Inhibiting the Segregation of Germanium in Silver Nanolayers
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aouani, H.; Wenger, J.; Gerard, D.; Rigneault, H.; Devaux, E.; Ebbesen, T.W.; Mahdavi, F.; Xu, T.; Blair, S. Crucial role of the adhesion layer on the plasmonic fluorescence enhancement. ACS Nano 2009, 3, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Wu, W.; Logeeswaran, V.J.; Yu, Z.; Islam, M.S.; Wang, S.Y.; Williams, R.S.; Fang, N.X. A smooth optical superlens. Appl. Phys. Lett. 2010, 96, 043102. [Google Scholar] [CrossRef] [Green Version]
- Melpignano, P.; Cioarec, C.; Clergereaux, R.; Gherardi, N.; Villeneuve, C.; Datas, L. E−beam deposited ultra−smooth silver thin film on glass with different nucleation layers: An optimization study for OLED micro−cavity application. Org. Electron. 2010, 11, 1111–1119. [Google Scholar] [CrossRef]
- Boltasseva, A.; Atwater, H.A. Low−loss plasmonic metamaterials. Science 2011, 331, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Cioarec, C.; Melpignano, P.; Gherardi, N.; Clergereaux, R.; Villeneuve, C. Ultrasmooth silver thin film electrodes with high polar liquid wettability for OLED microcavity application. Langmuir 2011, 27, 3611–3617. [Google Scholar] [CrossRef]
- Špačková, N.; Wrobel, P.; Bocková, M.; Homola, J. Optical biosensors based on plasmonic nanostructures: A review. Proc. IEEE 2016, 104, 2380–2408. [Google Scholar] [CrossRef]
- Stefaniuk, T.; Olivier, N.; Belardini, A.; McPolin, C.P.T.; Sibilia, C.; Wronkowska, A.A.; Wronkowski, A.; Szoplik, T.; Zayats, A.V. Self−assembled silver−germanium nanolayer metamaterial with the enhanced nonlinear response. Adv. Opt. Mater. 2017, 5, 1700753. [Google Scholar] [CrossRef]
- Zhao, G.; Shen, W.; Jeong, E.; Lee, S.-G.; Yu, S.M.; Bae, T.-S.; Lee, G.-H.; Han, S.Z.; Tang, J.; Choi, E.-A.; et al. Ultrathin silver film electrodes with ultralow optical and electrical losses for flexible organic photovoltaics. ACS Appl. Mater. Interfaces 2018, 10, 27510–27520. [Google Scholar] [CrossRef]
- Mahapatro, A.K.; Scott, A.; Manning, A.; Janes, D.B. Gold surface with sub−nm roughness realized by evaporation on a molecular adhesion monolayer. Appl. Phys. Lett. 2006, 88, 151917. [Google Scholar] [CrossRef] [Green Version]
- Oates, T.W.H.; Ryves, L.; Bilek, M.M.M. Dielectric functions of a growing silver film determined using dynamic in situ spectroscopic ellipsometry. Opt. Express 2008, 16, 2302–2314. [Google Scholar] [CrossRef]
- Logeeswaran, V.J.; Kobayashi, N.P.; Islam, M.S.; Wu, W.; Chaturvedi, P.; Fang, N.X.; Wang, S.Y.; Williams, R.S. Ultrasmooth silver thin films deposited with a germanium nucleation layer. Nano Lett. 2009, 9, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, B.; Leong, E.S.P.; Yang, P.; Zong, Y.; Si, G.; Teng, J.; Maier, S.A. Enhanced surface plasmon resonance on a smooth silver film with a seed growth layer. ACS Nano 2010, 4, 3139–3146. [Google Scholar] [CrossRef] [PubMed]
- Ke, L.; Lai, S.C.; Liu, H.; Peh, C.K.N.; Wang, B.; Teng, J.H. Ultrasmooth silver thin film on PEDOT: PSS nucleation layer for extended surface plasmon propagation. ACS Appl. Mater. Interfaces 2012, 4, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Flötotto, D.; Wang, Z.M.; Jeurgens, L.P.H.; Bischoff, E.; Mittemeijer, E.J. Effect of adatom surface diffusivity on microstructure and intrinsic stress evolutions during Ag film growth. J. Appl. Phys. 2012, 112, 043503. [Google Scholar] [CrossRef] [Green Version]
- Formica, N.; Ghosh, D.S.; Carrilero, A.; Chen, T.L.; Simpson, R.E.; Pruneri, V. Ultrastable and atomically smooth ultrathin silver films grown on a copper seed layer. ACS Appl. Mater. Interfaces 2013, 5, 3048–3053. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, T.; Ekinci, Y.; Martin, O.J.F.; Sigg, H. Engineering metal adhesion layers that do not deteriorate plasmon resonances. ACS Nano 2013, 7, 2751–2757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefaniuk, T.; Wróbel, P.; Górecka, E.; Szoplik, T. Optimum temperature for deposition of ultrasmooth silver nanolayers. Nanoscale Res. Lett. 2014, 9, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Stefaniuk, T.; Wróbel, P.; Trautman, P.; Szoplik, T. Ultrasmooth metal nanolayers for plasmonic applications: Surface roughness and specific resistivity. Appl. Opt. 2014, 53, B237–B241. [Google Scholar] [CrossRef]
- Wróbel, P.; Stefaniuk, T.; Trzcinski, M.; Wronkowska, A.A.; Wronkowski, A.; Szoplik, T. Ge wetting layer increases ohmic plasmon losses in Ag film due to segregation. ACS Appl. Mater. Interfaces 2015, 7, 8999–9005. [Google Scholar] [CrossRef]
- Leandro, L.; Malureanu, R.; Rozlosnik, N.; Lavrinienko, A. Ultrathin, ultrasmooth gold layer on dielectrics without the use of additional metallic adhesion layers. ACS Appl. Mater. Interfaces 2015, 7, 5797–5802. [Google Scholar] [CrossRef] [Green Version]
- Sukham, J.; Takayama, O.; Lavrinenko, A.V.; Malureanu, R. High−quality ultrathin gold layers with an APTMS adhesion for optimal performance of surface plasmon polariton−based devices. ACS Appl. Mater. Interfaces 2017, 9, 25049–25056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krayer, L.J.; Kim, J.; Munday, J.N. Near−perfect absorption throughout the visible using ultra−thin metal films on index−near−zero substrates. Opt. Mater. Express 2019, 9, 330–338. [Google Scholar] [CrossRef]
- Cancellieri, C.; Klyatskina, E.; Chiodi, M.; Janczak−Rusch, J.; Jeurgens, L.P.H. The effect of interfacial Ge and RF−bias on the microstructure and stress evolution upon annealing of Ag/AlN multilayers. Appl. Sci. 2018, 8, 2403. [Google Scholar] [CrossRef] [Green Version]
- Ariosa, D.; Cancellieri, C.; Araullo−Peters, V.; Chiodi, M.; Klyatskina, E.; Janczak−Rusch, J.; Jeurgens, L.P.H. Modeling of interface and internal disorder applied to XRD analysis of Ag−based nano−multilayers. ACS Appl. Mater. Interfaces 2018, 10, 20938–20949. [Google Scholar] [CrossRef]
- Parmigiani, F.; Kay, E.; Huang, T.; Perrin, J.; Jurich, M.; Swalen, J.D. Optical and electrical properties of thin silver films grown under ion bombardment. Phys. Rev. B Condens. Matter Mater. Phys. 1986, 33, 879–888. [Google Scholar] [CrossRef]
- Tsuda, Y.; Omoto, H.; Tanaka, K.; Ohsaki, H. The underlayer effects on the electrical resistivity of Ag thin film. Thin Solid Films 2006, 502, 223–227. [Google Scholar] [CrossRef]
- Yakubovsky, D.I.; Arsenin, A.V.; Stebunov, Y.V.; Fedyanin, D.Y.; Volkov, V.S. Optical constants and structural properties of thin gold films. Opt. Express 2017, 25, 25574–25587. [Google Scholar] [CrossRef] [Green Version]
- Wynblatt, P.; Ku, R.C. Surface energy and solute strain energy effects in surface segregation. Surf. Sci. 1977, 65, 511–531. [Google Scholar] [CrossRef]
- Seah, M.P. Quantitative prediction of surface segregation. J. Catal. 1979, 57, 450–457. [Google Scholar] [CrossRef]
- Wachs, L.; Miller, T.; Chiang, T.C. Evidence for germanium segregation on thin films of Ag on Ge(111). Phys. Rev. B 1986, 33, 8870–8873. [Google Scholar] [CrossRef]
- Olesinski, R.W.; Abbaschian, G.J. The Ag−Ge (silver germanium) system. Bull. Alloy Phase Diagr. 1988, 9, 58–64. [Google Scholar] [CrossRef]
- Herzig, C.; Divinski, S.V. Grain boundary diffusion in metals: Recent developments. Mater. Trans. 2003, 44, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Wynblatt, P.; Chatain, D. Anisotropy of segregation at grain boundaries and surfaces. Metall. Mater. Trans. A 2006, 37, 2595–2620. [Google Scholar] [CrossRef]
- Wynblatt, P. Interfacial segregation effects in wetting phenomena. Annu. Rev. Mater. Res. 2008, 38, 173–196. [Google Scholar] [CrossRef]
- Lončarić, M.; Sancho-Parramon, J.; Pavlovič, M.; Zorc, H.; Dubček, P.; Turković, A.; Bernstorff, S.; Jakopič, G.; Haase, A. Optical and structural characterization of silver islands films on glass substrates. Vacuum 2009, 84, 188–192. [Google Scholar] [CrossRef]
- Stefaniuk, T.; Ciesielski, A.; Wrobel, P.; Wronkowska, A.A.; Wronkowski, A.; Skowronski, L.; Szoplik, T. Optical parameters of 10 nm to 100 nm thick silver films. Rom. Rep. Phys. 2015, 67, 1331–1333. [Google Scholar]
- Ciesielski, A.; Skowronski, L.; Trzcinski, M.; Górecka, E.; Kierdaszuk, J.; Szoplik, T. Growth model and structure evolution of Ag films deposited on Ge. Beilstein J. Nanotechnol. 2018, 9, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Ciesielski, A.; Skowronski, L.; Trzcinski, M.; Szoplik, T. Controlling the optical parameters of self−assembled silver films with wetting layers and annealing. Appl. Surf. Sci. 2017, 421, 349–356. [Google Scholar] [CrossRef]
- Ciesielski, A.; Skowronski, L.; Pacuski, W.; Szoplik, T. Permittivity of Ge, Te and Se thin films in the 200–1500 nm spectral range. Predicting the segregation effects in silver. Mater. Sci. Semicond. Process. 2018, 81, 64–67. [Google Scholar] [CrossRef]
- Ciesielski, A.; Skowronski, L.; Trzcinski, M.; Górecka, E.; Trautman, P.; Szoplik, T. Evidence of germanium segregation in gold thin films. Surf. Sci. 2018, 674, 73–78. [Google Scholar] [CrossRef]
- Ciesielski, A.; Skowronski, L.; Trzcinski, M.; Górecka, E.; Pacuski, W.; Szoplik, T. Interaction of Te and Se interlayers with Ag or Au nanofilms in sandwich structures. Beilstein J. Nanotechnol. 2019, 10, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lejček, P. Grain Boundary Segregation in Metals; Springer Science & Business Media: Berlin, Germany, 2010. [Google Scholar]
- Mehrer, H. Diffusion in Solids: Fundamentals, Methods, Materials, Diffusion−Controlled Processes; Springer Science & Business Media: Berlin, Germany, 2007. [Google Scholar]
- Paul, A.; Laurila, T.; Vuorinen, V.; Divinski, S.V. Thermodynamics, Diffusion and the Kirkendall Effect in Solids; Springer International Publishing: Cham, Switzerland, 2014. [Google Scholar]
- Lejček, P.; Hofmann, S. On the relationship between entropy and enthalpy of grain boundary segregation. Interface Sci. 2001, 9, 221–230. [Google Scholar] [CrossRef]
- Lejček, P.; Hofmann, S. Thermodynamics of grain boundary segregation and applications to anisotropy, Compensation Effect and Prediction. Crit. Rev. Solid State 2008, 33, 133–163. [Google Scholar] [CrossRef]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements; Butterworth−Heinemann: Oxford, England, 1997. [Google Scholar]
- The Photographic Periodic Table of the Elements. Available online: https://periodictable.com/ (accessed on 17 March 2020).
- Cordero, B.; Gómez, V.; Platero−Prats, A.E.; Revés, M.; Echeverría, J.; Cremades, E.; Barragán, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 21, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Pyykkö, P.; Atsumi, M. Molecular Single−Bond Covalent Radii for Elements 1−118. Chem. Eur. J. 2009, 15, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Mechanical Properties, Elastic Constants, Lattice Vibrations of Germanium (Ge). Available online: http://www.ioffe.ru/SVA/NSM/Semicond/Ge/mechanic.html (accessed on 17 March 2020).
- Skriver, H.L.; Rosengaard, N.M. Surface energy and work function of metals. Phys. Rev. B 1992, 46, 7157–7168. [Google Scholar] [CrossRef] [Green Version]
- Jaccodine, R.J. Surface Energy of Germanium and Silicon. J. Electrochem. Soc. 1963, 110, 524–527. [Google Scholar] [CrossRef]
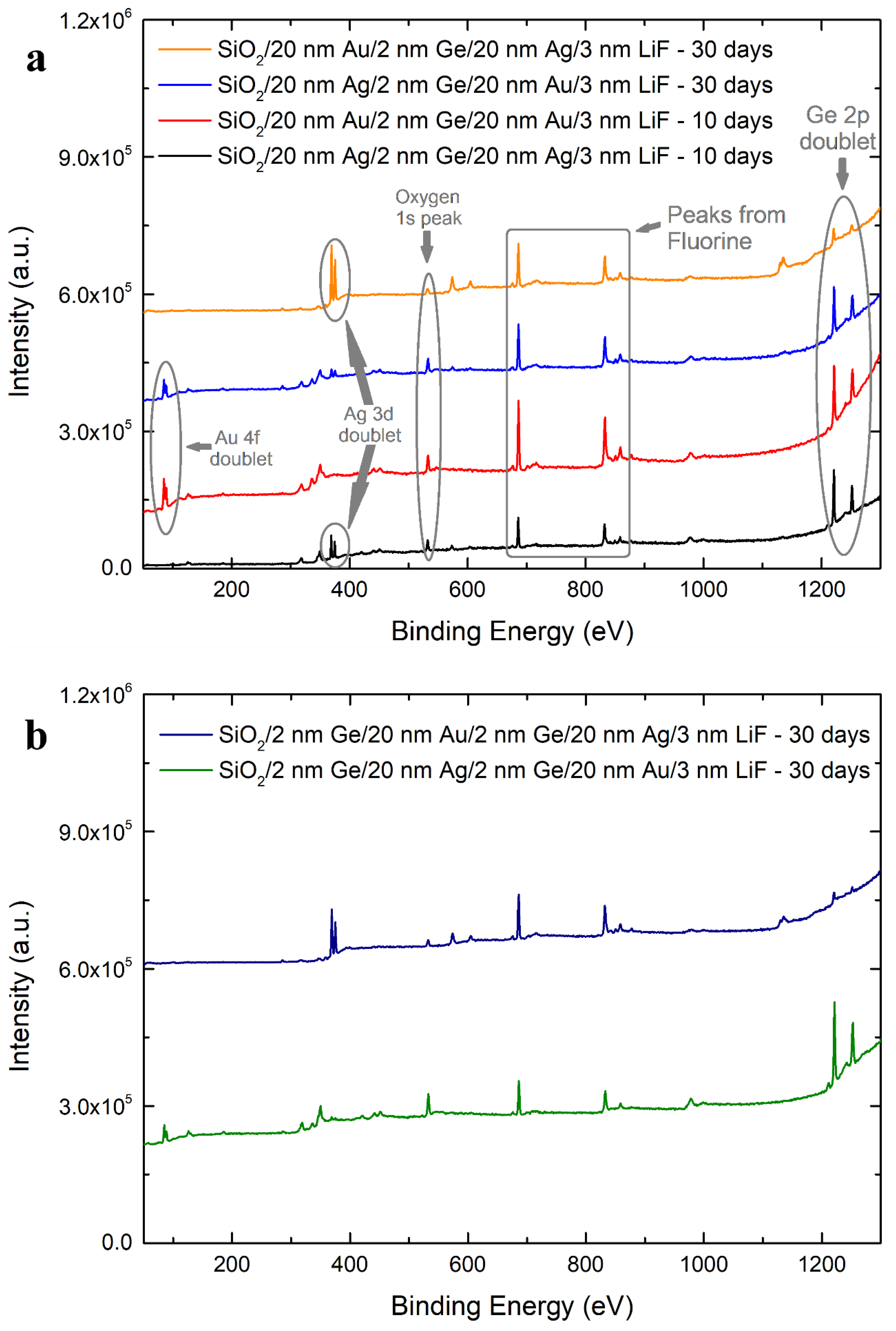
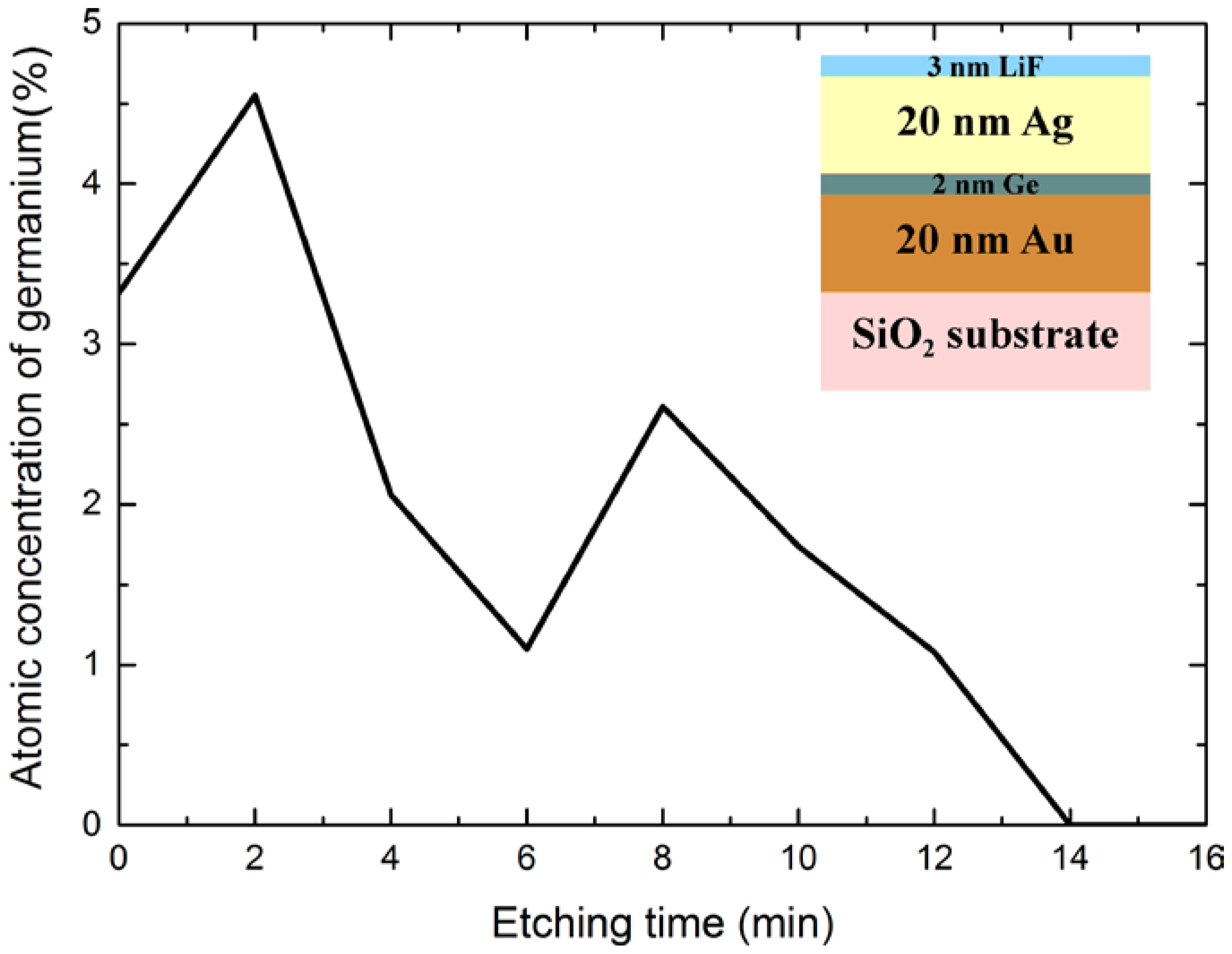
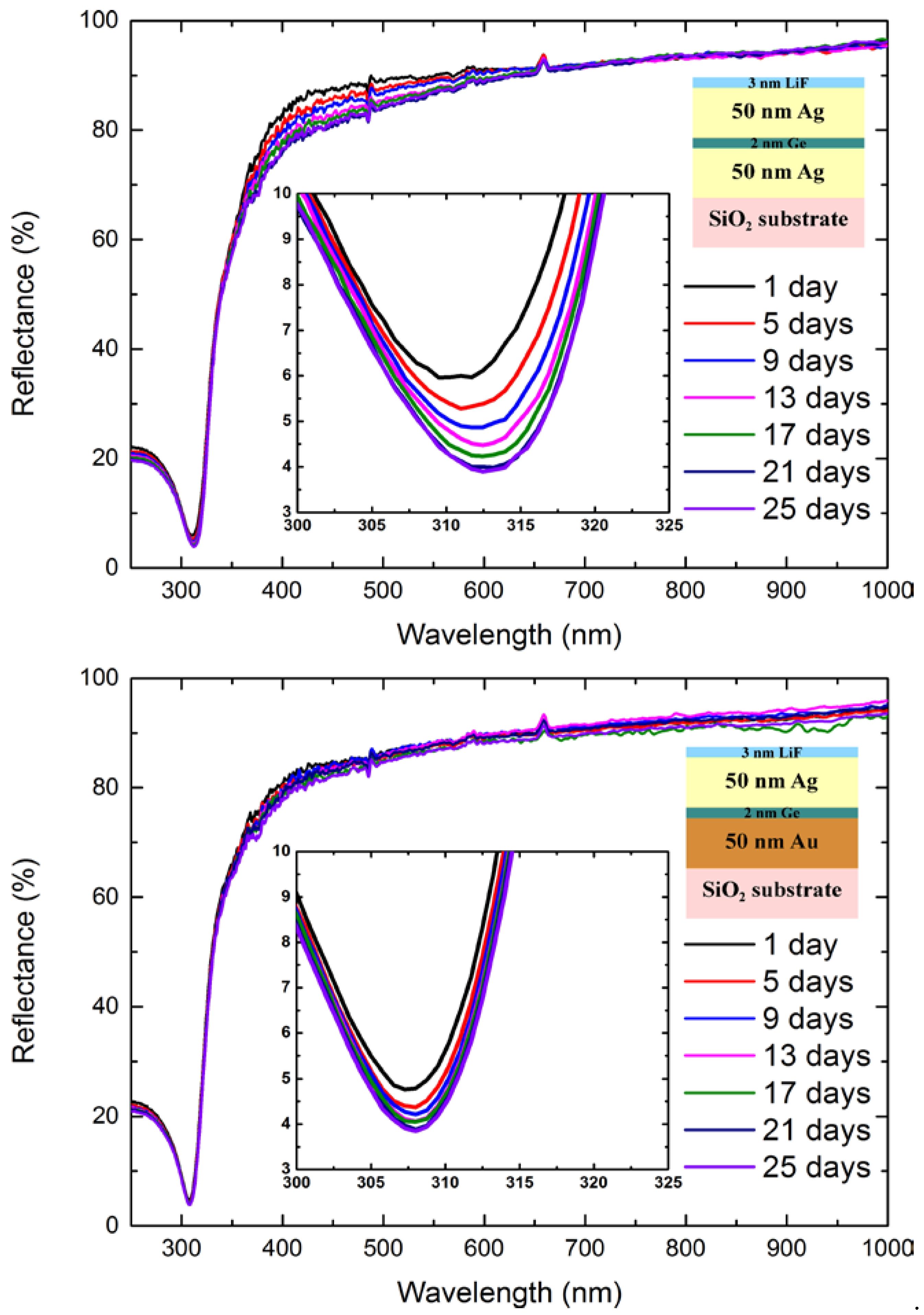
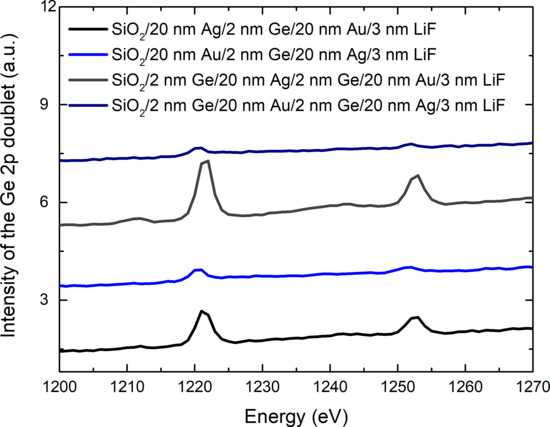
| Sample | Surface Ge−to−Metal Ratio |
|---|---|
| SiO2/20 nm Ag/2 nm Ge/20 nm Ag/3 nm LiF (measured 10 days after deposition) | 1.77 |
| SiO2/20 nm Au/2 nm Ge/20 nm Au/3 nm LiF (measured 10 days after deposition) | 1.09 |
| SiO2/20 nm Ag/2 nm Ge/20 nm Au/3 nm LiF (measured 30 days after deposition) | 1.63 |
| SiO2/20 nm Au/2 nm Ge/20 nm Ag/3 nm LiF (measured 30 days after deposition) | 0.19 |
| SiO2/2 nm Ge/20 nm Ag/2 nm Ge/20 nm Au/3 nm LiF (measured 30 days after deposition) | 3.07 |
| SiO2/2 nm Ge/20 nm Au/2 nm Ge/20 nm Ag/3 nm LiF (measured 30 days after deposition) | 0.18 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciesielski, A.; Trzcinski, M.; Szoplik, T. Inhibiting the Segregation of Germanium in Silver Nanolayers. Crystals 2020, 10, 262. https://doi.org/10.3390/cryst10040262
Ciesielski A, Trzcinski M, Szoplik T. Inhibiting the Segregation of Germanium in Silver Nanolayers. Crystals. 2020; 10(4):262. https://doi.org/10.3390/cryst10040262
Chicago/Turabian StyleCiesielski, Arkadiusz, Marek Trzcinski, and Tomasz Szoplik. 2020. "Inhibiting the Segregation of Germanium in Silver Nanolayers" Crystals 10, no. 4: 262. https://doi.org/10.3390/cryst10040262
APA StyleCiesielski, A., Trzcinski, M., & Szoplik, T. (2020). Inhibiting the Segregation of Germanium in Silver Nanolayers. Crystals, 10(4), 262. https://doi.org/10.3390/cryst10040262