Flux Single Crystal Growth of BaFe12−xTixO19 with Titanium Gradient
Abstract
1. Introduction
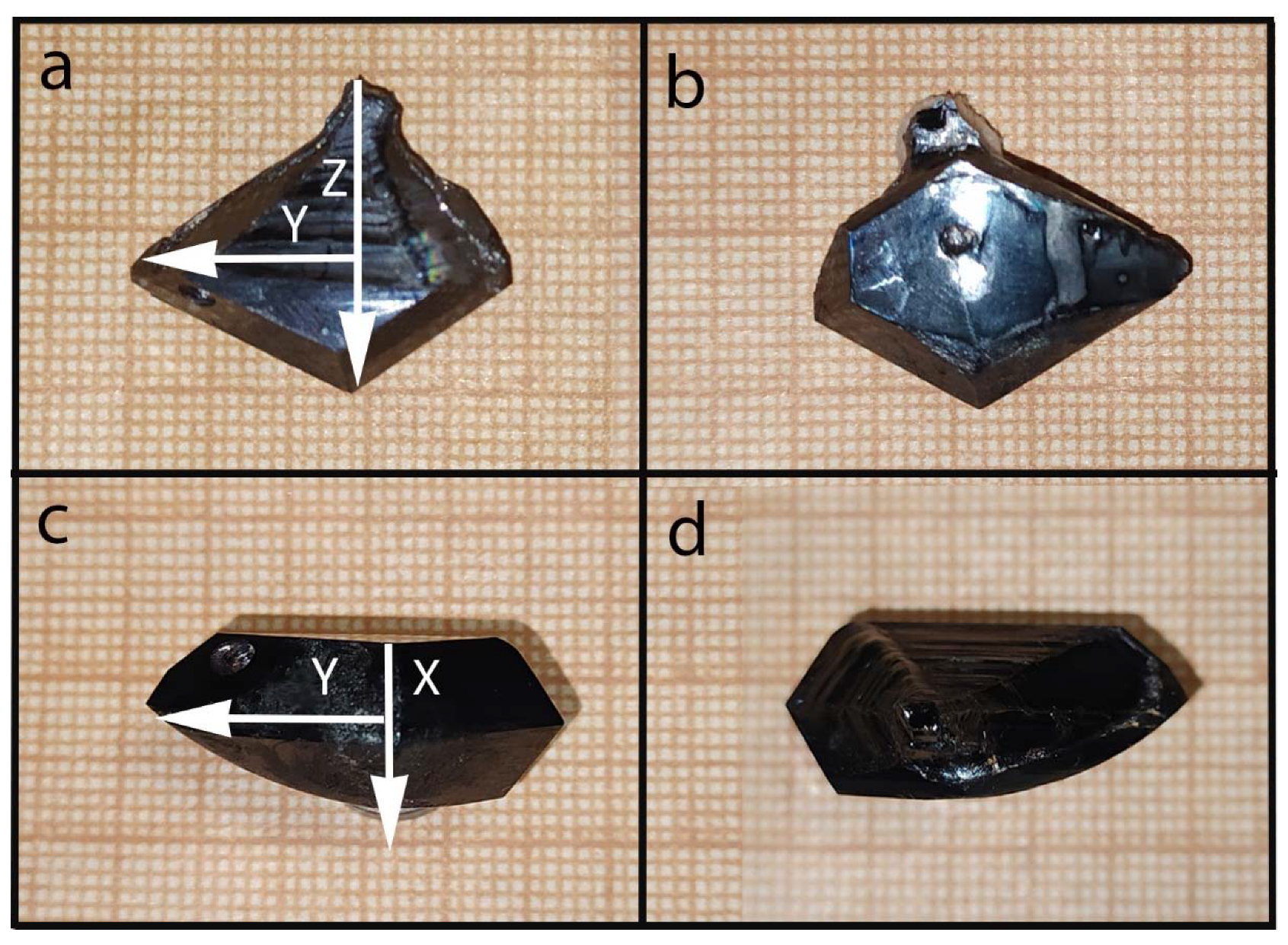
2. Materials and Methods
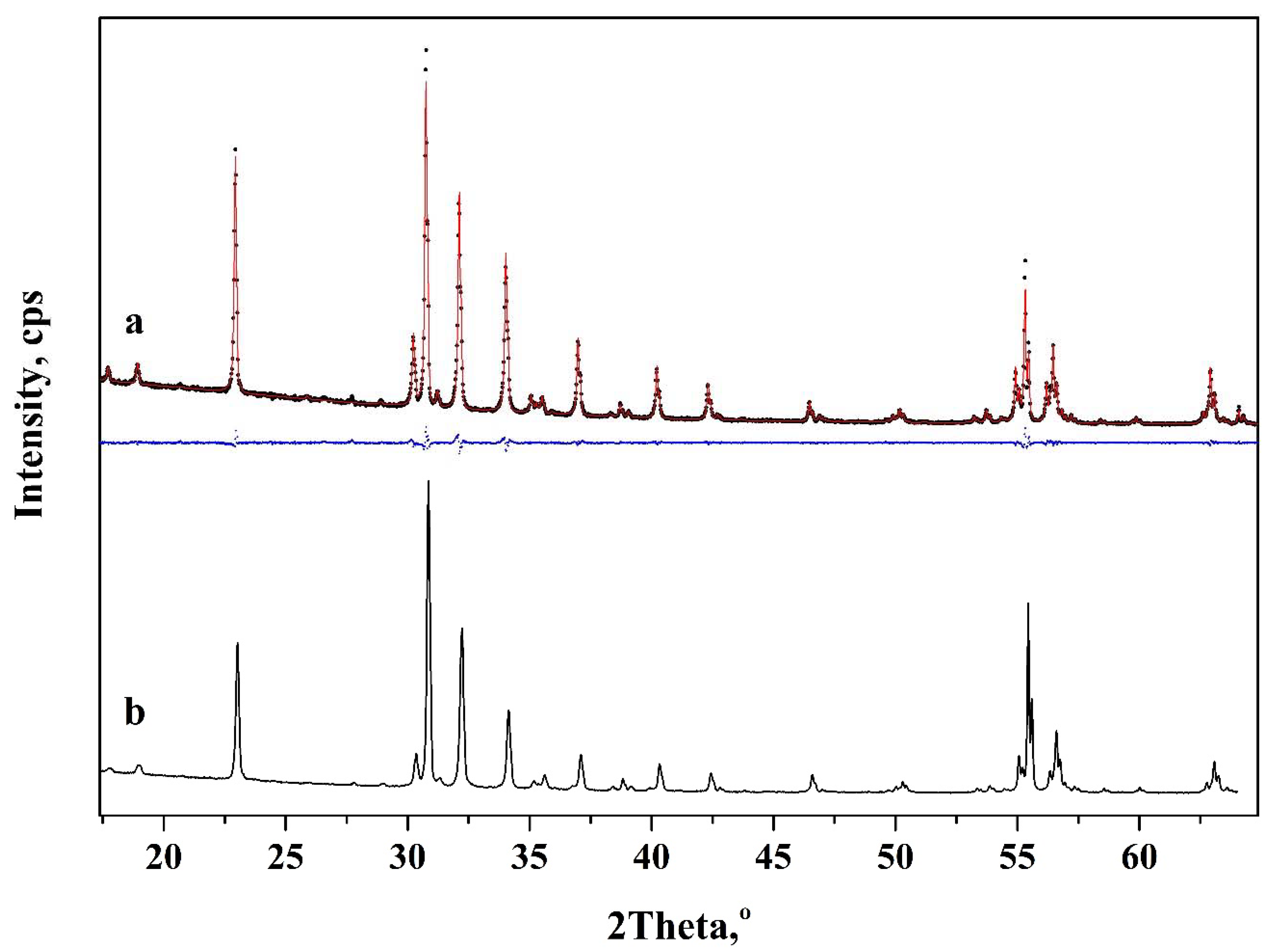
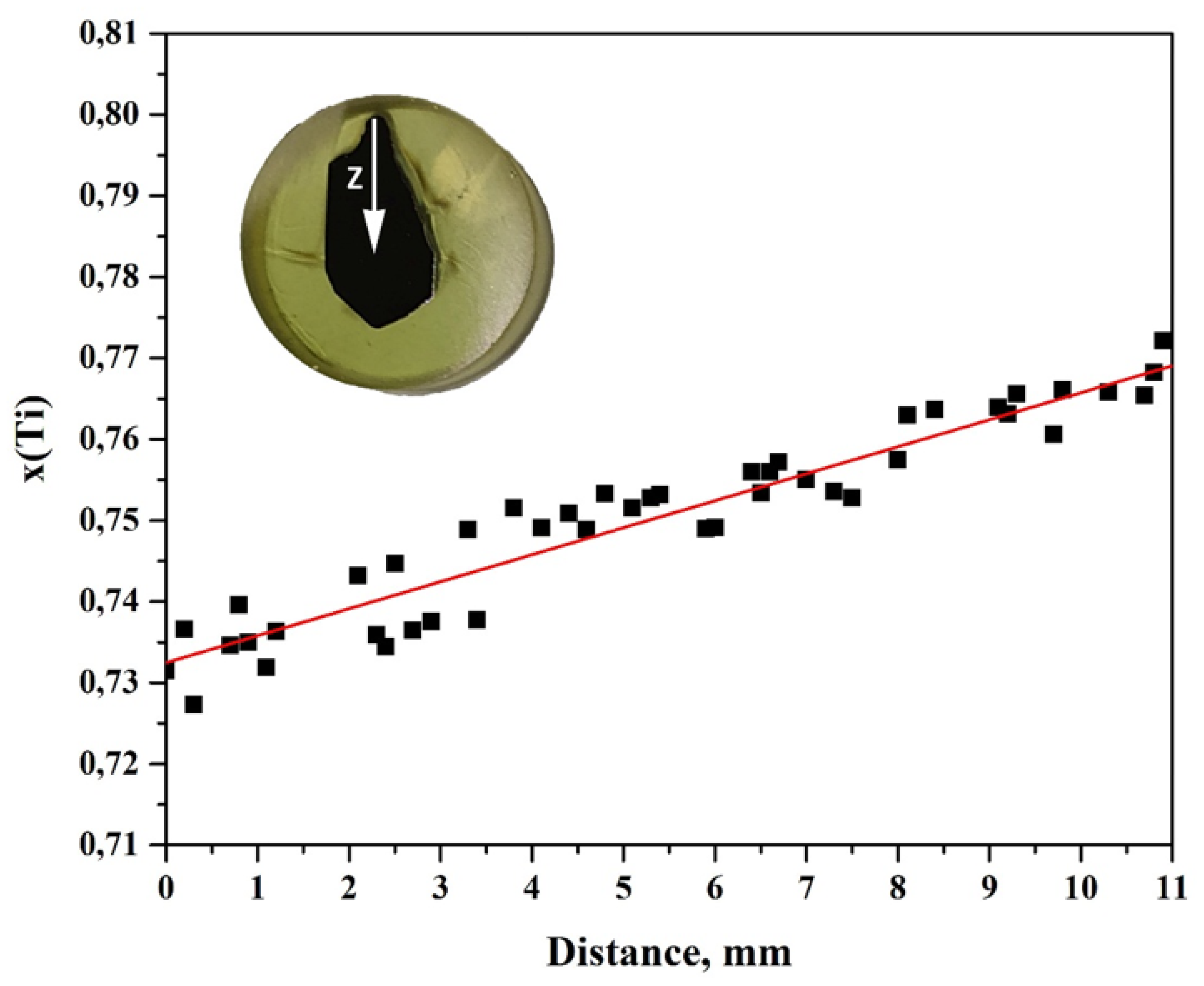
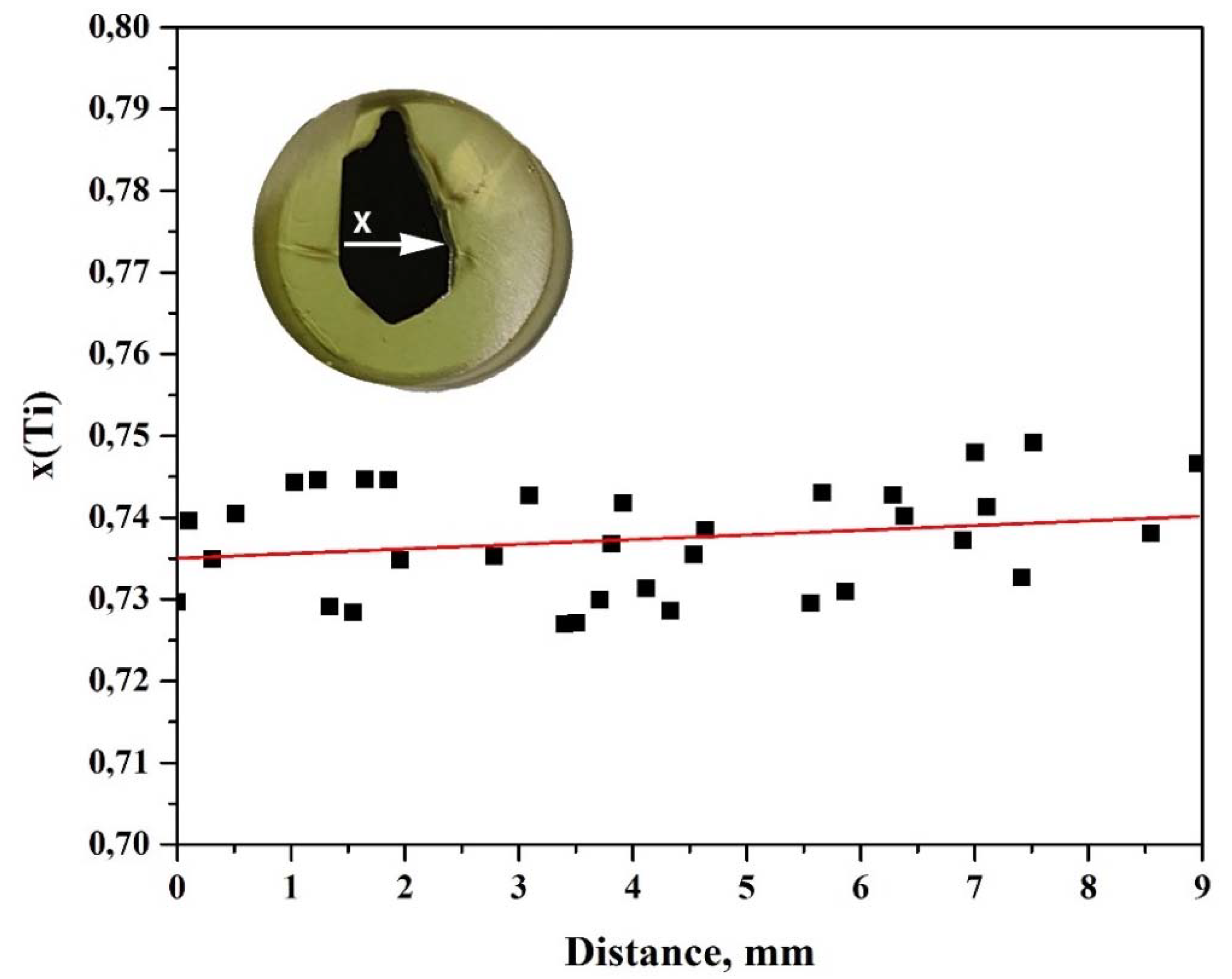
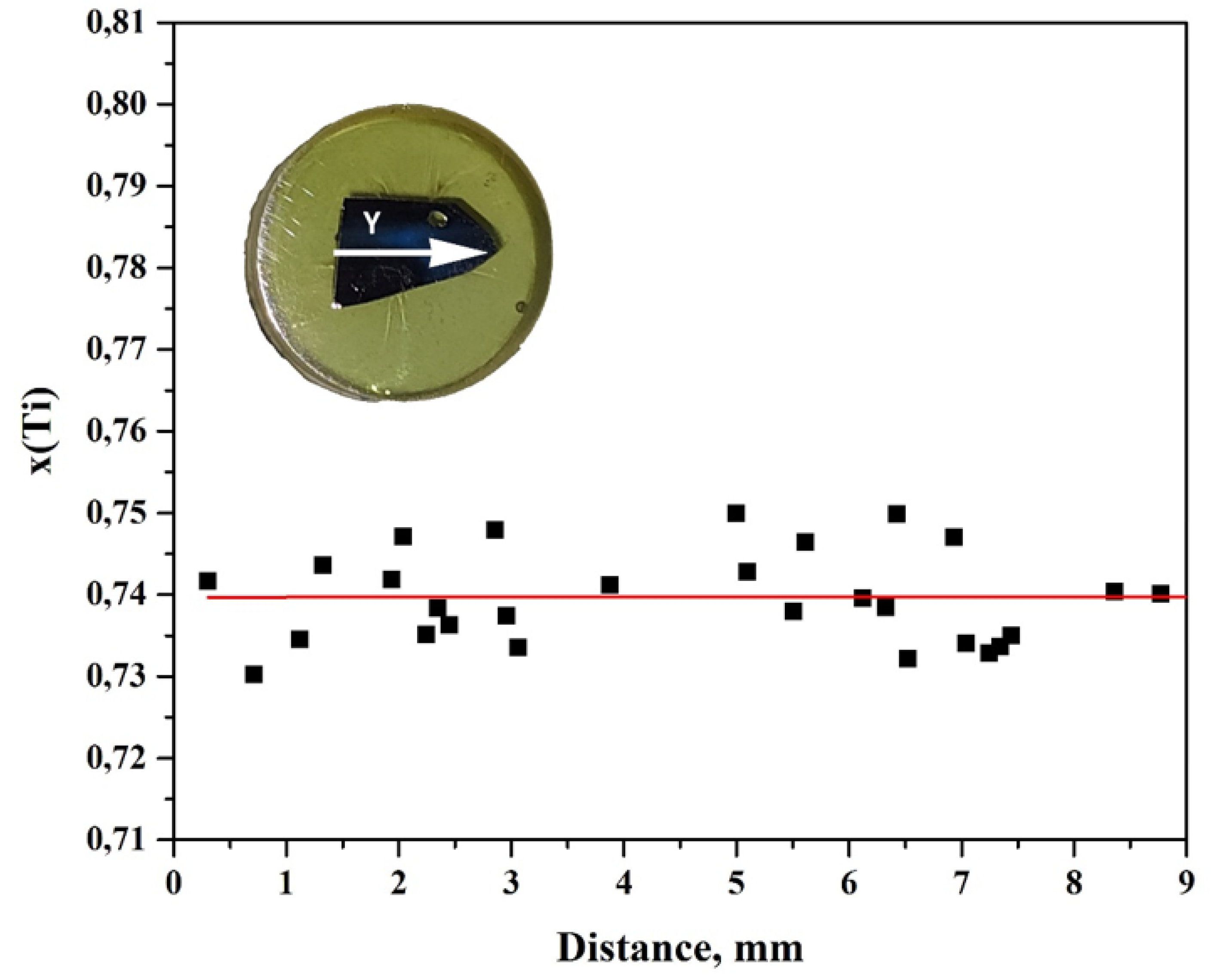
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, W.; Peng, B.; Zhang, W.; Zhou, S.; Schmid, H.T. Ultra large coercivity in barium ferrite thin films prepared by magnetron sputtering. J. Magn. Magn. Mater. 2010, 322, 1859–1862. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, W.; Peng, B.; Zhang, W. Properties of barium hexa-ferrite thin films dependent on sputtering pressure. Appl. Surf. Sci. 2011, 257, 2689–2693. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Ustinova, I.A.; Ustinov, A.B.; Gudkova, S.A.; Zherebtsov, D.A.; Trofimov, E.A.; Zabeivorota, N.S.; Mikhailov, G.G.; Niewa, R. Millimeter-wave Characterization of Aluminum Substituted Barium Lead Hexaferrite Single Crystals Grown from PbO–B2O3 Flux. Ceram. Int. 2017, 17, 15800–15804. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Klygach, D.S.; Zhivulin, V.E.; Malkin, A.I.; Vakhitov, M.G.; Gudkova, S.A.; Galimov, D.M.; Zherebtsov, D.A.; Trofimov, E.A.; Knyazev, N.S.; et al. Electromagnetic properties of BaFe12O19:Ti at centimeter wavelengths. J. Alloys Compd. 2018, 755, 177–183. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Tarasova, A.Y.; Zherebtsov, D.A.; Gudkova, S.A.; Galimov, D.M.; Zhivulin, V.E.; Trofimov, E.A.; Nemrava, S.; Perov, N.S.; Isaenko, L.I.; et al. Magnetic and structural properties of barium hexaferrite BaFe12O19 from various growth techniques. Materials 2017, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, O.V.; Vinnik, D.A.; Trofimov, E.A. The Poly-Substituted M-Type Hexaferrite Crystals Growth. Mater. Sci. Forum 2019, 946, 186–191. [Google Scholar] [CrossRef]
- Vinnik, D.; Zhivulin, V.; Trofimov, E.; Starikov, A.; Zherebtsov, D.; Zaitseva, O.; Gudkova, S.; Taskaev, S.; Klygach, D.; Vakhitov, M.; et al. Extremely Polysubstituted Magnetic Material Based on Magnetoplumbite with a Hexagonal Structure: Synthesis, Structure, Properties, Prospects. Nanomaterials 2019, 9, 559. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Trofimov, E.A.; Zhivulin, V.E.; Zaitseva, O.V.; Gudkova, S.A.; Starikov, A.Y.; Zherebtsov, D.A.; Kirsanova, A.A.; Häßner, M.; Niewa, R. High-entropy oxide phases with magnetoplumbite structure. Ceram. Int. 2019, 45, 12942–12948. [Google Scholar] [CrossRef]
- Song, Y.Y.; Ordóñez-Romero, C.L.; Wu, M. Millimeter wave notch filters based on ferromagnetic resonance in hexagonal barium ferrites. Appl. Phys. Lett. 2009, 95, 142506. [Google Scholar] [CrossRef]
- Harris, V.G. Modern microwave ferrites. IEEE Trans. Mag. 2012, 48, 1075–1104. [Google Scholar] [CrossRef]
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Marino-Castellanos, P.A.; Anglada-Rivera, J.; Cruz-Fuentes, A.; Lora-Serrano, R. Magnetic and microstructural properties of the Ti4+-doped Barium hexaferrite. J. Magn. Mater. 2004, 280, 214–220. [Google Scholar] [CrossRef]
- Geok, B.T.; Nagalingam, S.; Jefferson, D.A. Preparation and studies of Co(II) and Co(III)-substituted barium ferrite prepared by sol–gel method. Mater. Chem. Phys. 2007, 101, 158–162. [Google Scholar] [CrossRef]
- Xie, Y.; Honga, X.; Wangc, X.; Zhao, J.; Gaob, Y.; Ling, Y.; Yan, S.; Shi, L.; Zhang, K. Preparation and electromagnetic properties of La-doped barium-ferrite/polythiophene composites. Synth. Met. 2012, 162, 1643–1647. [Google Scholar] [CrossRef]
- Li, W.; Qiao, X.; Li, M.; Liu, T.; Peng, H.X. La and Co substituted M-type barium ferrites processed by sol–gel combustion synthesis. Mater. Res. Bull. 2013, 48, 4449–4453. [Google Scholar] [CrossRef]
- Kakizakia, K.; Taguchi, H.; Hiratsuka, N. Magnetic properties of La–Co substituted barium ferrite thin films withlarge magnetic anisotropy. J. Magn. Magn. Mater. 2004, 272, 2241–2243. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, J.; Wang, H.; Yan, S.; Wang, L.; Zhang, Q. Er3+−substituted W-type barium ferrite: Preparation and electromagnetic properties. J. Rare Earths 2010, 28, 940–943. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Turchenko, V.O.; Bobrikov, I.A.; Trukhanov, S.V.; Balagurov, A.I.; Kazakevich, I.S. Crystal structure and magnetic properties of BaFe12−xAlxO19 (x = 01–1.2). J. Magn. Magn. Matter. 2015, 393, 253–259. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Trukhanov, A.V.; Slimani, Y.; You, K.Y.; Trukhanov, S.V.; Trukhanova, E.L.; Esa, F.; Sadaqat, A.; Chaudhary, K.; Zdorovets, M.; et al. Correlation between composition and electrodynamics properties in nanocomposites based on hard/soft ferrimagnetics with strong exchange coupling. Nanomaterials 2019, 9, 202. [Google Scholar] [CrossRef]
- Gambino, R.J.; Leonard, F. Growth of barium ferrite single crystals. J. Am. Ceram. Soc. 1961, 44, 221–224. [Google Scholar] [CrossRef]
- Obradors, X.; Collomb, A.; Pernet, M.; Samaras, D.; Joubert, J.C. X-ray analysis of the structural and dynamic properties of BaFe12O19 hexagonal ferrite at room temperature. J. Solid State Chem. 1985, 56, 171–181. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Zherebtsov, D.A.; Mashkovtseva, L.S.; Nemrava, S.; Perov, N.S.; Semisalova, A.S.; Krivtsov, I.V.; Isaenko, L.I.; Mikhailov, G.G.; Niewa, R. Ti-Substituted BaFe12O19 Single Crystal Growth and Characterization. Cryst. Growth Des. 2014, 14, 5834–5839. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Zhivulin, V.E.; Starikov, A.Y.; Gudkova, S.A.; Trofimov, E.A.; Trukhanov, A.V.; Trukhanov, S.V.; Turchenko, V.A.; Matveev, V.V.; Lahderanta, E.; et al. Influence of titanium substitution on structure, magnetic and electric properties of barium hexaferrites BaFe12−xTixO19. J. Magn. Magn. Mater. 2020, 498, 166117. [Google Scholar] [CrossRef]
| Mass Fraction of Components | |||
|---|---|---|---|
| BaCO3 | Fe2O3 | TiO2 | Na2CO3 |
| 0.1335 | 0.4864 | 0.1623 | 0.2176 |
| Formula | a, Å | c, Å | Volume, Å3 |
|---|---|---|---|
| BaFe11.25Ti0.75O19 | 5.9014(1) | 23.224(1) | 700.4(1) |
| BaFe12O19 | 5.8935(3) | 23.193(3) | 697.6(3) |
| BaFe12O19 [21] | 5.8920(1) | 23.183(1) | 696.9(1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhivulin, V.E.; Trofimov, E.A.; Zaitseva, O.V.; Zherebtsov, D.A.; Uchaev, D.A.; Vinnik, D.A. Flux Single Crystal Growth of BaFe12−xTixO19 with Titanium Gradient. Crystals 2020, 10, 264. https://doi.org/10.3390/cryst10040264
Zhivulin VE, Trofimov EA, Zaitseva OV, Zherebtsov DA, Uchaev DA, Vinnik DA. Flux Single Crystal Growth of BaFe12−xTixO19 with Titanium Gradient. Crystals. 2020; 10(4):264. https://doi.org/10.3390/cryst10040264
Chicago/Turabian StyleZhivulin, Vladimir E., Evgeny A. Trofimov, Olga V. Zaitseva, Dmitry A. Zherebtsov, Danil A. Uchaev, and Denis A. Vinnik. 2020. "Flux Single Crystal Growth of BaFe12−xTixO19 with Titanium Gradient" Crystals 10, no. 4: 264. https://doi.org/10.3390/cryst10040264
APA StyleZhivulin, V. E., Trofimov, E. A., Zaitseva, O. V., Zherebtsov, D. A., Uchaev, D. A., & Vinnik, D. A. (2020). Flux Single Crystal Growth of BaFe12−xTixO19 with Titanium Gradient. Crystals, 10(4), 264. https://doi.org/10.3390/cryst10040264





