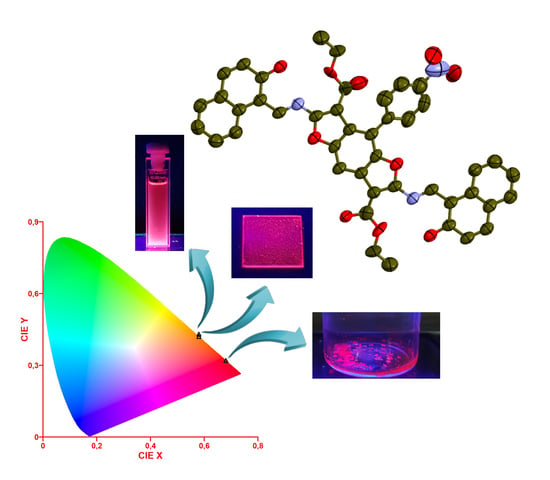
A Novel DR/NIR T-Shaped AIEgen: Synthesis and X-Ray Crystal Structure Study
Abstract
1. Introduction
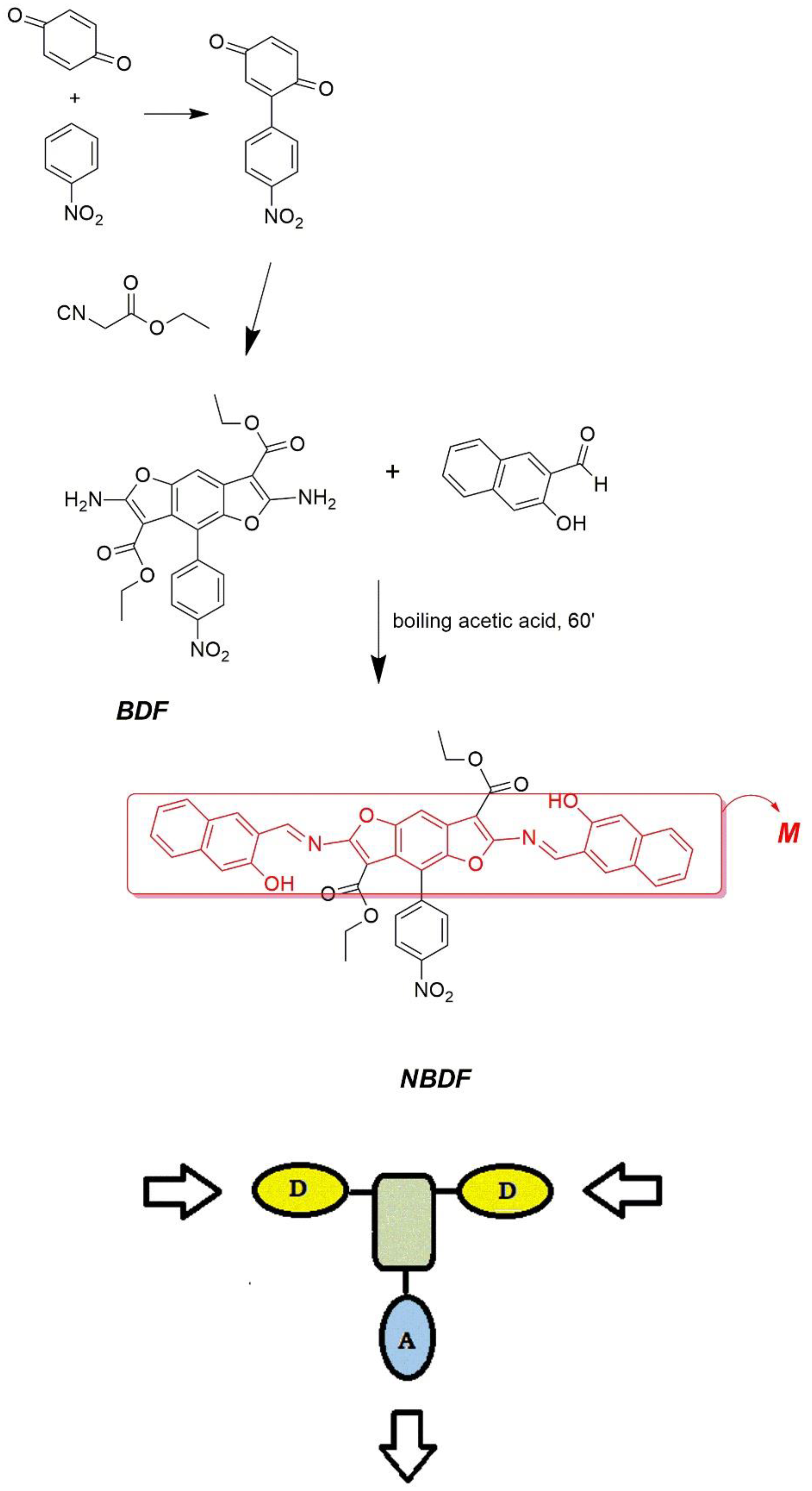
2. Experimental
2.1. Synthesis of NBDF
2.2. X-Ray Crystallography
3. Results and Discussion
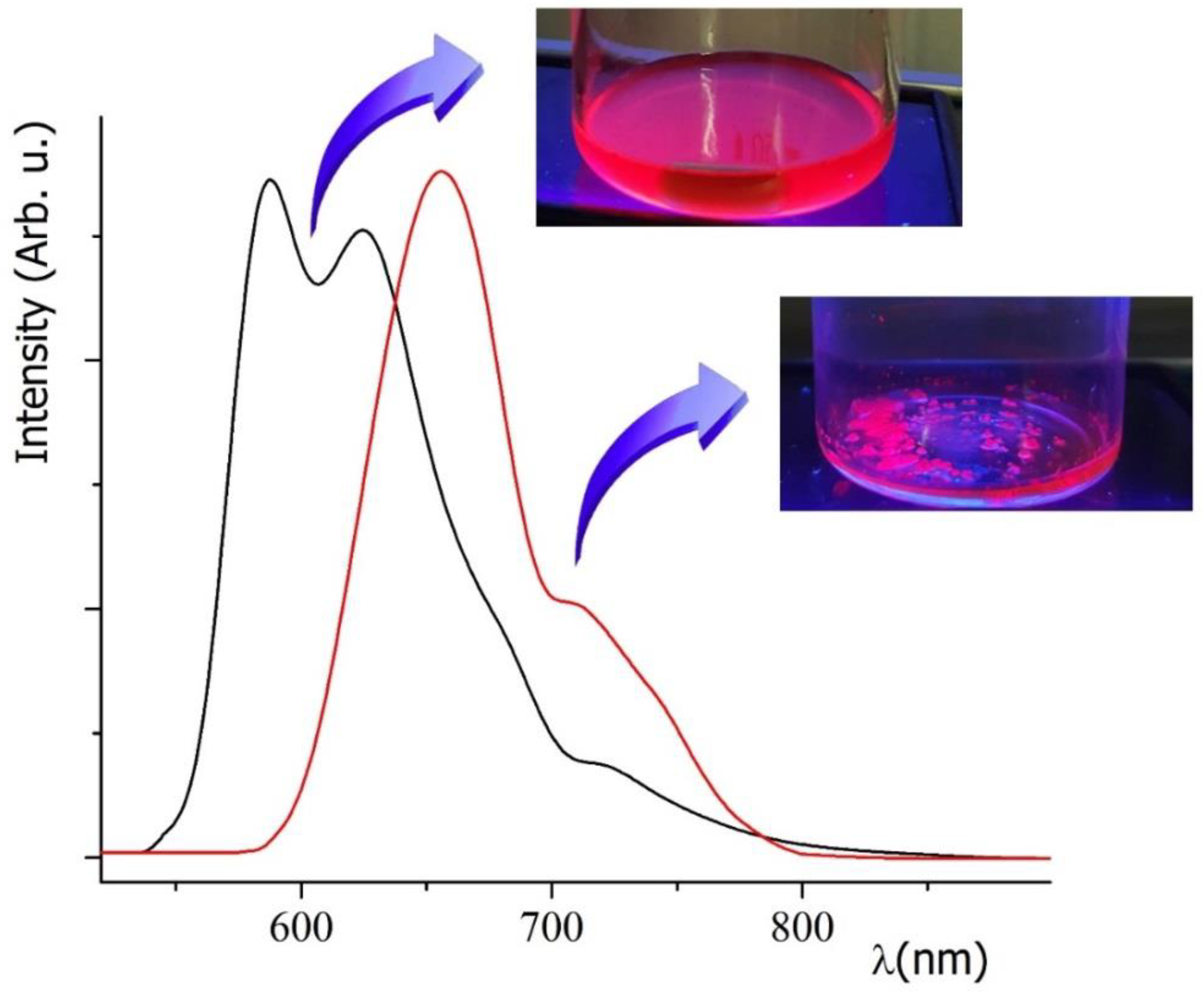
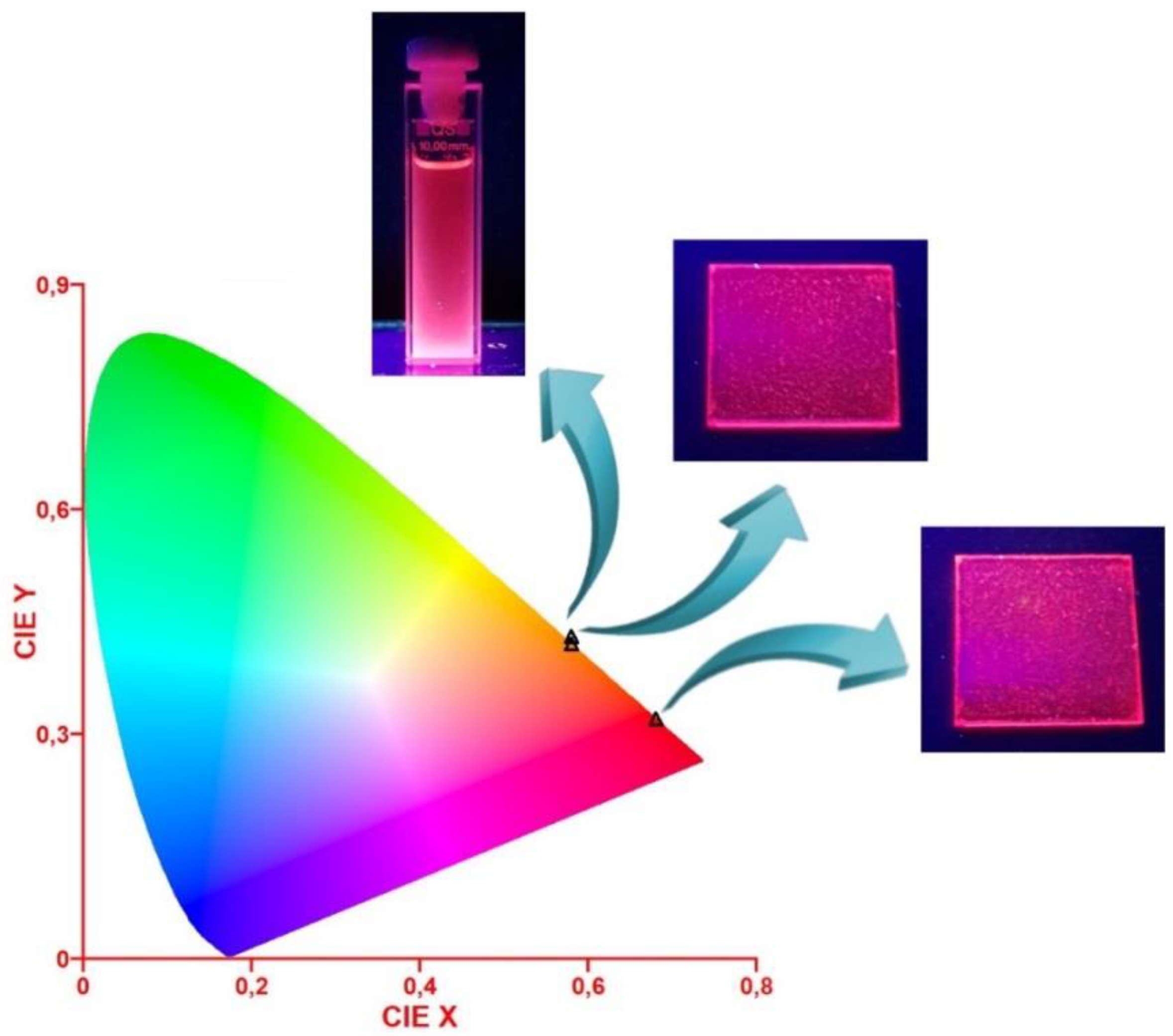
3.1. Spectroscopic Behavior
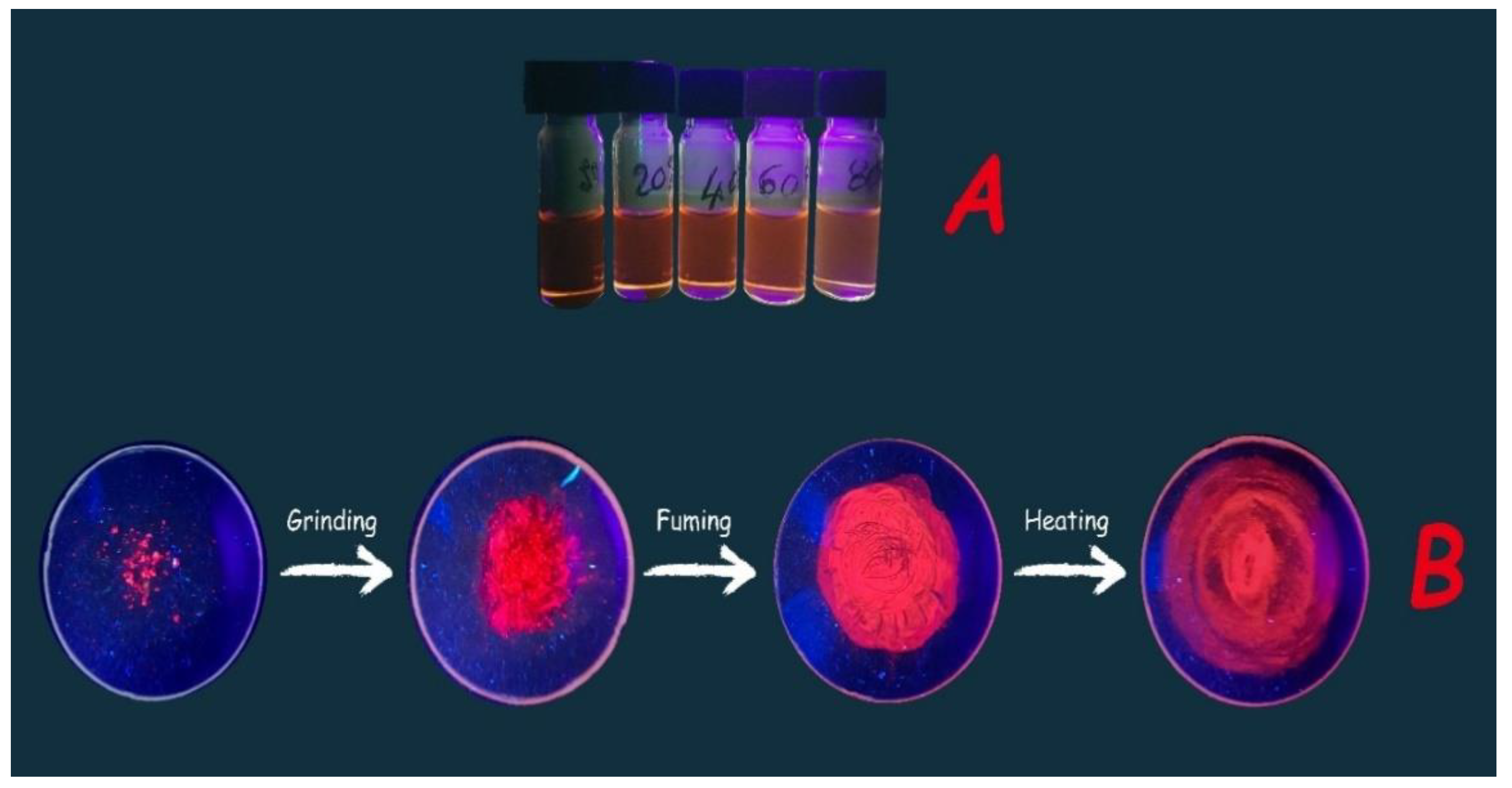
3.2. Production of a Red Emissive Polymeric Layer
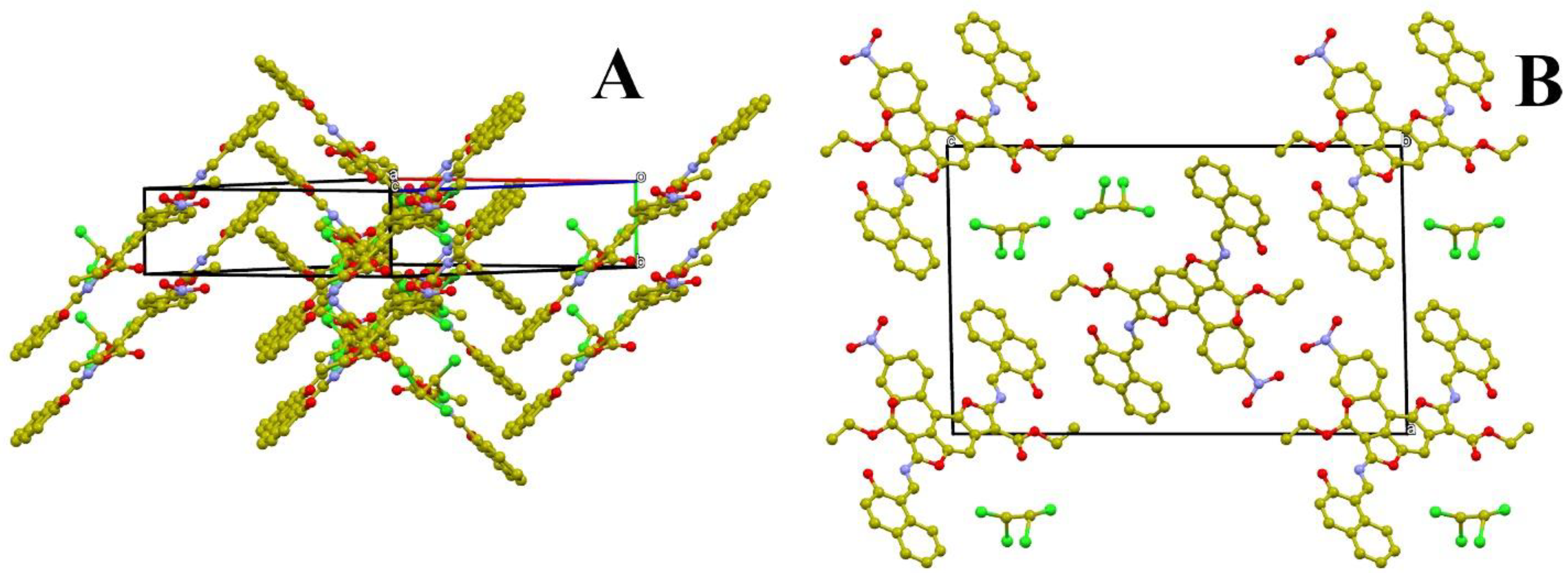
3.3. Single-Crystal X-Ray Structure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, W.; Pan, Y.; Xiao, R.; Peng, Q.; Zhang, S.; Ma, D.; Li, F.; Shen, F.; Wang, Y.; Yang, B.; et al. Employing ∼100% Excitons in OLEDs by Utilizing a Fluorescent Molecule with Hybridized Local and Charge-Transfer Excited State. Adv. Funct. Mater. 2014, 24, 1609–1614. [Google Scholar] [CrossRef]
- Wang, S.; Yan, X.; Cheng, Z.; Zhang, H.; Liu, Y.; Wang, Y. Highly Efficient Near-Infrared Delayed Fluorescence Organic Light Emitting Diodes Using a Phenanthrene-Based Charge-Transfer Compound. Angew. Chem. 2015, 54, 13068–13072. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhang, S.; Wang, R.; Li, W.; Shen, F.; Yang, B.; Ma, Y. Highly efficient near-infrared organic light-emitting diode based on a butterfly-shaped donor-acceptor chromophore with strong solid-state fluorescence and a large proportion of radiative excitons. Angew. Chem. 2014, 53, 2119–2123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Song, J.; Qu, J.; Li, B.; Zhu, W.; Wong, W.-Y. Near-Infrared Emitting Materials: Near-Infrared Emitting Materials via Harvesting Triplet Excitons: Molecular Design, Properties, and Application in Organic Light Emitting Diodes (Advanced Optical Materials 18/2018). Adv. Opt. Mater. 2018, 6, 1870070. [Google Scholar] [CrossRef]
- Anthony, S.P. Organic Solid-State Fluorescence: Strategies for Generating Switchable and Tunable Fluorescent Materials. ChemPlusChem 2012, 77, 518–531. [Google Scholar] [CrossRef]
- Kwok, R.T.; Leung, C.W.; Lam, J.W.; Tang, B.Z. Biosensing by luminogens with aggregation-induced emission characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef]
- Li, K.; Liu, Y.; Li, Y.; Feng, Q.; Hou, H.; Tang, B.Z. 2,5-bis(4-alkoxycarbonylphenyl)-1,4-diaryl-1,4-dihydropyrrolo[3,2-b]pyrrole (AAPP) AIEgens: Tunable RIR and TICT characteristics and their multifunctional applications. Chem. Sci. 2017, 8, 7258–7267. [Google Scholar] [CrossRef]
- Lu, H.; Zheng, Y.; Zhao, X.; Wang, L.; Ma, S.; Han, X.; Xu, B.; Tian, W.; Gao, H. Highly Efficient Far Red/Near-Infrared Solid Fluorophores: Aggregation-Induced Emission, Intramolecular Charge Transfer, Twisted Molecular Conformation, and Bioimaging Applications. Angew. Chem. 2016, 55, 155–159. [Google Scholar] [CrossRef]
- Borbone, F.; Carella, A.; Caruso, U.; Roviello, G.; Tuzi, A.; Dardano, P.; Lettieri, S.; Maddalena, P.; Barsella, A. Large second-order NLO activity in poly(4-vinylpyridine) grafted with PdII and CuII chromophoric complexes with tridentate bent ligands containing heterocycles. Eur. J. Inorg. Chem. 2008, 1846–1853. [Google Scholar] [CrossRef]
- Hong, Y.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Diana, R.; Panunzi, B.; Concilio, S.; Marrafino, F.; Shikler, R.; Caruso, T.; Caruso, U. The Effect of Bulky Substituents on Two π-Conjugated Mesogenic Fluorophores. Their Organic Polymers and Zinc-Bridged Luminescent Networks. Polymers 2019, 11, 1379. [Google Scholar] [CrossRef] [PubMed]
- Diana, R.; Panunzi, B.; Piotto, S.; Caruso, T.; Caruso, U. Solid-state fluorescence of two zinc coordination polymers from bulky dicyano-phenylenevinylene and bis-azobenzene cores. Inorg. Chem. Commun. 2019, 110, 107602. [Google Scholar] [CrossRef]
- Iida, A.; Yamaguchi, S. Intense solid-state blue emission with a small Stokes’ shift: Pi-stacking protection of the diphenylanthracene skeleton. Chem. Commun. 2009, 3002–3004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Chen, S.; Lam, J.W.; Lu, P.; Zhong, Y.; Wong, K.S.; Kwok, H.S.; Tang, B.Z. Creation of highly efficient solid emitter by decorating pyrene core with AIE-active tetraphenylethene peripheries. Chem. Commun. 2010, 46, 2221–2223. [Google Scholar] [CrossRef]
- Jiménez, Á.J.; Lin, M.-J.; Burschka, C.; Becker, J.; Settels, V.; Engels, B.; Würthner, F. Structure–property relationships for 1,7-diphenoxy-perylene bisimides in solution and in the solid state. Chem. Sci. 2014, 5, 608–619. [Google Scholar] [CrossRef]
- Shimizu, M.; Fukui, H.; Natakani, M.; Sakaguchi, H. Aggregation-Induced Orange-to-Red Fluorescence of 2,5-Bis(diarylamino)terephthalic Acid Dithioesters. Eur. J. Org. Chem. 2016, 2016, 5950–5956. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 1740–1741. [Google Scholar] [CrossRef]
- Wan, Q.; Tong, J.; Zhang, B.; Li, Y.; Wang, Z.; Tang, B.Z. Exploration of High Efficiency AIE-Active Deep/Near-Infrared Red Emitters in OLEDs with High-Radiance. Adv. Opt. Mater. 2019, 8, 1901520. [Google Scholar] [CrossRef]
- Englman, R.; Jortner, J. The energy gap law for radiationless transitions in large molecules. Mol. Phys. 1970, 18, 145–164. [Google Scholar] [CrossRef]
- Bixon, M.; Jortner, J.; Cortes, J.; Heitele, H.; Michel-Beyerle, M.E. Energy Gap Law for Nonradiative and Radiative Charge Transfer in Isolated and in Solvated Supermolecules. J. Phys.Chem. 1994, 98, 7289–7299. [Google Scholar] [CrossRef]
- Burshtein, A.I.; Krissinel, E. Free Energy Gap Law under Diffusion Control. J. Phys.Chem. 1996, 100, 3005–3015. [Google Scholar] [CrossRef]
- Yang, W.; Liu, C.; Gao, Q.; Du, J.; Shen, P.; Liu, Y.; Yang, C. A morphology and size-dependent ON-OFF switchable NIR-emitting naphthothiazolium cyanine dye: AIE-active CIEE effect. Opt. Mater. 2017, 66, 623–629. [Google Scholar] [CrossRef]
- Guo, Z.H.; Lei, T.; Jin, Z.X.; Wang, J.Y.; Pei, J. T-shaped donor-acceptor molecules for low-loss red-emission optical waveguide. Org. Lett. 2013, 15, 3530–3533. [Google Scholar] [CrossRef]
- Guo, Z.; Park, S.; Yoon, J.; Shin, I. Recent progress in the development of near-infrared fluorescent probes for bioimaging applications. Chem. Soc. Rev. 2014, 43, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Zhen, S.; Wang, S.; Li, S.; Luo, W.; Gao, M.; Ng, L.G.; Goh, C.C.; Qin, A.; Zhao, Z.; Liu, B.; et al. Efficient Red/Near-Infrared Fluorophores Based on Benzo[1,2-b:4,5-b′]dithiophene 1,1,5,5-Tetraoxide for Targeted Photodynamic Therapy and In Vivo Two-Photon Fluorescence Bioimaging. Adv. Funct. Mater. 2018, 28, 1706945. [Google Scholar] [CrossRef]
- Green, O.; Gnaim, S.; Blau, R.; Eldar-Boock, A.; Satchi-Fainaro, R.; Shabat, D. Near-Infrared Dioxetane Luminophores with Direct Chemiluminescence Emission Mode. J. Am. Chem. Soc. 2017, 139, 13243–13248. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Ding, D.; Liu, J.; Yuan, W.Z.; Hu, Y.; Liu, B.; Tang, B.Z. Biocompatible Nanoparticles with Aggregation-Induced Emission Characteristics as Far-Red/Near-Infrared Fluorescent Bioprobes for In Vitro and In Vivo Imaging Applications. Adv. Funct. Mater. 2012, 22, 771–779. [Google Scholar] [CrossRef]
- Qian, J.; Tang, B.Z. AIE Luminogens for Bioimaging and Theranostics: From Organelles to Animals. Chem 2017, 3, 56–91. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Z.; Wang, H.; Zhou, B.; Zhu, S.; Zhong, Y.; Wang, J.; Wan, H.; Antaris, A.; Ma, R.; et al. Rational Design of Molecular Fluorophores for Biological Imaging in the NIR-II Window. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Han, X.; Bai, Q.; Yao, L.; Liu, H.; Gao, Y.; Li, J.; Liu, L.; Liu, Y.; Li, X.; Lu, P.; et al. Highly Efficient Solid-State Near-Infrared Emitting Material Based on Triphenylamine and Diphenylfumaronitrile with an EQE of 2.58% in Nondoped Organic Light-Emitting Diode. Adv. Funct. Mater. 2015, 25, 7521–7529. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Cai, M.; Li, Y.; Zhang, D.; Qiu, Y.; Duan, L. Towards highly efficient red thermally activated delayed fluorescence materials by the control of intra-molecular pi-pi stacking interactions. Nanotechnology 2016, 27, 094001. [Google Scholar] [CrossRef] [PubMed]
- Porzio, W.; Destri, S.; Giovanella, U.; Pasini, M.; Motta, T.; Natali, D.; Sampietro, M.; Campione, M. Fluorenone–thiophene derivative for organic field effect transistors: A combined structural, morphological and electrical study. Thin Solid Films 2005, 492, 212–220. [Google Scholar] [CrossRef]
- Ekbote, A.; Jadhav, T.; Misra, R. T-Shaped donor–acceptor–donor type tetraphenylethylene substituted quinoxaline derivatives: Aggregation-induced emission and mechanochromism. New J. Chem. 2017, 41, 9346–9353. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural changes accompanying intramolecular electron transfer: Focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef]
- Kanibolotsky, A.L.; Forgie, J.C.; McEntee, G.J.; Talpur, M.M.; Skabara, P.J.; Westgate, T.D.; McDouall, J.J.; Auinger, M.; Coles, S.J.; Hursthouse, M.B. Controlling the conformational changes in donor-acceptor [4]-dendralenes through intramolecular charge-transfer processes. Chemistry 2009, 15, 11581–11593. [Google Scholar] [CrossRef][Green Version]
- Soni, J.N.; Soman, S.S. Synthesis and antimicrobial evaluation of amide derivatives of benzodifuran-2-carboxylic acid. Eur. J. Med. Chem. 2014, 75, 77–81. [Google Scholar] [CrossRef]
- Xie, F.; Zhu, H.; Zhang, H.; Lang, Q.; Tang, L.; Huang, Q.; Yu, L. In vitro and in vivo characterization of a benzofuran derivative, a potential anticancer agent, as a novel Aurora B kinase inhibitor. Eur. J. Med. Chem. 2015, 89, 310–319. [Google Scholar] [CrossRef]
- Roviello, G.N.; Roviello, V.; Musumeci, D.; Pedone, C. Synthesis of a novel benzodifuran derivative and its molecular recognition of poly rA RNA. Biol. Chem. 2013, 394, 1235–1239. [Google Scholar] [CrossRef]
- Carella, A.; Roviello, V.; Iannitti, R.; Palumbo, R.; La Manna, S.; Marasco, D.; Trifuoggi, M.; Diana, R.; Roviello, G.N. Evaluating the biological properties of synthetic 4-nitrophenyl functionalized benzofuran derivatives with telomeric DNA binding and antiproliferative activities. Int. J. Biol. Macromol. 2019, 121, 77–88. [Google Scholar] [CrossRef]
- Shariat, M.; Abdollahi, S. Synthesis of benzoxazinone derivatives: A new route to 2 (N phthaloylmethyl)-4H-3,1-benzoxazin-4-one. Molecules 2004, 9, 705–712. [Google Scholar] [CrossRef]
- Papadopoulos, E.P.; Torres, C.D. A Simple Preparation of 2-Aryl-4H-3,1-benzoxazin-4-ones. Heterocycles 1982, 19, 1039. [Google Scholar] [CrossRef]
- Caruso, U.; Panunzi, B.; Roviello, G.N.; Roviello, G.; Tingoli, M.; Tuzi, A. Synthesis, structure and reactivity of amino-benzodifurane derivatives. C. R. Chim. 2009, 12, 622–634. [Google Scholar] [CrossRef]
- Beagley, B.; Flowers, W.T.; Hafees, A.; Pritchard, R.G. p- and o-Bis(N,N-diacetylamino)benzene. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 1971–1973. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Marrafino, F.; Piotto, S.; Caruso, U. Novel Dicyano-Phenylenevinylene Fluorophores for Low-Doped Layers: A Highly Emissive Material for Red OLEDs. Polymers 2019, 11, 1751. [Google Scholar] [CrossRef]
- Panunzi, B.; Concilio, S.; Diana, R.; Shikler, R.; Nabha, S.; Piotto, S.; Sessa, L.; Tuzi, A.; Caruso, U. Photophysical Properties of Luminescent Zinc(II)‒Pyridinyloxadiazole Complexes and their Glassy Self-Assembly Networks. Eur. J. Inorg. Chem. 2018, 2018, 2709–2716. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Palma, S.D.; Fusco, S.; Nabha, S.; Panunzi, B.; Shikler, R. High solid state photoluminescence quantum yields and effective color tuning in polyvinylpyridine based zinc(II) metallopolymers. Macromol. Chem. Phys. 2015, 216, 1516–1522. [Google Scholar] [CrossRef]
- Obushak, M.D.; Martyak, R.L.; Matiychuk, V.S. Synthesis of heterocycles on the basis of arylation products of unsaturated compounds. Part 9. Dialkyl 2,6-diamino-4-arylfuro[2′,3′:4,5]benzo[b]furan-3,7-dicarboxylates from 2-aryl-1,4-benzoquinones and cyanoacetic esters. Pol. J. Chem. 2002, 76, 1419–1424. [Google Scholar] [CrossRef]
- Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 133–144. [Google Scholar] [CrossRef]
- Evans, P.R. An introduction to data reduction: Space-group determination, scaling and intensity statistics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 282–292. [Google Scholar] [CrossRef]
- Burla, M.C.; Carrozzini, B.; Cascarano, G.L.; Giacovazzo, C.; Polidori, G. Solving proteins at non-atomic resolution by direct methods: Update. J. Appl. Crystallogr. 2017, 50, 1048–1055. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGXandORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Müller, P.; Herbst-Irmer, R.; Spek, A.L.; Schneider, T.R. Crystal Structure Refinement: A Crystallographer’s Guide to SHELXL; Oxford University Press: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Felouat, A.; D’Aleo, A.; Charaf-Eddin, A.; Jacquemin, D.; Le Guennic, B.; Kim, E.; Lee, K.J.; Woo, J.H.; Ribierre, J.C.; Wu, J.W.; et al. Tuning the Direction of Intramolecular Charge Transfer and the Nature of the Fluorescent State in a T-Shaped Molecular Dyad. J. Phys. Chem. A 2015, 119, 6283–6295. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. A symmetrical azo-based fluorophore and the derived salen multipurpose framework for emissive layers. Inorg. Chem. Commun. 2019, 104, 186–189. [Google Scholar] [CrossRef]
- Diana, R.; Panunzi, B.; Shikler, R.; Nabha, S.; Caruso, U. Highly efficient dicyano-phenylenevinylene fluorophore as polymer dopant or zinc-driven self-assembling building block. Inorg. Chem. Commun. 2019, 104, 145–149. [Google Scholar] [CrossRef]
- Panunzi, B.; Borbone, F.; Capobianco, A.; Concilio, S.; Diana, R.; Peluso, A.; Piotto, S.; Tuzi, A.; Velardo, A.; Caruso, U. Synthesis, spectroscopic properties and DFT calculations of a novel multipolar azo dye and its zinc(II) complex. Inorg. Chem. Commun. 2017, 84, 103–108. [Google Scholar] [CrossRef]
- Mishra, V.R.; Ghanavatkar, C.W.; Sekar, N. ESIPT clubbed azo dyes as deep red emitting fluorescent molecular rotors: Photophysical properties, pH study, viscosity sensitivity, and DFT studies. J. Lumin. 2019, 215, 116689. [Google Scholar] [CrossRef]
- Warde, U.; Sekar, N. NLOphoric mono-azo dyes with negative solvatochromism and in-built ESIPT unit from ethyl 1,3-dihydroxy-2-naphthoate: Estimation of excited state dipole moment and pH study. Dyes Pigment. 2017, 137, 384–394. [Google Scholar] [CrossRef]
- Chen, W.; Wright, B.D.; Pang, Y. Rational design of a NIR-emitting Pd(II) sensor via oxidative cyclization to form a benzoxazole ring. Chem. Commun. 2012, 48, 3824–3826. [Google Scholar] [CrossRef] [PubMed]
- Panunzi, B.; Diana, R.; Concilio, S.; Sessa, L.; Shikler, R.; Nabha, S.; Tuzi, A.; Caruso, U.; Piotto, S. Solid-state highly efficient dr mono and poly-dicyano-phenylenevinylene fluorophores. Molecules 2018, 23, 1505. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.A.; Hisaindee, S.; Saleh, N. Spectroscopic studies of keto–enol tautomeric equilibrium of azo dyes. RSC Adv. 2015, 5, 18097–18110. [Google Scholar] [CrossRef]
- Satam, M.A.; Raut, R.K.; Sekar, N. Fluorescent azo disperse dyes from 3-(1,3-benzothiazol-2-yl)naphthalen-2-ol and comparison with 2-naphthol analogs. Dyes Pigments 2013, 96, 92–103. [Google Scholar] [CrossRef]
- Borbone, F.; Caruso, U.; Concilio, S.; Nabha, S.; Piotto, S.; Shikler, R.; Tuzi, A.; Panunzi, B. From cadmium(II)-aroylhydrazone complexes to metallopolymers with enhanced photoluminescence. A structural and DFT study. Inorg. Chim. Acta 2017, 458, 129–137. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, W.; Hao, X.; Redshaw, C.; Chen, L.; Sun, W.-H. 6-Benzhydryl-4-methyl-2-(1H-benzoimidazol-2-yl)phenol ligands and their zinc complexes: Syntheses, characterization and photoluminescence behavior. Inorg. Chim. Acta 2012, 392, 345–353. [Google Scholar] [CrossRef]
- Panunzi, B.; Diana, R.; Caruso, U. A Highly Efficient White Luminescent Zinc (II) Based Metallopolymer by RGB Approach. Polymers 2019, 11, 1712. [Google Scholar] [CrossRef]
- Roviello, A.; Borbone, F.; Carella, A.; Diana, R.; Roviello, G.; Panunzi, B.; Ambrosio, A.; Maddalena, P. High quantum yield photoluminescence of new polyamides containing oligo-PPV amino derivatives and related oligomers. J. Polym. Sci. Part. A Polym. Chem. 2009, 47, 2677–2689. [Google Scholar] [CrossRef]
- Sk, B.; Khodia, S.; Patra, A. T and V-shaped donor-acceptor-donor molecules involving pyridoquinoxaline: large Stokes shift, environment-sensitive tunable emission and temperature-induced fluorochromism. Chem. Commun. 2018, 54, 1786–1789. [Google Scholar] [CrossRef]
- Feng, S.; Gong, S.; Feng, G. Aggregation-induced emission and solid fluorescence of fluorescein derivatives. Chem. Commun. 2020, 56, 2511–2513. [Google Scholar] [CrossRef]
- Salimimarand, M.; La, D.D.; Kobaisi, M.A.; Bhosale, S.V. Flower-like superstructures of AIE-active tetraphenylethylene through solvophobic controlled self-assembly. Sci. Rep. 2017, 7, 42898. [Google Scholar] [CrossRef] [PubMed]
- Salimimarand, M.; La, D.; Bhosale, S.; Jones, L.; Bhosale, S. Influence of Odd and Even Alkyl Chains on Supramolecular Nanoarchitecture via Self-Assembly of Tetraphenylethylene-Based AIEgens. Appl. Sci. 2017, 7, 1119. [Google Scholar] [CrossRef]
- Lee, C.S.; Park, J.T.; Kim, J.H. Structural color-tunable mesoporous bragg stack layers based on graft copolymer self-assembly for high-efficiency solid-state dye-sensitized solar cells. J. Power Sources 2016, 324, 637–645. [Google Scholar] [CrossRef]


| NBDF | |
|---|---|
| CCDC number | 1988060 |
| Formula probe and solvent | C44H31N3O10·C2H2Cl4 |
| Temperature (K) | 100 |
| Wavelength (Å) | 0.7000 |
| Crystal system | Monoclinic |
| Space group | P 1 21/n 1 |
| a (Å) | 16.413(6) |
| b (Å) | 4.779(1) |
| c (Å) | 25.891(4) |
| β (°) | 91.172(11) |
| R-merge (last shell: 0.87−0.82 Å) | 0.070 (0.559) |
| CC(1/2) | 0.998 (0.895) |
| I/σ(I) | 8.4 (1.3) |
| Completeness (%) | 71.9 |
| Estimated mosaicity (°) | 0.25 |
| Volume | 2030.4 Å3 |
| Z | 2 |
| Calculated density | 1.519 g/cm3 |
| θ range for data collection (°) | 1.550 to 25.397 |
| Reflections collected / unique | 18565/2855 |
| R(int) | 0.0643 |
| Data / restraints / parameters | 2855/70/381 |
| R1 indices (I > 2σ(I), 1913) | 0.1275 (0.1538, all data) |
| wR2 | 0.345 (0.371, all data) |
| F(000) | 922 |
| Largest diff. peak and hole | 0.82 and -0.38 e-/Å3 |
| Goodness-of-fit on F2 | 1.53 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diana, R.; Caruso, U.; Di Costanzo, L.; Bakayoko, G.; Panunzi, B. A Novel DR/NIR T-Shaped AIEgen: Synthesis and X-Ray Crystal Structure Study. Crystals 2020, 10, 269. https://doi.org/10.3390/cryst10040269
Diana R, Caruso U, Di Costanzo L, Bakayoko G, Panunzi B. A Novel DR/NIR T-Shaped AIEgen: Synthesis and X-Ray Crystal Structure Study. Crystals. 2020; 10(4):269. https://doi.org/10.3390/cryst10040269
Chicago/Turabian StyleDiana, Rosita, Ugo Caruso, Luigi Di Costanzo, Gelsomina Bakayoko, and Barbara Panunzi. 2020. "A Novel DR/NIR T-Shaped AIEgen: Synthesis and X-Ray Crystal Structure Study" Crystals 10, no. 4: 269. https://doi.org/10.3390/cryst10040269
APA StyleDiana, R., Caruso, U., Di Costanzo, L., Bakayoko, G., & Panunzi, B. (2020). A Novel DR/NIR T-Shaped AIEgen: Synthesis and X-Ray Crystal Structure Study. Crystals, 10(4), 269. https://doi.org/10.3390/cryst10040269