Optimized Corrosion Performance of AISI 1345 Steel in Hydrochloric Acid Through Thermo-Mechanical Cyclic Annealing Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
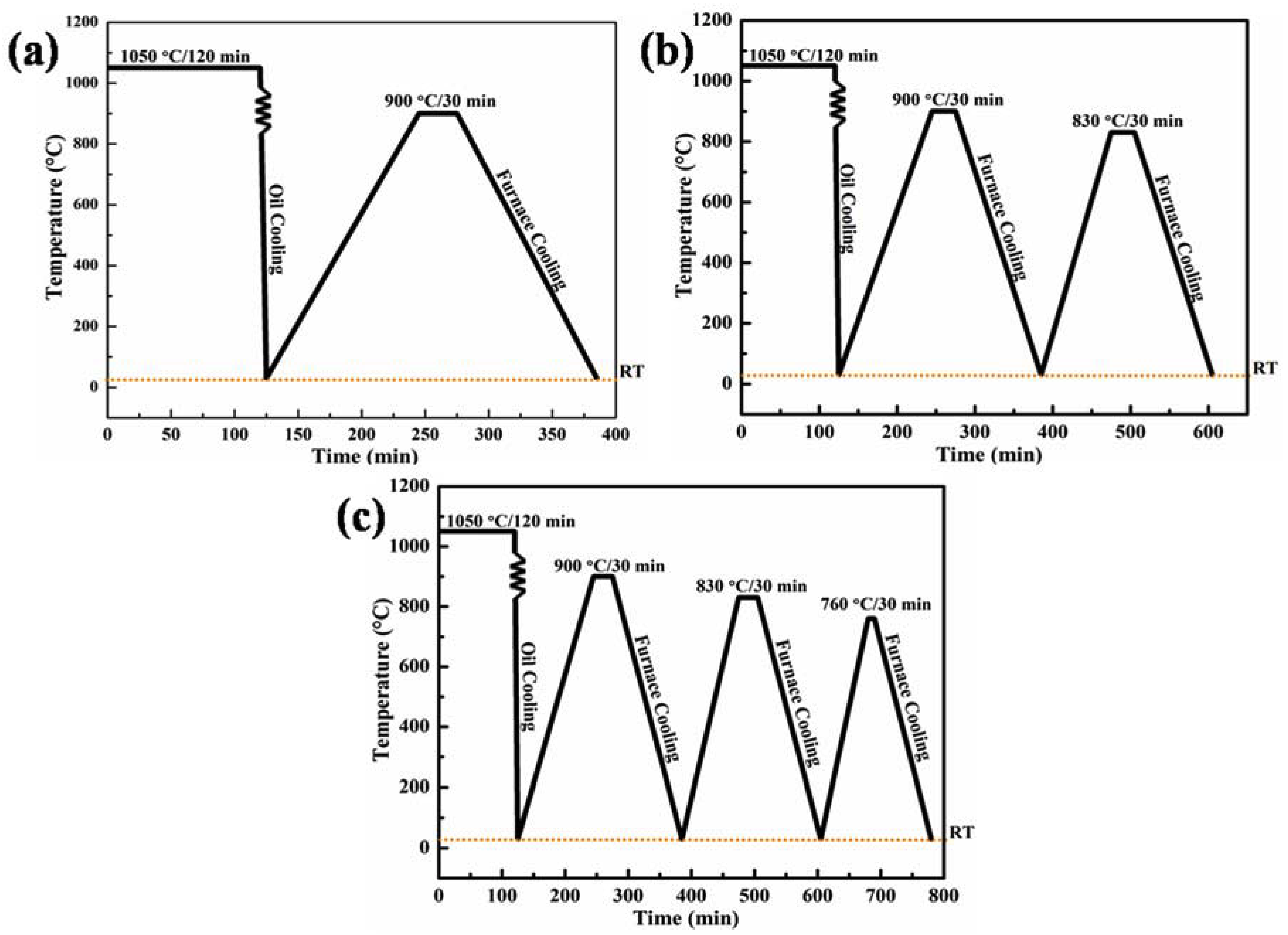
2.2. Thermo-Mechanical Cyclic Annealing Processes
2.3. Microstructure Analysis
2.4. Vickers Hardness Testing
2.5. Electrochemical Analysis
2.6. Immersion Corrosion Analysis
3. Results and Discussion
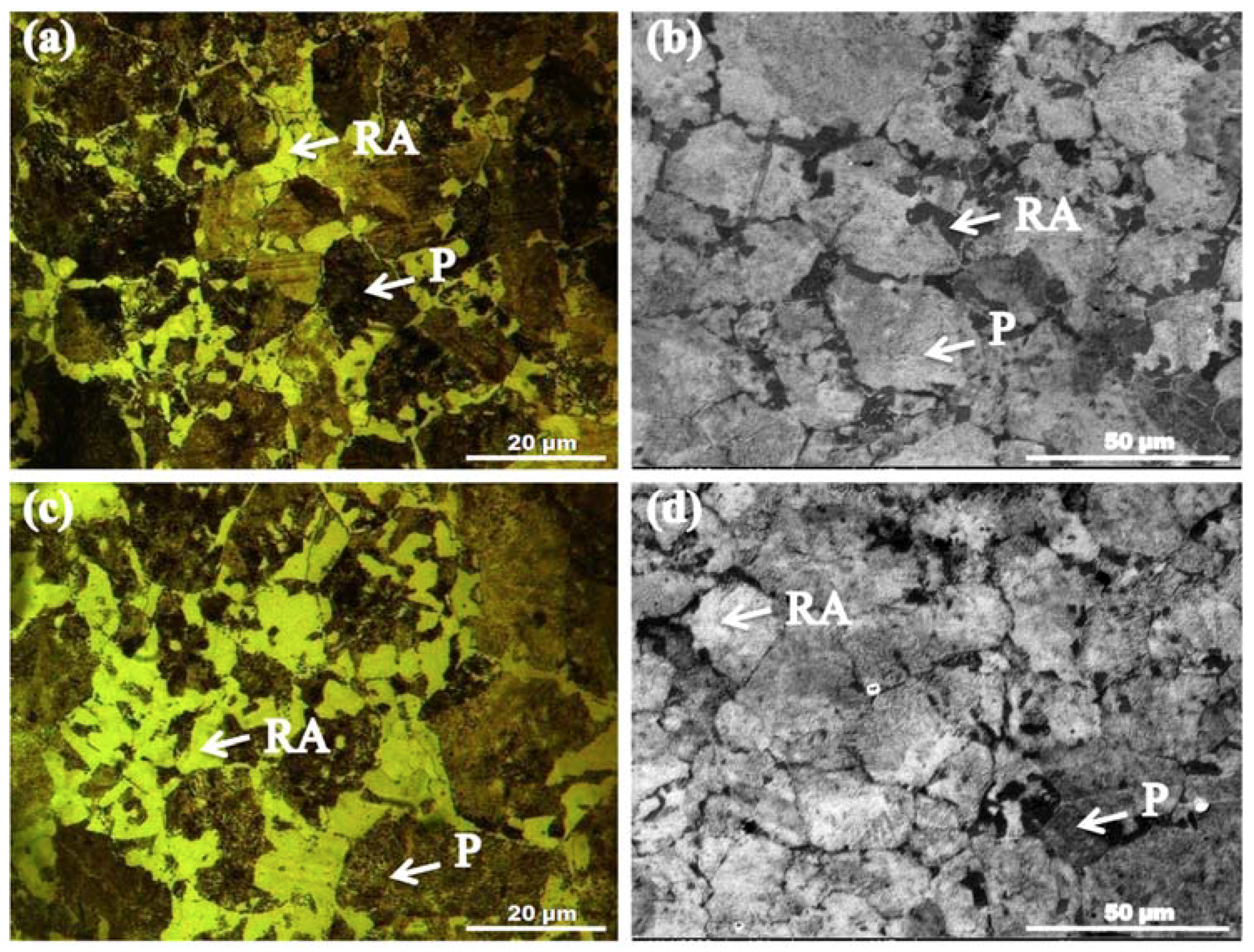
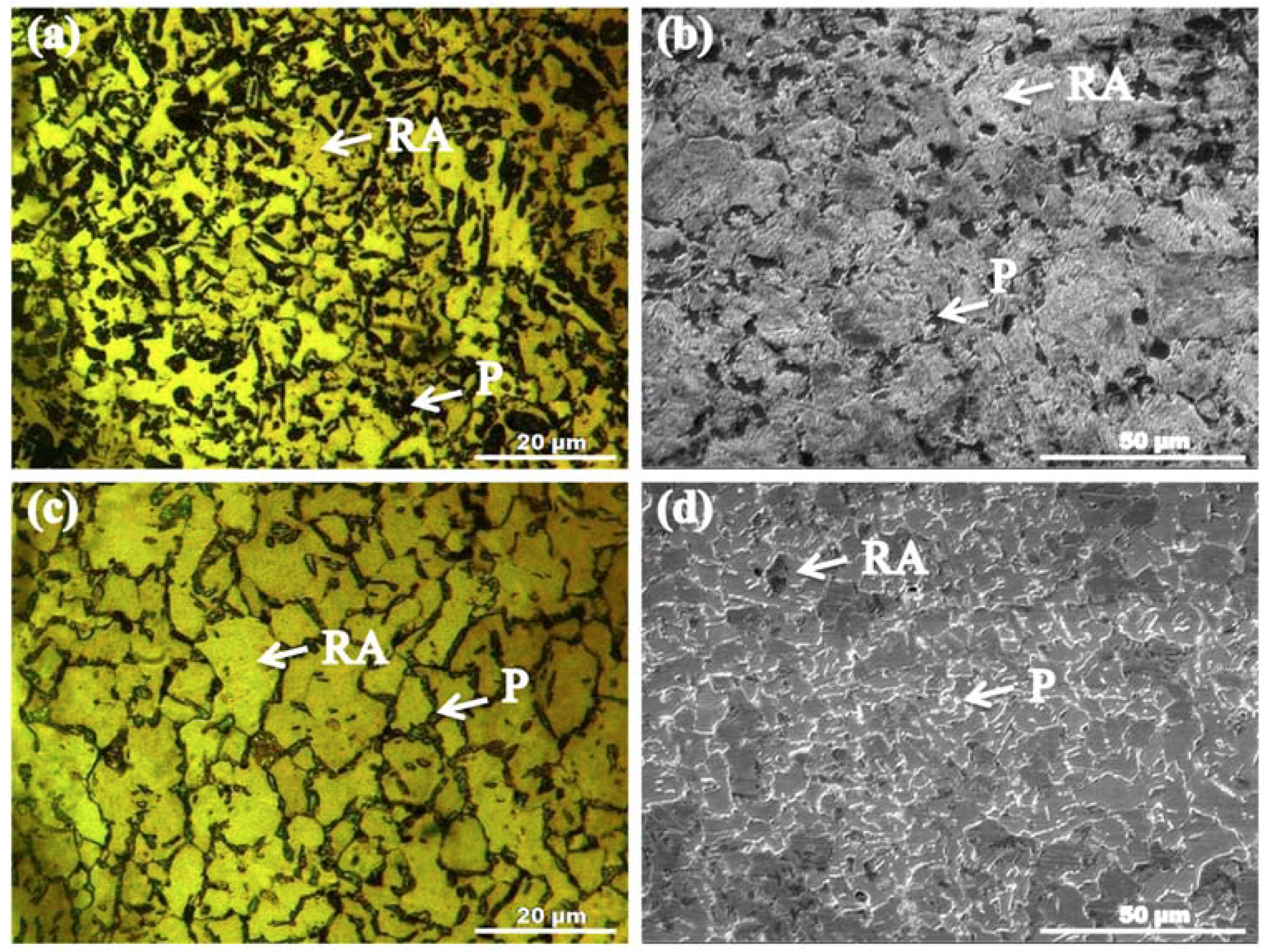
3.1. Microstructure Evolution
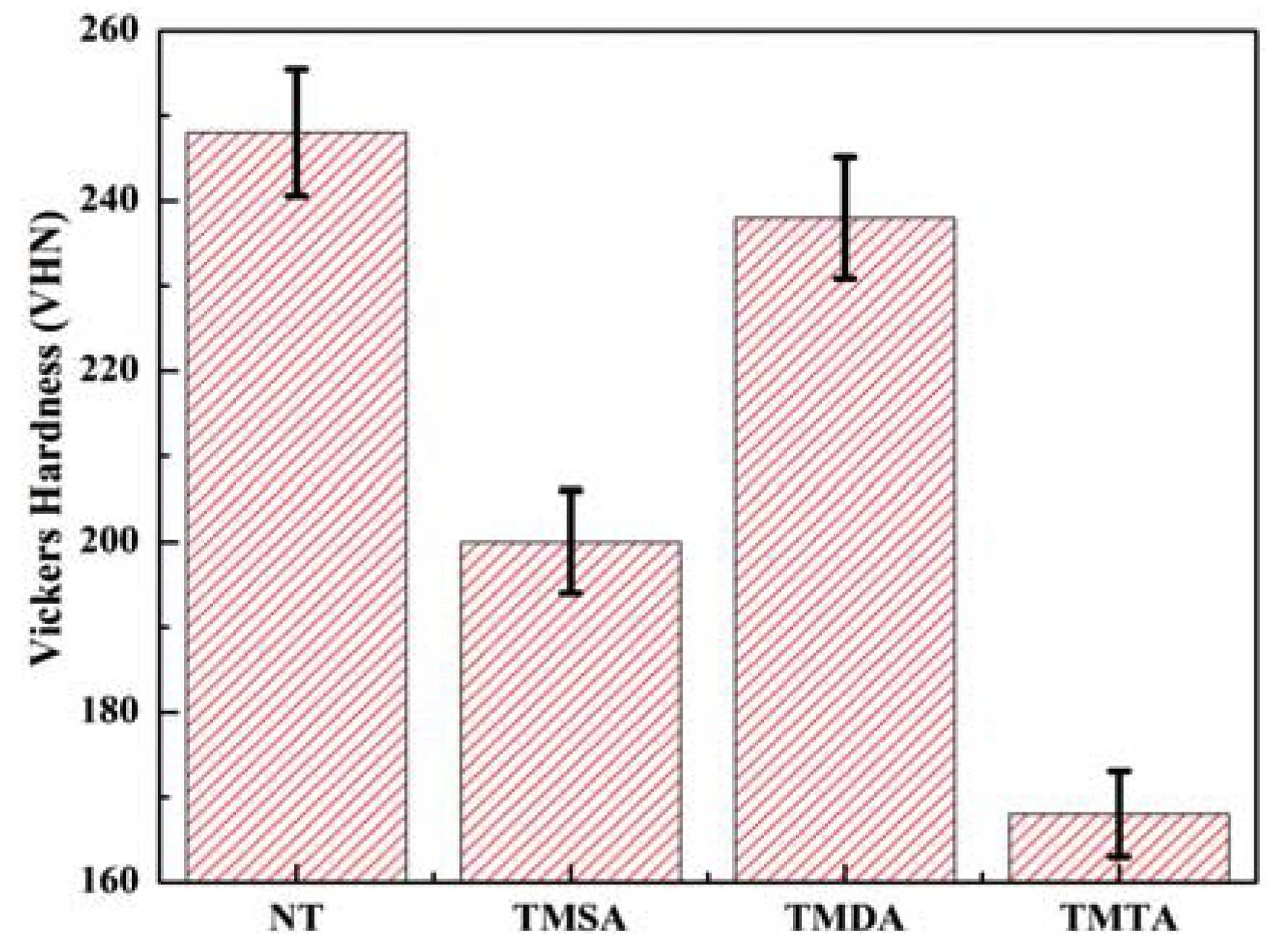
3.2. Vickers Hardness
3.3. Electrochemical Properties
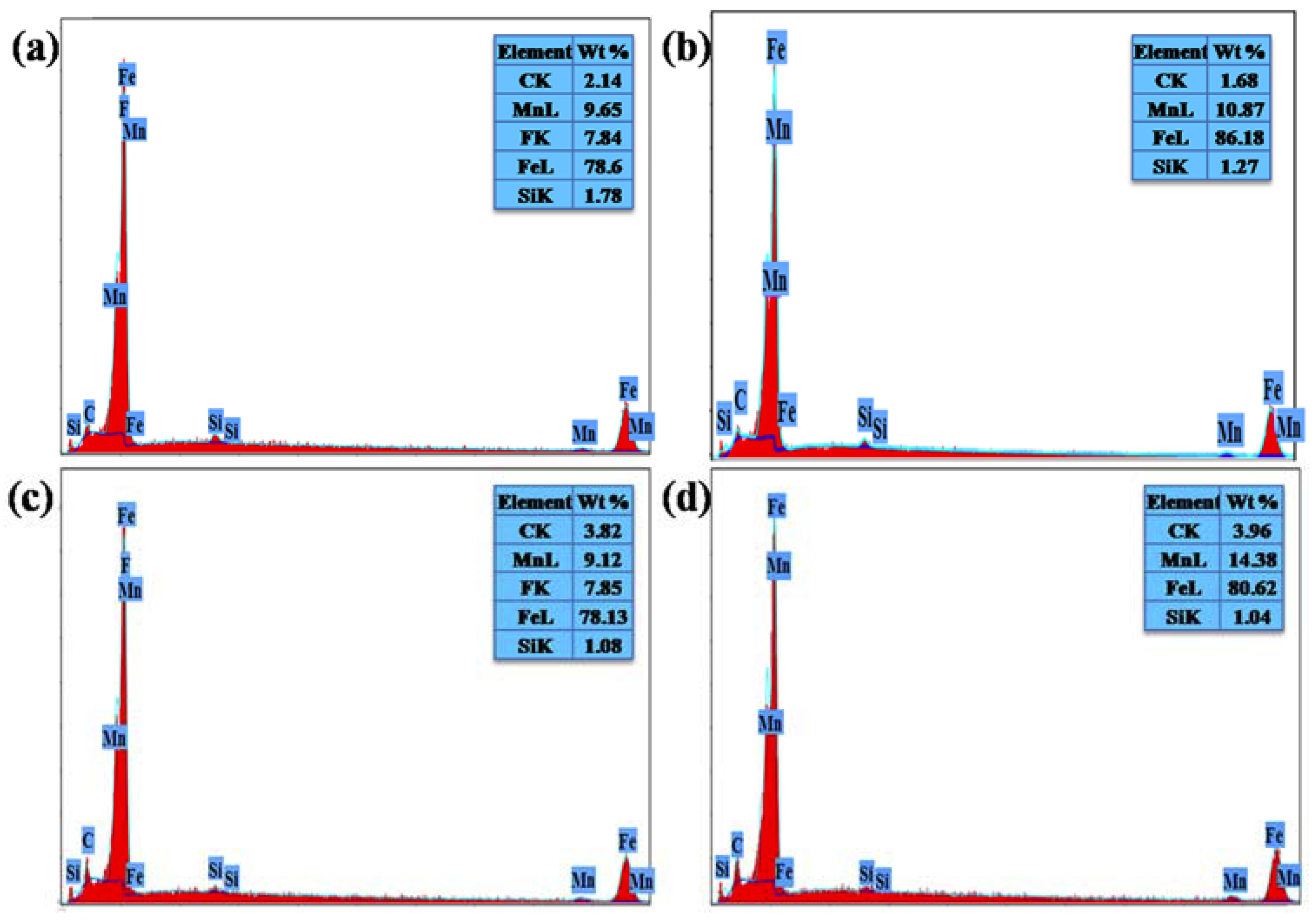
3.4. Immersion Corrosion Properties
4. Conclusions
- i.
- Dual-phase microstructures comprising retained austenite and pearlite phases were achieved after all TMCA treatments of AISI 1345 steel. Secondary phase particles precipitation was observed after TMSA treatment. Variations in grain size, fractions of both phases, and C and Mn percentages in both phases were observed with varying TMCA treatments. TMTA treated sample exhibited maximum fractions of retained austenite and minimum fractions of pearlite along grain boundaries. This pearlite contained the highest percentages of C (3.96%) and Mn (14.38%) among all samples. TMDA treatment provided maximum (26.7 µm) whereas, TMSA minimum (254 µm) grain refinement of microstructure.
- ii.
- TMDA and TMTA treatments significantly improved the electrochemical properties of AISI 1345 steel in 5 vol% HCl solution compared to non-treated steel. Both treatments caused a three-fold reduction in Icorr (6.610, 6.060 µA.cm−2) as well as in corrosion rate (3.025, 2.771 mpy) attributed to the reduced fractions of pearlite and in turn number of corrosion cells. On the other hand, TMSA treatment exhibited very poor electrochemical properties among all samples.
- iii.
- The surface of the TMTA treated sample was observed to be partially covered with a very thin crack-free oxide layer having the lowest oxygen percentages (8.16% and 42%) in the EDS spectrum and EDS map among all samples. These features proved that TMTA treated sample underwent a minimum corrosion attack of the HCl solution. On the other hand, the surface of TMSA treated sample was fully covered with thick, spongy oxide layer having clear micro-cracks and highest oxygen percentages (50.51% and 68%) in the EDS spectrum and EDS map among all treated samples. These features proved that TMSA treated sample underwent intense corrosion attack of the HCl solution. Results of electrochemical analysis and immersion corrosion analysis were in good agreement.
Author Contributions
Funding
Conflicts of Interest
References
- Merimi, I.; Benkaddour, R.; Lgaz, H.; Rezki, N.; Messali, M.; Jeffali, F.; Oudda, H.; Hammouti, B. Insights into corrosion inhibition behavior of a triazole derivative for mild steel in hydrochloric acid solution. Mater. Today-Proc. 2019, 13, 1008–1022. [Google Scholar] [CrossRef]
- Boutouil, A.; Laamari, M.R.; Elazhary, I.; Bahsis, L.; Anane, H.; Stiriba, S.E. Towards a deeper understanding of the inhibition mechanism of a new 1,2,3-triazole derivative for mild steel corrosion in the hydrochloric acid solution using coupled experimental and theoretical methods. Mater. Chem. Phys. 2020, 241, 122420. [Google Scholar] [CrossRef]
- Nadi, I.; Belattmania, Z.; Sabour, B.; Reani, A.; Sahibed-dine, A.; Jama, C.; Bentiss, F. Sargassum muticum extract based on alginate biopolymer as a newefficient biological corrosion inhibitor for carbon steel in hydrochloric acid pickling environment: Gravimetric, electrochemical and surface studies. Int. J. Biol. Macromol. 2019, 141, 137–149. [Google Scholar] [CrossRef]
- Bouhlal, F.; Labjar, N.; Abdoun, F.; Mazkour, A.; Idrissi, M.S.; Mahi, M.E.; Lotfi, E.M.; Skalli, A.; Hajjaji, S.E. Chemical and electrochemical studies of the inhibition performance ofhydro-alcoholic extract of used coffee grounds (HECG) for the corrosion of C38 steel in 1 M hydrochloric acid. Egypt. J. Pet. 2019. [Google Scholar] [CrossRef]
- Ahmed, M.H.O.; Amiery, A.A.A.; Majedy, Y.K.A.; Kadhum, A.A.H.; Mohamad, A.B.; Gaaz, T.S. Synthesis and characterization of a novel organic corrosion inhibitor for mild steel in 1 M hydrochloric acid. Results Phys. 2018, 8, 728–733. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, D.; Chen, H.; Guo, S.; Yang, Q.; Chen, L.; Zhao, H.; Wang, L. A high-efficiency corrosion inhibitor of N-doped citric acid-based carbon dots for mild steel in hydrochloric acid environment. J. Hazard. Mater. 2020, 381, 121019. [Google Scholar] [CrossRef] [PubMed]
- Lgaz, H.; Saha, S.K.; Chaouiki, A.; Bhat, K.S.; Salghi, R.; Shubhalaxmi; Banerjee, P.; Ali, I.H.; Khan, M.I.; Chung, I.M. Exploring the potential role of pyrazoline derivatives in corrosion inhibition of mild steel in hydrochloric acid solution: Insights from experimental and computational studies. Constr. Build. Mater. 2020, 233, 117320. [Google Scholar] [CrossRef]
- El-Etre, A.Y.; Ali, A.I. A novel green inhibitor for C-steel corrosion in 2.0 mol·L−1 hydrochloric acid solution. Chin. J. Chem. Eng. 2017, 25, 373–380. [Google Scholar] [CrossRef]
- Devika, B.G.; Doreswamy, B.H.; Tandon, H.C. Corrosion behaviour of metal complexes of antipyrine based azo dye ligand for soft-cast steel in 1 M hydrochloric acid. J. King Saud Univ. Sci. 2020, 32, 881–890. [Google Scholar] [CrossRef]
- Ishak, A.; Adams, F.V.; Madu, J.O.; Joseph, I.V.; Olubambi, P.A. corrosion inhibition of mild steel in 1M hydrochloric acid using haematostaphisbarteri leaves extract. Procedia Manuf. 2019, 35, 1279–1285. [Google Scholar] [CrossRef]
- Sarvghad, M.; Aguila, D.D.; Will, G. Optimized corrosion performance of a carbon steel in dilute sulfuric acid through heat treatment. Appl. Surf. Sci. 2019, 491, 460–468. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, W.; Shi, H.; Li, G.; Ding, Y. The effects of stabilizing treatment on microstructure and corrosion resistance of 316Ti stainless steel. Eng. Fail. Anal. 2019, 105, 961–969. [Google Scholar] [CrossRef]
- Kong, D.; Dong, C.; Ni, X.; Zhang, L.; Yao, J.; Man, C.; Cheng, X.; Xiao, K.; Li, X. Mechanical properties and corrosion behavior of selective laser melted 316L stainless steel after different heat treatment processes. J. Mater. Sci. Technol. 2019, 35, 1499–1507. [Google Scholar] [CrossRef]
- Sarkar, S.; Mukherjee, S.; Kumar, C.S.; Nath, A.K. Effects of heat treatment on microstructure, mechanical and corrosion properties of 15-5 PH stainless steel parts built by selective laser melting process. J. Manuf. Process. 2020, 50, 279–294. [Google Scholar] [CrossRef]
- Sudhakar, I.; Vardhan, Y.V.; Sruthi, P.; Harshit, P.V.S.; Kumar, S.S.; Prakash, V.V. Study on Corrosion Behavior of Heat Treated High Carbon Low Alloy Steel. Mater. Today-Proc. 2018, 5, 3919–3925. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Cheng, X.; Wang, H.; Huang, Z. Effect of heat treatment on microstructure, mechanical and corrosion properties of austenitic stainless steel 316 L using arc additive manufacturing. Mater. Sci. Eng. A 2018, 715, 307–314. [Google Scholar] [CrossRef]
- Sharma, L.; Chhibber, R. Microstructure evolution and electrochemical corrosion behaviour of API X70 linepipe steel in different environments. Int. J. Press. Vessel. Pip. 2019, 171, 51–59. [Google Scholar] [CrossRef]
- Cerdán, C.E.; Ooi, S.W.; Joshi, G.R.; Morana, R.; Bhadeshia, H.K.D.H.; Akid, R. Effect of tempering heat treatment on the CO2 corrosion resistance of quench-hardened Cr-Mo low-alloy steels for oil and gas applications. Corros. Sci. 2019, 154, 36–48. [Google Scholar] [CrossRef]
- Behbahani, K.M.; Najafisayar, P.; Pakshir, M.; Shahsavari, M. An electrochemical study on the effect of stabilization and sensitization heat treatments on the intergranular corrosion behaviour of AISI 321H austenitic stainless steel. Corros. Sci. 2018, 138, 28–41. [Google Scholar] [CrossRef]
- Luo, H.; Yu, Q.; Dong, C.; Sha, G.; Liu, Z.; Liang, J.; Wang, L.; Han, G.; Li, X. Influence of the aging time on the microstructure and electrochemical behaviour of a 15-5PH ultra-high strength stainless steel. Corros. Sci. 2018, 139, 185–196. [Google Scholar] [CrossRef]
- Wang, L.; Dong, C.; Man, C.; Kong, D.; Xiao, K.; Li, X. Enhancing the corrosion resistance of selective laser melted 15-5PH martensite stainless steel via heat treatment. Corros. Sci. 2020, 166, 108427. [Google Scholar] [CrossRef]
- Chandra, K.; Kain, V.; Tewari, R. Microstructural and electrochemical characterisation of heat-treated 347 stainless steel with different phases. Corros. Sci. 2013, 67, 118–129. [Google Scholar] [CrossRef]
- Kadowaki, M.; Muto, I.; Katayama, H.; Masuda, H.; Sugawara, Y.; Hara, N. Effectiveness of an intercritical heat-treatment on localized corrosion resistance at the microstructural boundaries of medium-carbon steel. Corros. Sci. 2019, 154, 159–177. [Google Scholar] [CrossRef]
- Ganesh, P.; Kumar, A.V.; Thinaharan, C.; Krishna, N.G.; George, R.P.; Parvathavarthini, N.; Rai, S.K.; Kaul, R.; Mudali, U.K.; Kukreja, L.M. Enhancement of intergranular corrosion resistance of type 304 stainless steel through a novel surface thermo-mechanical treatment. Surf. Coat. Technol. 2013, 232, 920–927. [Google Scholar] [CrossRef]
- Hafeez, M.A.; Inam, A.; Farooq, A. Mechanical and corrosion properties of medium carbon low alloy steel after cyclic quenching and tempering heat–treatments. Mater. Res. Express 2020, 7, 016553. [Google Scholar] [CrossRef]
- Prakash, P.; Vanaja, J.; Srinivasan, N.; Parameswaran, P.; Rao, G.V.S.N.; Laha, K. Effect of thermo-mechanical treatment on tensile properties of reduced activation ferritic-martensitic steel. Mater. Sci. Eng. A 2018, 724, 171–180. [Google Scholar] [CrossRef]
- Li, S.; Eliniyaz, Z.; Sun, F.; Shen, Y.; Zhang, L.A.; Shan, A. Effect of thermo-mechanical treatment on microstructure and mechanical properties of P92 heat resistant steel. Mater. Sci. Eng. A 2013, 559, 882–888. [Google Scholar] [CrossRef]
- Ghosh, A.; Ghosh, M. Tensile and impact behaviour of thermo mechanically treated and micro-alloyed medium carbon steel bar. Constr. Build. Mater. 2018, 192, 657–670. [Google Scholar] [CrossRef]
- Balan, K.P.; Reddy, A.V.; Sarma, D.S. Effect of single and double austenitization treatments on the microstructure and mechanical properties of 16Cr-2Ni Steel. J. Mater. Eng. Perform. 1999, 8, 385–393. [Google Scholar] [CrossRef]
- Chang, E.; Chang, C.Y.; Liu, C.D. The effects of double austenitization on the mechanical properties of a 0.34C containing low-alloy Ni-Cr-Mo-V steel. Metall. Mater. Trans. A 1991, 25, 545–555. [Google Scholar] [CrossRef]
- Pandey, C.; Mahapatra, M.M.; Kumar, P.; Kumar, P.; Saini, N.; Thakare, J.G.; Kumar, S. Study on effect of double austenitization treatment on fracture morphology tensile tested nuclear grade P92 steel. Eng. Fail. Anal. 2019, 96, 158–167. [Google Scholar] [CrossRef]
- Salunkhe, S.; Fabijanic, D.; Nayak, J.; Hodgson, P. Effect of single and double austenitization treatments on the microstructure and hardness of AISI D2 tool steel. Mater. Today-Proc. 2015, 2, 1901–1906. [Google Scholar] [CrossRef]
- Cao, Z.; Shi, Z.; Yu, F.; Sugimoto, K.; Cao, W.; Weng, Y. Effects of double quenching on fatigue properties of high carbon bearing steel with extra-high purity. Int. J. Fatigue 2019, 128, 105176. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, C.; Zhou, T.; Liu, D.; Fang, X.; Li, H.; Suo, J. Effects of Ti and a twice-quenching treatment on the microstructure and ductile brittle transition temperature of 9CrWVTiN steels. Mater. Des. 2015, 88, 675–682. [Google Scholar] [CrossRef]
- Arlazarov, A.; Gouné, M.; Bouaziz, O.; Hazotte, A.; Petitgand, G.; Barges, P. Evolution of microstructure and mechanical properties of medium Mn steels during double annealing. Mater. Sci. Eng. A 2012, 542, 31–39. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Wang, F.M.; Tao, S.F.; Zhao, D.Q.; Zhang, X.Y. Structure and texture formation during single and double cold rolling and annealing processes for Nb+Ti added interstitial-free steels. J. Iron Steel Res. Int. 2013, 20, 42–49. [Google Scholar] [CrossRef]
- Han, J.; Kang, S.H.; Lee, S.J.; Lee, Y.K. Fabrication of bimodal-grained Al-free medium Mn steel by double intercritical annealing and its tensile properties. J. Alloys Compd. 2016, 681, 580–588. [Google Scholar] [CrossRef]
- Saha, R.; Ray, R.K.; Bhattacharjee, D. Attaining deep drawability and non-earing properties in Ti + Nb interstitial-free steels through double cold rolling and annealing. Scr. Mater. 2007, 57, 257–260. [Google Scholar] [CrossRef]
- Eskandari, M.; Kermanpur, A.; Najafizadeh, A. Formation of nano-grained structure in a 301 stainless steel using a repetitive thermo-mechanical treatment. Mater. Lett. 2009, 63, 1442–1444. [Google Scholar] [CrossRef]
- Prakash, P.; Vanaja, J.; Palaparti, D.P.R.; Reddy, G.V.P.; Laha, K.; Rao, G.V.S.N. Tensile flow and work hardening behavior of reduced activation ferritic martensitic steel subjected to thermo-mechanical treatment. J. Nucl. Mater. 2019, 520, 19–26. [Google Scholar] [CrossRef]
- Hafeez, M.A.; Farooq, A. Microstructural, mechanical and tribological investigation of 30CrMnSiNi2A ultra-high strength steel under various tempering temperatures. Mater. Res. Express 2018, 5, 016505. [Google Scholar] [CrossRef]
- Hafeez, M.A. Effect of microstructural transformation during tempering on mechanical properties of quenched and tempered 38CrSi steel. Mater. Res. Express. 2019, 6, 086552. [Google Scholar] [CrossRef]
- Hafeez, M.A.; Inam, A.; Arshad, M.A. Investigation on microstructural, mechanical, and electrochemical properties of water, brine quenched and tempered low carbon steel. Mater. Res. Express 2019, 6, 096524. [Google Scholar] [CrossRef]
- Inam, A.; Imtiaz, Y.; Hafeez, M.A.; Munir, S.; Ali, Z.; Ishtiaq, M.; Hassan, M.H.; Maqbool, A.; Haider, W. Effect of tempering time on microstructure, mechanical, and electrochemical properties of quenched–partitioned–tempered Advanced High Strength Steel (AHSS). Mater. Res. Express 2019, 6, 126509. [Google Scholar] [CrossRef]
- Roberge, P.R. Handbook of Corrosion Engineering; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Revie, R.W.; Uhlig, H.H. Corrosion and Corrosion Control, 4th ed.; John Wiley and Sons Inc.: New York, NY, USA, 2008. [Google Scholar]
- Hafeez, M.A.; Farooq, A. Effect of heat treatments on the mechanical and electrochemical behavior of 38CrSi and AISI 4140 steels. Metallogr. Microstruct. Anal. 2019, 8, 479–487. [Google Scholar] [CrossRef]
- Hafeez, M.A. Mechanical investigation on mechanical properties and immersion corrosion performance of 0.35%C–10.5%Mn steel processed by austenite reverted transformation (ART) annealing process. Metallogr. Microstruct. Anal. 2020, 1–10. [Google Scholar] [CrossRef]
- Lytle, D.A.; Sorg, T.J.; Frietch, C. Accumulation of arsenic in drinking water distribution systems. Environ. Sci. Technol. 2004, 38, 5365–5372. [Google Scholar] [CrossRef]
- Sarin, P.; Snoeyink, V.L.; Bebee, J.; Jim, K.K.; Beckett, M.A.; Kriven, W.M.; Clement, J.A. Iron release from corroded iron pipes in drinking water distribution systems: Effect of dissolved oxygen. Water Res. 2004, 38, 1259–1269. [Google Scholar] [CrossRef]
- Gerke, T.L.; Maynard, J.B.; Schock, M.R.; Lytle, D.L. Physiochemical characterization of five iron tubercles from a single drinking water distribution system: Possible new insights on their formation and growth. Corros. Sci. 2008, 50, 2030–2039. [Google Scholar] [CrossRef]
- Peng, C.Y.; Korshin, G.V.; Valentine, R.L.; Hill, A.S.; Friedman, M.J.; Reiber, S.H. Characterization of elemental and structural composition of corrosion scales and deposits formed in drinking water distribution systems. Water Res. 2010, 44, 4570–4580. [Google Scholar] [CrossRef]
- Li, M.J.; Liu, Z.W.; Chen, Y.C.; Hai, Y. Characteristics of iron corrosion scales and water quality variations in drinking water distribution systems of different pipe materials. Water Res. 2016, 106, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Sarin, P.; Snoeyink, V.L.; Bebee, J.; Kriven, W.M.; Clement, J.A. Physico-chemical characteristics of corrosion scales in old iron pipes. Water Res. 2001, 35, 2961–2969. [Google Scholar] [CrossRef]
- Antunes, R.A.; Costa, I.; Faria, D.L. Characterization of corrosion products formed on steels in the first months of atmospheric exposure. Mater. Res. 2003, 6, 403–408. [Google Scholar] [CrossRef]
- Gheno, T.; Monceau, D.; Zhang, J.; Young, D.J. Carburisation of ferritic Fe-Cralloys by low carbon activity gases. Corros. Sci. 2011, 53, 2767–2777. [Google Scholar] [CrossRef]
- Maslar, J.E.; Hurst, W.S.; Bowers, W.J.; Hendricks, J.H.; Aquino, M.I.; Levin, I. In situ Raman spectroscopic investigation of chromium surfaces under hydrothermal conditions. Appl. Surf. Sci. 2001, 180, 102–118. [Google Scholar] [CrossRef]
- Yang, F.; Shi, B.Y.; Gu, J.N.; Wang, D.; Yang, M. Morphological and physicochemical characteristics of iron corrosion scales formed under different water source histories in a drinking water distribution system. Water Res. 2012, 46, 5423–5433. [Google Scholar] [CrossRef]



| Raw Materials | Wt. (kg) | C | Si | Mn | S | P | Fe |
|---|---|---|---|---|---|---|---|
| Mild Steel Scrap | 50.0 | 0.10 | 0.30 | 0.25 | 0.002 | 0.002 | Bal. |
| Carburizer | 0.08 | 98.0 | – | – | 0.015 | – | – |
| Ferro-Silicon | 0.11 | – | 70.0 | – | – | – | Bal. |
| Ferro-Manganese | 1.92 | 6.00 | 0.97 | 60.0 | – | – | Bal. |
| AISI 1345 | 52.1 | 0.45 | 0.37 | 1.78 | 0.017 | 0.002 | Bal. |
| Samples | βa mV/Decades | βc mV/Decades | Icorr (µA.cm−2) | Ecorr (mV) | Corrosion Rate (mpy) |
|---|---|---|---|---|---|
| NT | 81.40 | 179.1 | 20 | −480 | 9.143 |
| TMSA | 69.20 | 143.7 | 35.60 | −469 | 16.27 |
| TMDA | 61.30 | 142.3 | 6.610 | −647 | 3.025 |
| TMTA | 65.90 | 138.3 | 6.060 | −522 | 2.771 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafeez, M.A.; Inam, A.; Hassan, M.U.; Umer, M.A.; Usman, M.; Hanif, A. Optimized Corrosion Performance of AISI 1345 Steel in Hydrochloric Acid Through Thermo-Mechanical Cyclic Annealing Processes. Crystals 2020, 10, 265. https://doi.org/10.3390/cryst10040265
Hafeez MA, Inam A, Hassan MU, Umer MA, Usman M, Hanif A. Optimized Corrosion Performance of AISI 1345 Steel in Hydrochloric Acid Through Thermo-Mechanical Cyclic Annealing Processes. Crystals. 2020; 10(4):265. https://doi.org/10.3390/cryst10040265
Chicago/Turabian StyleHafeez, Muhammad Arslan, Aqil Inam, Misbah Ul Hassan, Malik Adeel Umer, Muhammad Usman, and Asad Hanif. 2020. "Optimized Corrosion Performance of AISI 1345 Steel in Hydrochloric Acid Through Thermo-Mechanical Cyclic Annealing Processes" Crystals 10, no. 4: 265. https://doi.org/10.3390/cryst10040265
APA StyleHafeez, M. A., Inam, A., Hassan, M. U., Umer, M. A., Usman, M., & Hanif, A. (2020). Optimized Corrosion Performance of AISI 1345 Steel in Hydrochloric Acid Through Thermo-Mechanical Cyclic Annealing Processes. Crystals, 10(4), 265. https://doi.org/10.3390/cryst10040265







