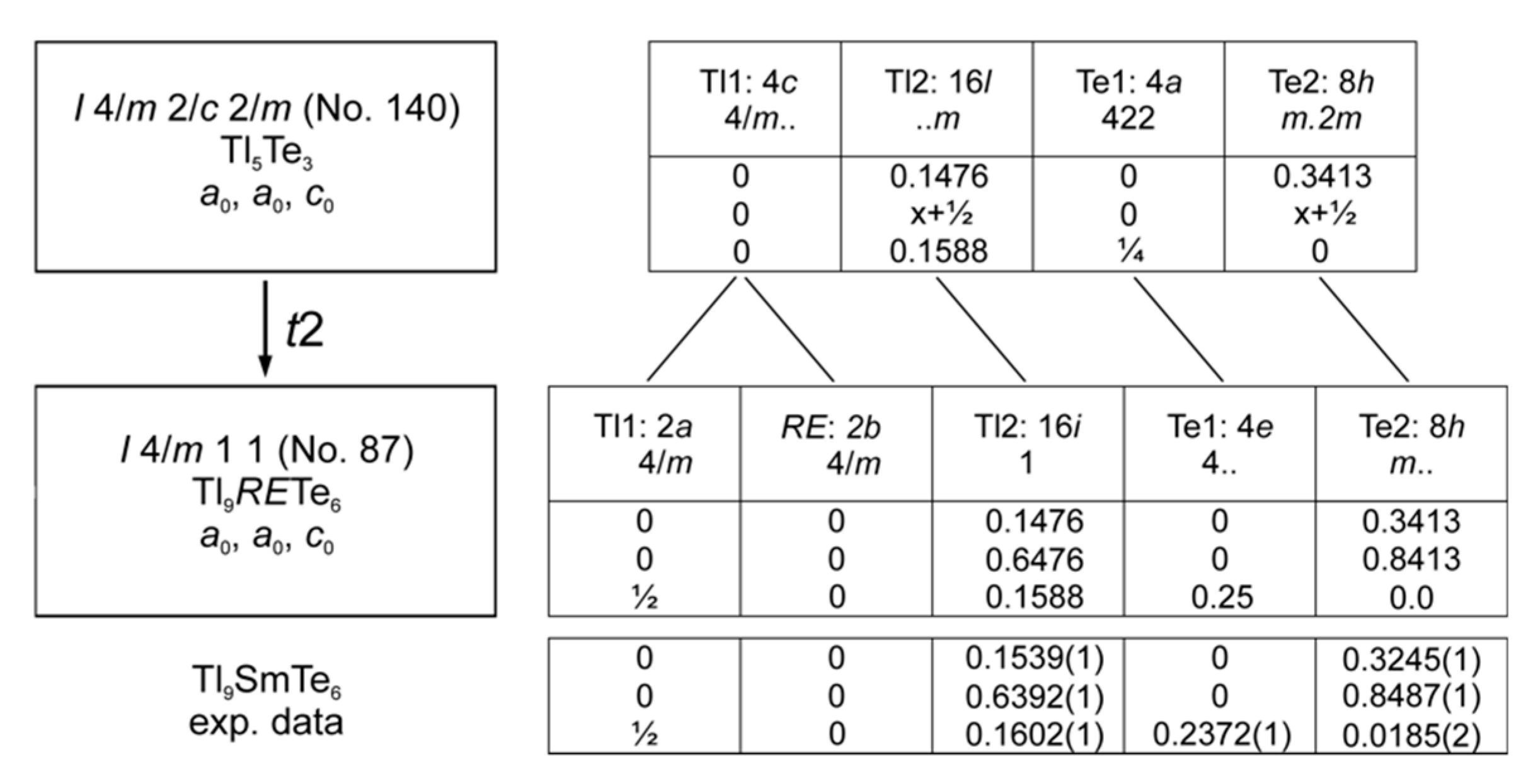
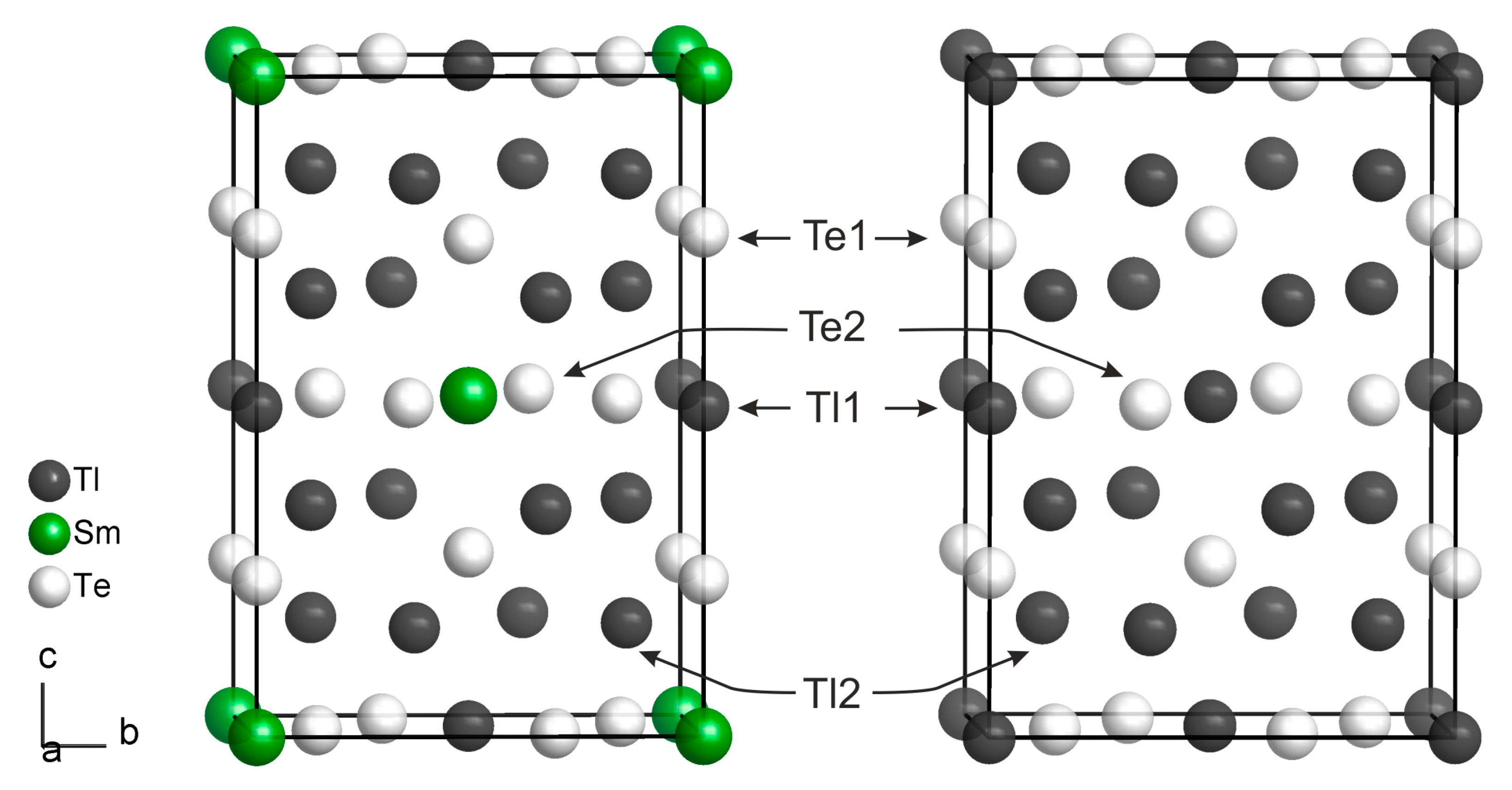
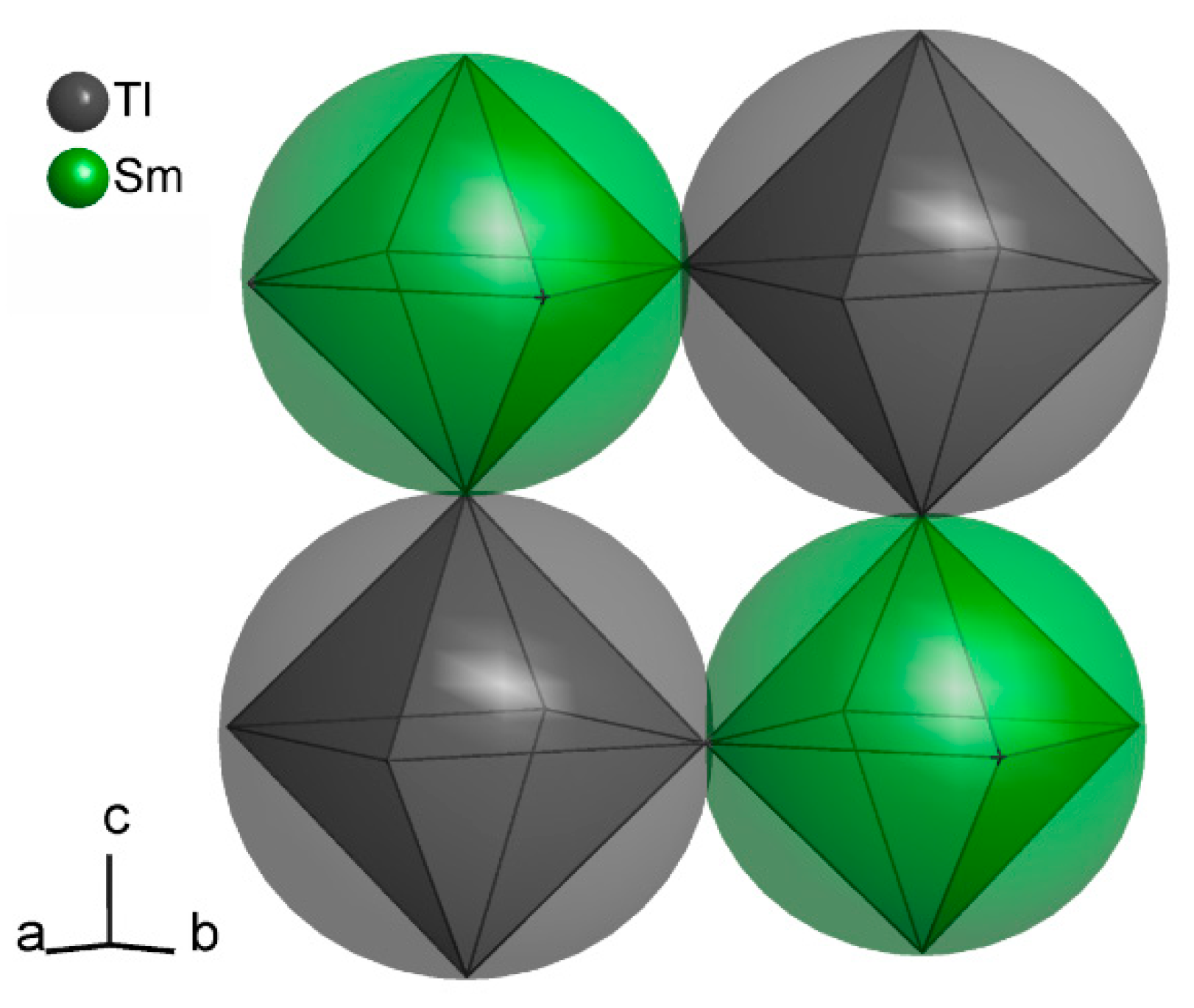
2.4. Crystal Structure Determination
2.5. Electronic Structure Calculations
2.6. Magnetic Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wölfing, B.; Kloc, C.; Teubner, J.; Bucher, E. High performance thermoelectric Tl9BiTe6 with an extremely low thermal conductivity. Phys. Rev. B 2001, 86, 4350–4353. [Google Scholar]
- Yamanaka, S.; Kosuka, A.; Korosaki, K. Thermoelectric properties of Tl9BiTe6. J. Alloys Compd. 2003, 352, 275–278. [Google Scholar] [CrossRef]
- Guo, Q.; Kleinke, H. Thermoelectric properties of hot-pressed Tl9LnTe6 (Ln = La, Ce, Pr, Nd, Sm, Gd, Tb) and Tl10−xLaxTe6 (0.90 ≤ x ≤ 1.05). J. Alloys Compd. 2015, 630, 37–42. [Google Scholar] [CrossRef]
- Shi, Y.; Sturm, C.; Kleinke, H. Chalcogenides as thermoelectric materials. J. Solid State Chem. 2019, 270, 273–279. [Google Scholar] [CrossRef]
- Sankar, C.R.; Bangarigadu-Sanasy, S.; Assoud, A.; Kleinke, H. Syntheses, crystal structures and thermoelectric properties of two new thallium tellurides: Tl4ZrTe4 and Tl4HfTe4. J. Mater. Chem. 2010, 20, 7485–7490. [Google Scholar] [CrossRef]
- Bangarigadu-Sanasy, S.; Sankar, R.; Assoud, A.; Kleinke, H. Crystal structures and thermoelectric properties of the series Tl10−xLaxTe6 with 0.2 ≤ x ≤ 1.15. Dalton Trans. 2011, 40, 862–867. [Google Scholar] [CrossRef]
- Kuropatwa, B.A.; Assoud, A.; Kleinke, H. Phase range and physical properties of the thallium tin tellurides Tl10−xSnxTe6 (x ≤ 2.2). J. Alloys Compd. 2011, 509, 6768–6772. [Google Scholar] [CrossRef]
- Sankar, C.R.; Bangarigadu-Sanasy, S.; Kleinke, H. Thermoelectric Properties of TlGdQ2 (Q = Se, Te) and Tl9GdTe6. J. Electron. Mater. 2012, 41, 1663–1666. [Google Scholar] [CrossRef]
- Bangarigadu-Sanasy, S.; Sankar, C.R.; Schlender, P.; Kleinke, H. Thermoelectric properties of Tl10−xLnxTe6, with Ln= Ce, Pr, Nd, Sm, Gd, Tb, Dy, Ho and Er, and 0.25 ≤ x ≤ 1.32. J. Alloys Compd. 2013, 549, 126–134. [Google Scholar] [CrossRef]
- Bangarigadu-Sanasy, S.; Sankar, C.R.; Dube, P.A.; Greedan, J.E.; Kleinke, H. Magnetic properties of Tl9LnTe6, Ln= Ce, Pr, Tb and Sm. J. Alloys Compd. 2014, 589, 389–392. [Google Scholar] [CrossRef]
- Kuropatwa, B.A.; Guo, Q.; Assoud, A.; Kleinke, H. Optimization of the Telluride Tl10−x−ySnxBiyTe6 for the Thermoelectric Energy Conversion. Z. Anorg. Allg. Chem. 2014, 640, 774–780. [Google Scholar] [CrossRef]
- Arpino, K.E.; Wallace, D.C.; Nie, Y.F.; Birol, T.; King, P.D.C.; Chatterjee, S.; Uchida, M.; Koohpayeh, S.M.; Wen, J.-J.; Page, K.; et al. Evidence for topologically protected surface states and a superconducting phase in [Tl4](Tl1−xSnx)Te3 using photoemission, specific heat, and magnetization measurements, and density functional theory. Phys. Rev. Lett. 2014, 112, 017002. [Google Scholar] [CrossRef] [PubMed]
- Bhan, S.; Schubert, K. Kristallstruktur von Tl5Te3 und Tl2Te3. J. Less-Common Met. 1970, 20, 229–235. [Google Scholar] [CrossRef]
- Schewe, I.; Böttcher, P.; von Schnering, H.G. The crystal structure of Tl5Te3 and its relationship to the Cr5B3 type. Z. Kristallogr. 1989, 188, 287–298. [Google Scholar] [CrossRef]
- Berg, L.G.; Abdul’manov, A.G. Pseudobinary System Bi2Te3–Tl9BiTe6. Izv. Akad. Nauk SSSR Neorg. Mater. 1970, 12, 2192–2193. [Google Scholar]
- Voroshilov, Y.V.; Gurzan, M.I.; Kish, Z.Z.; Lada, L.V. Phase equilibria in the Tl-Pb-Te system and crystal structure of Tl4B4X3 and Tl9B5X6 compounds. Inorg. Mater. 1988, 24, 1265–1269. [Google Scholar]
- Doert, T.; Höffkes, S.; Klein, C.; Böttcher, P. The crystal structures of AgTl4Te3 and AgTl4Te3. Z. Kristallogr. Suppl. 1991, 3, 52. [Google Scholar]
- Bradtmöller, S.; Böttcher, P. Darstellung und Kristallstruktur von SnTl4Te3 und PbTl4Te3. Z. Anorg. Allg. Chem. 1993, 619, 1155–1160. [Google Scholar] [CrossRef]
- Bradtmöller, S.; Böttcher, P. Crystal structure of molybdenum tetrathallium tritelluride, MoTl4Te3. Z. Kristallogr. 1994, 209, 75. [Google Scholar] [CrossRef]
- Doert, T.; Böttcher, P. Crystal structure of antimony nonathallium hexatelluride, SbTl9Te6. Z. Kristallogr. 1994, 209, 96. [Google Scholar] [CrossRef]
- Bradtmöller, S.; Böttcher, P. Crystal structure of copper tetrathallium tritelluride, CuTl4Te3. Z. Kristallogr. 1994, 209, 97. [Google Scholar] [CrossRef]
- Tokura, Y.; Yasuda, K.; Tsukazaki, A. Magnetic topological insulators. Nat. Rev. Phys. 2019, 1, 126–143. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr.-Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- AZtech; Oxford Instruments: Oxford, UK, 2013.
- Apex Suite; Bruker-AXS: Madison, WI, USA, 2013.
- Sheldrick, G.M. (Ed.) Sadabs; Bruker AXS: Karlsruhe, Germany, 2002. [Google Scholar]
- Diamond 4; Version 4.6.1; Structure Visualization Software; Crystal Impact GbR: Bonn, Germany, 2019.
- The Elk FP–LAPW Code, Version 1.4.22. Available online: http://elk.sourceforge.net (accessed on 17 March 2019).
- Shick, A.B.; Liechtenstein, A.I.; Pickett, W.E. Implementation of the LDA+ U method using the full-potential linearized augmented plane-wave basis. Phys. Rev. B 1999, 60, 10763. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Nordell, K.J.; Miller, G.J. Electronic structure, superconductivity, and substitution patterns in TI5Te3. J. Alloys Comp. 1996, 241, 51–62. [Google Scholar] [CrossRef]
- Blundell, S. Magnetism in Condensed Matter; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
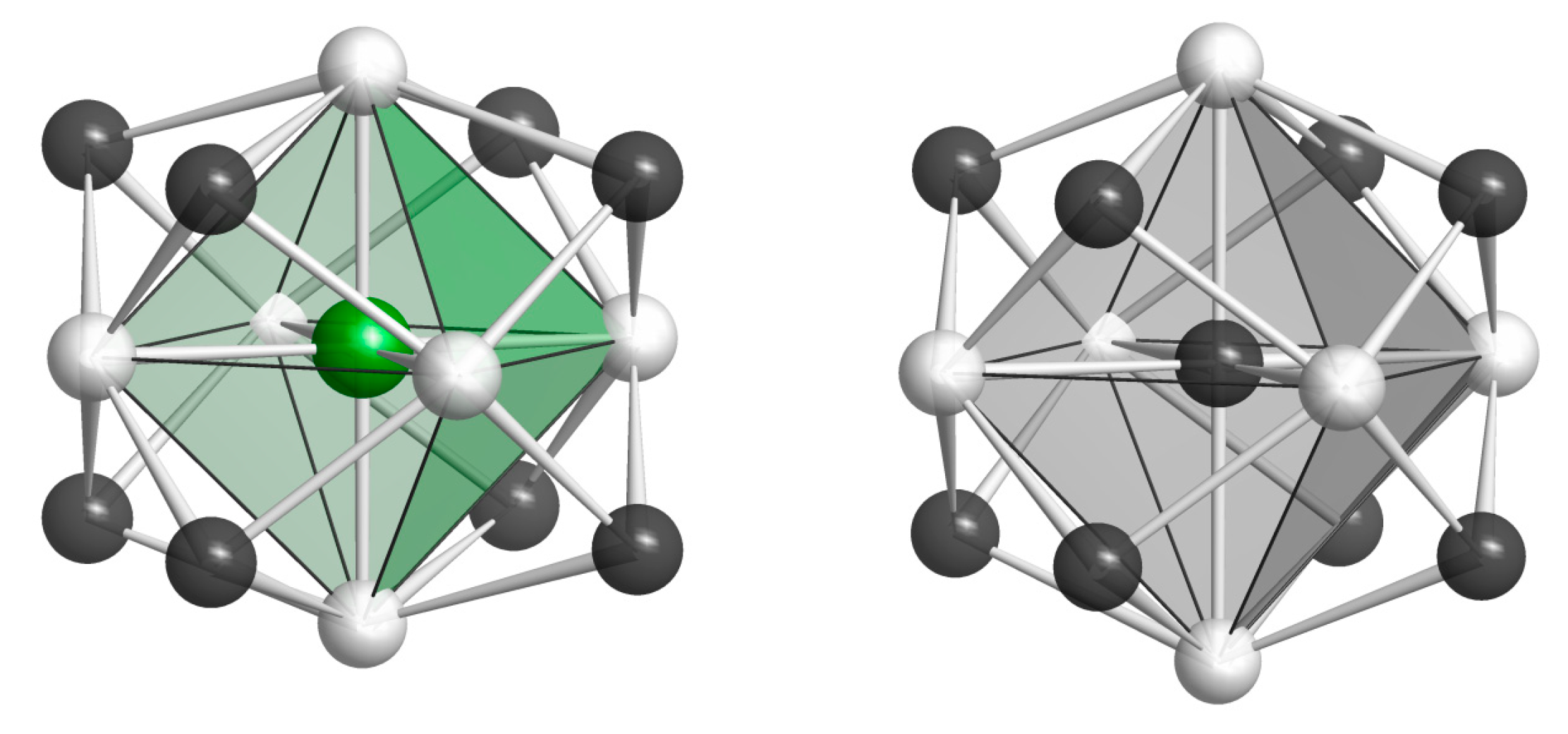
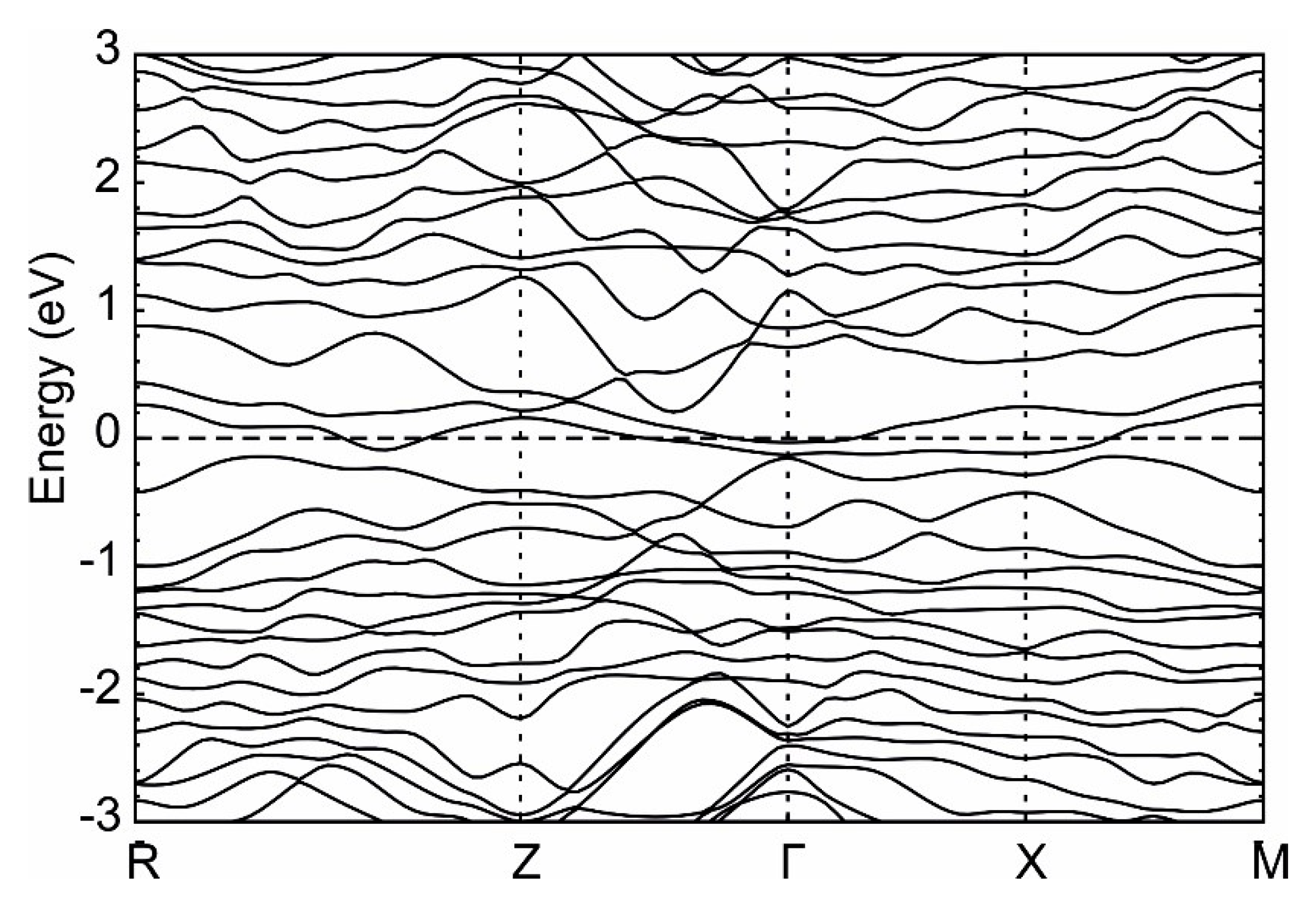
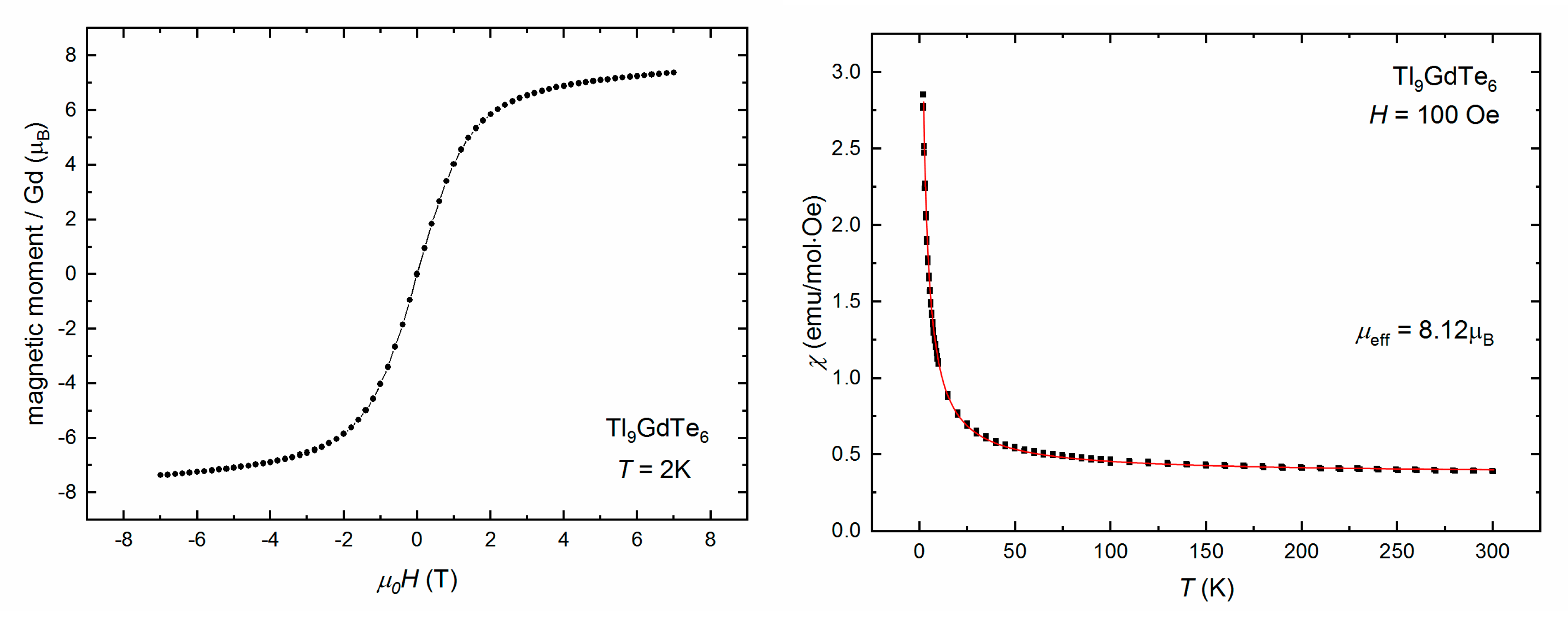
| Element | Tl9CeTe6 | Tl9SmTe6 | Tl9GdTe6 | Theor. |
|---|---|---|---|---|
| RE | 5.7(4) | 5.9(4) | 6.4(2) | 6.2 |
| Tl | 56.0(3) | 55.3(5) | 55.1(3) | 56.3 |
| Te | 38.3(3) | 38.8(2) | 38.5(4) | 37.5 |
| Chemical Composition | Tl9CeTe6 | Tl9SmTe6 | Tl9GdTe6 |
|---|---|---|---|
| Mr/g·mol−1, F(000) | 2745.1, 2189 | 2755.4, 2206 | 2762.3, 2210 |
| temperature/K | 295(1) | ||
| diffractometer type | Kappa Apex II CCD (Bruker-AXS) | ||
| wavelength/Å | 0.71069 (Mo Kα, graphite monochromator) | ||
| crystal system | tetragonal | ||
| space group (No.), Z | I4/m (no. 87), 2 | ||
| cell parameters *, a/pm | 890.48(2) | 886.50(5) | 887.00(3) |
| c/pm | 1313.42(5) | 1306.41(8) | 1306.56(5) |
| cell volume/106 pm3 | 1041.48(5) | 1026.7(1) | 1027.96(6) |
| density/g·cm−3 | 8.75 | 8.91 | 8.92 |
| Abs. coefficient/mm−1 | 79.69 | 81.48 | 81.75 |
| absorption correction | multi-scan (SADABS [26]) | ||
| - | 0.289/0.749 | 0.292/0.746 | 0.499/0.747 |
| crystal size / mm3 | 0.189 × 0.057 × 0.045 | 0.021 × 0.027 × 0.043 | 0.075 × 0.061 × 0.035 |
| measurement range | 2.8 ≤ θ ≤ 42.6 −13 ≤ h ≤ 15 −15 ≤ k ≤ 15 −15 ≤ l ≤ 24 | 2.8 ≤ θ ≤ 34.7 −11 ≤ h ≤ 14 −9 ≤ k ≤ 14 −20 ≤ l ≤ 20 | 2.8 ≤ θ ≤ 35.2 −14 ≤ h ≤ 13 −14 ≤ k ≤ 11 −17 ≤ l ≤ 19 |
| Rint | 0.0328 | 0.071 | 0.030 |
| refinement | JANA2006, full-matrix least squares on F2 [23] | ||
| reflections, with I > 3σ(I), parameters | 1693, 1458, 25 | 1153, 795, 24 | 1151, 830, 25 |
| extinction parameter | 0.118(2) | - | 0.0059(7) |
| goodness-of-fit | 1.5 | 1.1 | 1.2 |
| R1(obs), wR2(obs) | 0.031, 0.065 | 0.035, 0.062 | 0.026, 0.049 |
| R1(all), wR2(all) | 0.038, 0.066 | 0.059, 0.069 | 0.050, 0.055 |
| twin matrix | 0 1 0, 1 0 0, 0 0 −1 | ||
| twin volume fraction | 0.518(2) | 0.672(2) | 0.997(1) |
| Δρmin / Δρmax, e/106 pm3 | 2.57/−2.02 | 2.02/−3.38 | 2.29/−2.12 |
| Atom | x | y | z | Ueq, pm2 |
|---|---|---|---|---|
| Tl9CeTe6 | ||||
| Ce | 0 | 0 | 0 | 170(1) |
| Tl1 | 0 | 0 | ½ | 252(1) |
| Tl2 | 0.1557(1) | 0.6377(1) | 0.1600(1) | 335(1) |
| Te1 | 0 | 0 | 0.2403(1) | 120(1) |
| Te2 | 0.3284(1) | 0.8466(1) | 0 | 180(1) |
| Tl9SmTe6 | ||||
| Sm | 0 | 0 | 0 | 173(3) |
| Tl1 | 0 | 0 | ½ | 0273(3) |
| Tl2 | 0.1539(1) | 0.6392(1) | 0.1602(1) | 339(1) |
| Te1 | 0 | 0 | 0.2372(1) | 201(3) |
| Te2 | 0.3245(1) | 0.8487(1) | 0 | 185(2) |
| Tl9GdTe6 | ||||
| Gd | 0 | 0 | 0 | 173(3) |
| Tl1 | 0 | 0 | ½ | 273(3) |
| Tl2 | 0.1539(1) | 0.6392(1) | 0.1602(1) | 339(1) |
| Te1 | 0 | 0 | 0.2372(1) | 201(3) |
| Te2 | 0.3245(1) | 0.8487(1) | 0 | 185(2) |
| Tl9CeTe6 | |||||||
| Ce– | Te1 | 315.7(1) | 2× | Tl1– | Te1 | 341.1(1) | 2× |
| Te2 | 322.8(1) | 4× | Te2 | 344.4(1) | 4× | ||
| Tl2 | 409.2(1) | 8× | Tl2 | 391.4(1) | 8× | ||
| Tl2– | Tl2 | 352.3(1) | Tl2– | Te2 | 320.0(1) | ||
| 352.8(1) | 2× | 346.4(1) | |||||
| 370.2(1) | 347.6(1) | ||||||
| Te1 | 355.2(1) | ||||||
| 366.6(1) | |||||||
| Tl9SmTe6 | |||||||
| Sm– | Te1 | 309.9(1) | 2× | Tl1– | Te1 | 343.3(1) | 2× |
| Te2 | 317.5(1) | 4× | Te2 | 346.0(1) | 4× | ||
| Tl2 | 405.9(1) | 8× | Tl2 | 391.3(1) | 8× | ||
| Tl2– | Tl2 | 350.2(1) | Tl2– | Te2 | 318.1(1) | ||
| 350.3(1) | 2× | 343.6(1) | |||||
| 362.0(1) | 348.7(1) | ||||||
| Te1 | 356.8(1) | ||||||
| 362.0(1) | |||||||
| Tl9GdTe6 | |||||||
| Gd– | Te1 | 309.4(1) | 2× | Tl1– | Te1 | 343.9(1) | 2× |
| Te2 | 316.9(1) | 4× | Te2 | 346.9(1) | 4× | ||
| Tl2 | 405.7(1) | 8× | Tl2 | 392.0(1) | 8× | ||
| Tl2– | Tl2 | 350.1(1) | Tl2– | Te2 | 318.2(1) | ||
| 350.4(1) | 2× | 343.1(1) | |||||
| 368.2(1) | 349.7(1) | ||||||
| Te1 | 357.6(1) | ||||||
| 361.5(1) | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaeva, A.; Schönemann, R.; Doert, T. Syntheses, Crystal Structure and Magnetic Properties of Tl9RETe6 (RE = Ce, Sm, Gd). Crystals 2020, 10, 277. https://doi.org/10.3390/cryst10040277
Isaeva A, Schönemann R, Doert T. Syntheses, Crystal Structure and Magnetic Properties of Tl9RETe6 (RE = Ce, Sm, Gd). Crystals. 2020; 10(4):277. https://doi.org/10.3390/cryst10040277
Chicago/Turabian StyleIsaeva, Anna, Rico Schönemann, and Thomas Doert. 2020. "Syntheses, Crystal Structure and Magnetic Properties of Tl9RETe6 (RE = Ce, Sm, Gd)" Crystals 10, no. 4: 277. https://doi.org/10.3390/cryst10040277
APA StyleIsaeva, A., Schönemann, R., & Doert, T. (2020). Syntheses, Crystal Structure and Magnetic Properties of Tl9RETe6 (RE = Ce, Sm, Gd). Crystals, 10(4), 277. https://doi.org/10.3390/cryst10040277







