(Perylene)3-(TCNQF1)2: Yet Another Member in the Series of Perylene–TCNQFx Polymorphic Charge Transfer Crystals
Abstract
1. Introduction
2. Materials and Methods
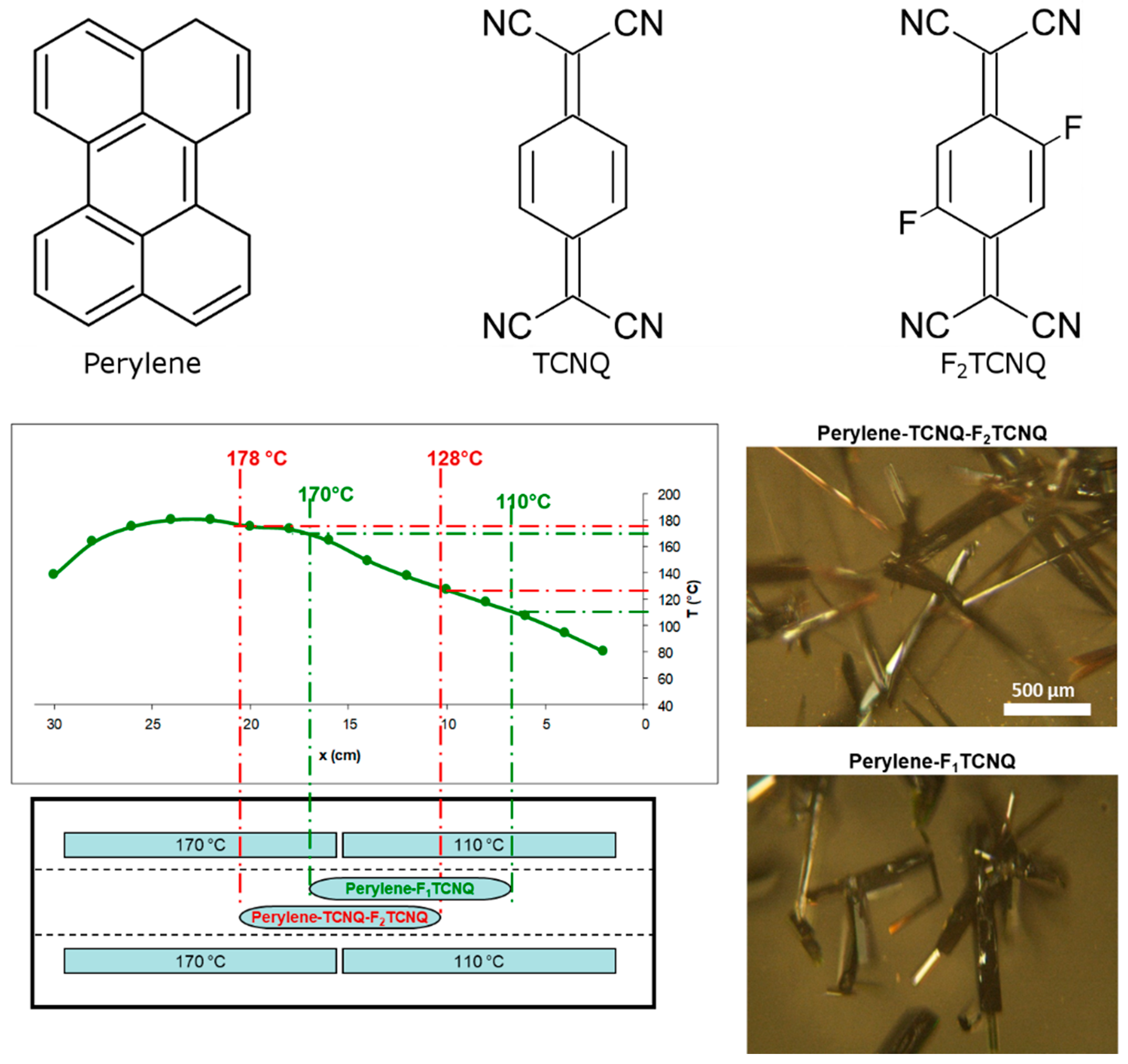
2.1. Sample Preparation and Crystal Growth
2.2. X-ray Diffraction Measurements
2.3. Spectroscopic Measurements
3. Results
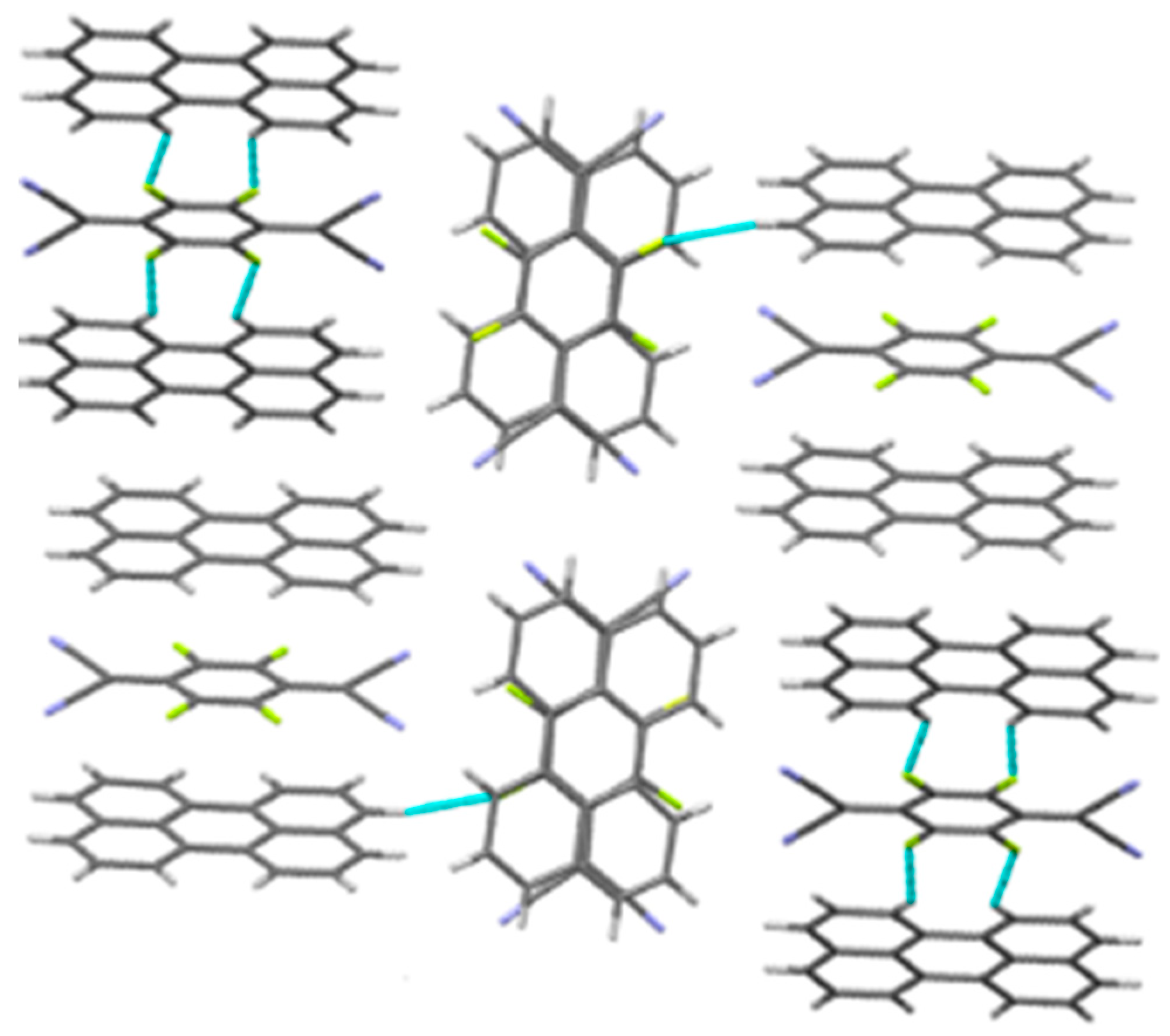
3.1. Crystal Structure
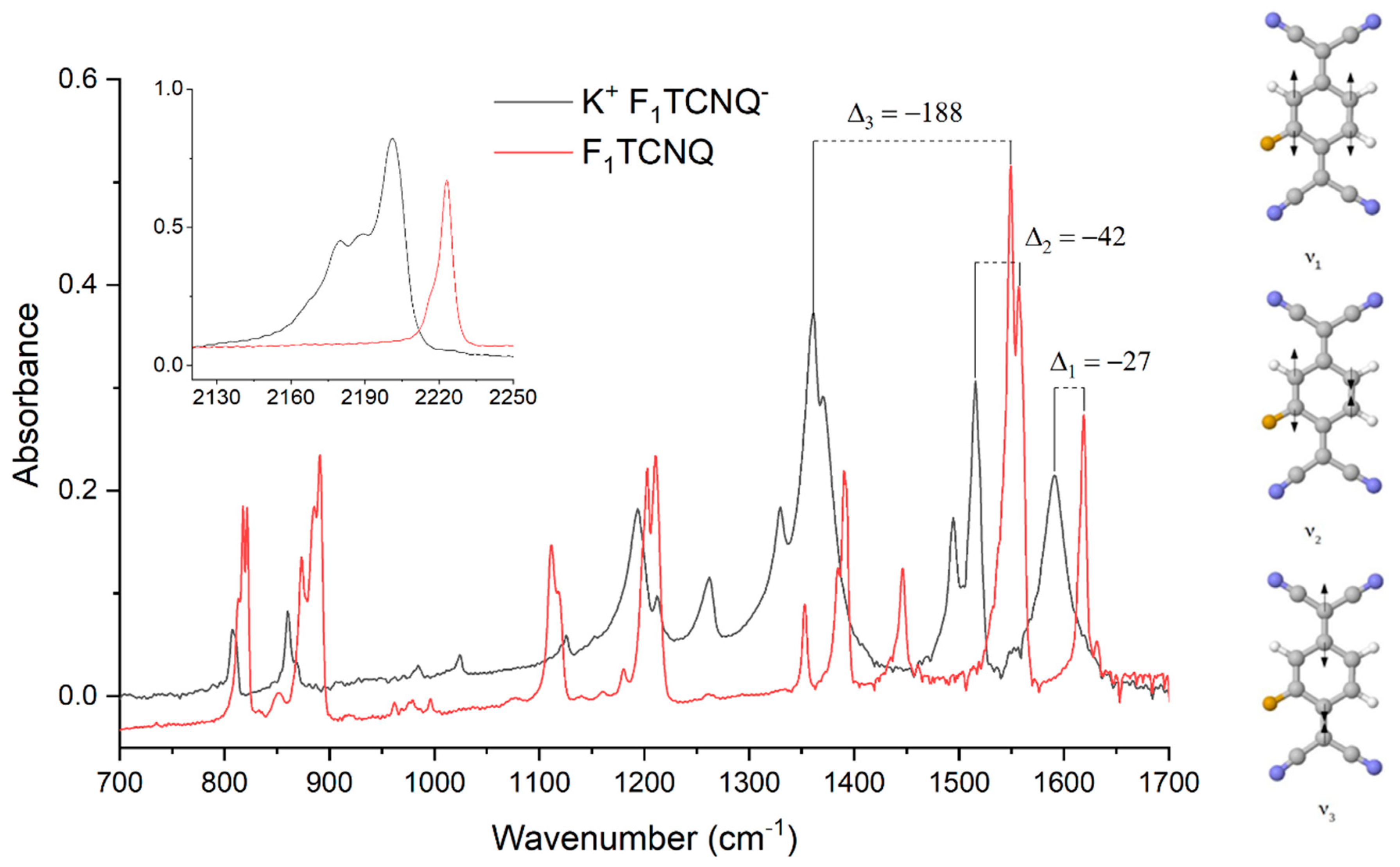
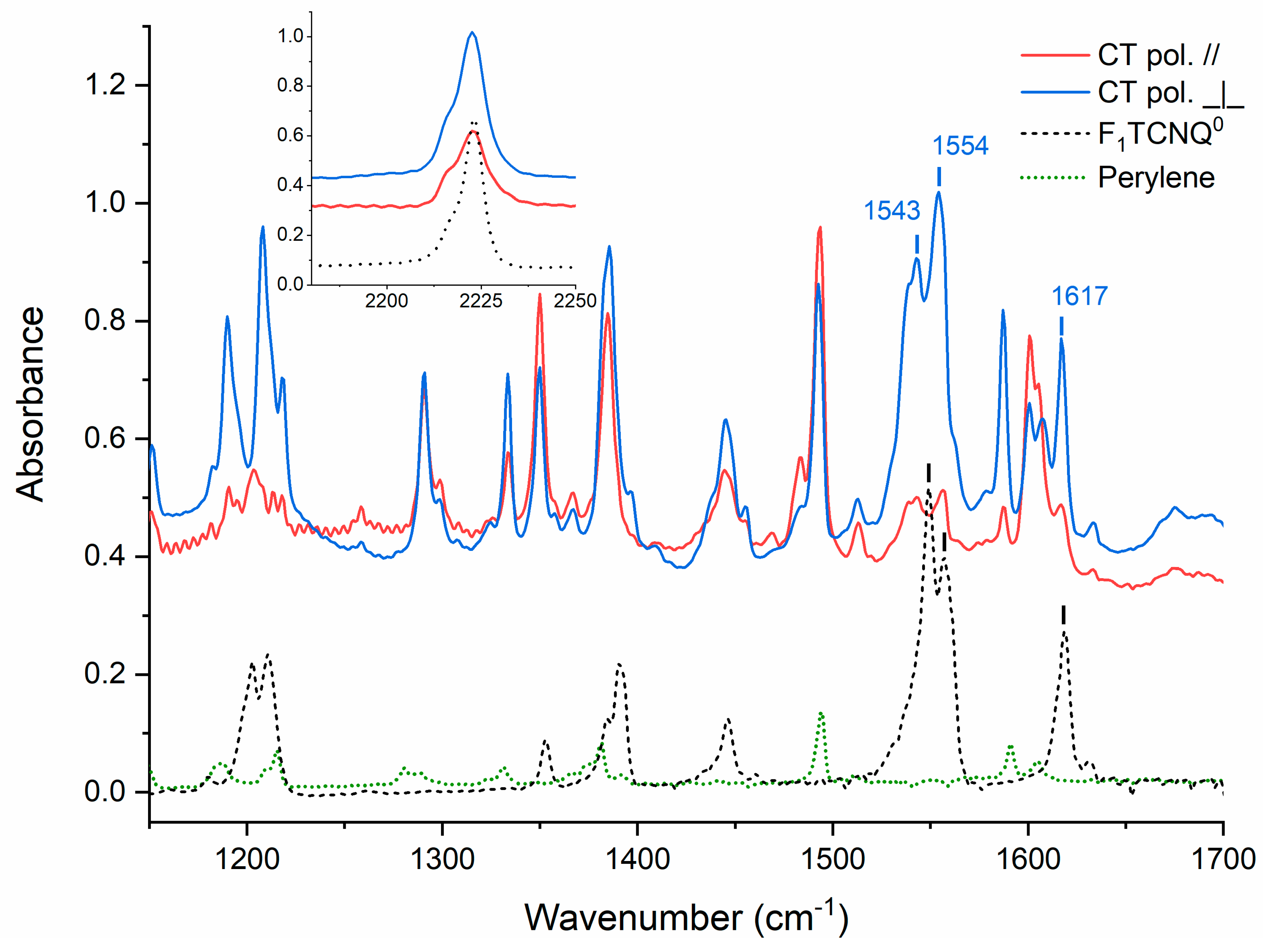
3.2. Determination of the Degree of CT
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, H.; Hu, P.; Ye, J.; Zhang, K.K.; Long, Y.; Hu, W.; Kloc, C. Tuning of the degree of charge transfer and the electronic properties in organic binary compounds by crystal engineering: A perspective. J. Mater. Chem. C 2018, 6, 1884–1902. [Google Scholar] [CrossRef]
- Sun, L.; Zhu, W.; Yang, F.; Li, B.; Ren, X.; Zhang, X.; Hu, W. Molecular cocrystals: Design, charge–Transfer and optoelectronic functionality. Phys. Chem. Chem. Phys. 2018, 20, 6009–6023. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yi, Y.; Li, Y.; Kim, E.G.; Coropceanu, V.; Brédas, J.L. Prediction of remarkable ambipolar charge—Transport characteristics in organic mixed-stack charge—Transfer crystals. J. Am. Chem. Soc. 2012, 134, 2340–2347. [Google Scholar] [CrossRef] [PubMed]
- Goetz, K.P.; Vermeulen, D.; Payne, M.E.; Kloc, C.; McNeil, L.E.; Jurchescu, O.D. Charge–Transfer complexes: New perspectives on an old class of compounds. J. Mater. Chem. C 2014, 2, 3065–3076. [Google Scholar] [CrossRef]
- Geng, H.; Zheng, X.; Shuai, Z.; Zhu, L.; Yi, Y. Understanding the charge transport and polarities in organic donor-acceptor mixed-stack crystals: Molecular insights from the super-exchange couplings. Adv. Mater. 2015, 27, 1443–1449. [Google Scholar] [CrossRef]
- Bernstein, J. Polymorphism in Molecular Crystals; Oxford University Press: New York, NY, USA, 2002. [Google Scholar]
- Goetz, K.P.; Tsutsumi, J.; Pookpanratana, S.; Chen, J.; Corbin, N.S.; Behera, R.K.; Coropceanu, V.; Richter, C.A.; Hacker, C.A.; Hasegawa, T.; et al. Polymorphism in the 1:1 Charge–Transfer Complex DBTTF–TCNQ and Its Effects on Optical and Electronic Properties. Adv. Electron. Mater. 2016, 2, 1–10. [Google Scholar] [CrossRef]
- Salzillo, T.; Campos, A.; Mas-Torrent, M. Solution-processed thin films of a charge transfer complex for ambipolar field-effect transistors. J. Mater. Chem. C 2019, 7, 10257–10263. [Google Scholar] [CrossRef]
- McCrone, W.C. Polymorphism in Physics and Chemistry of the Organic Solid State, Vol. II.; Fox, D., Labes, M.M., Weissenberg, A., Eds.; Interscience: New York, NY, USA, 1965; Volume II. [Google Scholar]
- Henderson, J.; Masino, M.; Hatcher, L.E.; Kociok-Köhn, G.; Salzillo, T.; Brillante, A.; Raithby, P.R.; Girlando, A.; Da Como, E. New Polymorphs of Perylene: Tetracyanoquinodimethane Charge Transfer Cocrystals. Cryst. Growth Des. 2018, 18, 2003–2009. [Google Scholar] [CrossRef]
- Vermeulen, D.; Zhu, L.Y.; Goetz, K.P.; Hu, P.; Jiang, H.; Day, C.S.; Jurchescu, O.D.; Coropceanu, V.; Kloc, C.; McNeil, L.E. Charge Transport Properties of Perylene − TCNQ Crystals: The Effect of Stoichiometry. J. Phys. Chem. C 2014, 118, 24688–24696. [Google Scholar] [CrossRef]
- Ishii, K.; Yakushi, K.; Kuroda, H.; Inokuchi, H. Reflection and Photoconduction Spectra of the Single Crystals of Perylene–TCNQ 1:1 and 3:1 Molecular Complexes. Bull. Chem. Soc. Jpn. 1984, 57, 3043–3047. [Google Scholar] [CrossRef]
- Salzillo, T.; Masino, M.; Kociok-Köhn, G.; Di Nuzzo, D.; Venuti, E.; Della Valle, R.G.; Vanossi, D.; Fontanesi, C.; Girlando, A.; Brillante, A.; et al. Structure, Stoichiometry, and Charge Transfer in Cocrystals of Perylene with TCNQ-Fx. Cryst. Growth Des. 2016, 16, 3028–3036. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, C.; Hao, W.; Zhao, H.; Li, S.; Shen, L.; Zhu, J.; Di, C.A. Impact of Stoichiometry and Fluorine Atoms on the Charge Transport of Perylene-F4TCNQ. J. Phys. Chem. Lett. 2019, 10, 3376–3380. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Du, K.; Wei, F.; Jiang, H.; Kloc, C. Crystal growth, HOMO-LUMO engineering, and charge transfer degree in perylene-FxTCNQ (x = 1, 2, 4) organic charge transfer binary compounds. Cryst. Growth Des. 2016, 16, 3019–3027. [Google Scholar] [CrossRef]
- Melby, L.R.; Harder, R.J.; Hertler, W.R.; Mahler, W.; Benson, R.E.; Mochel, W.E. Substituted Quinodimethans. II. Anion-Radical Derivatives and Complexes of 7, 7, 8, 8-Tetracyanoquinodimethan. J. Am. Chem. Soc. 1962, 84, 3374–3387. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-Ray Diffraction Data Collected in Oscillation Mode. Macromol. Crystallogr. 1997, 276, 307–326. [Google Scholar]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Meneghetti, M.; Pecile, C. Charge–transfer organic crystals: Molecular vibrations and spectroscopic effects of electron–molecular vibration coupling of the strong electron acceptor TCNQF4. J. Chem. Phys. 1986, 84, 4149–4162. [Google Scholar] [CrossRef]
- Girlando, A. Comment on Polymorphism in the 1:1 Charge–Transfer Complex DBTT-TCNQ and Its Effects on Optical and Electronic Properties. Adv. Electron. Mater. 2017, 3, 1600437. [Google Scholar] [CrossRef]
- Frackowiak, A.; Olejniczak, I.; Świetlik, R.; Jeannin, O.; Fourmigué, M. Temperature-Induced Neutral-Ionic Phase Transition in the (EDT-TTF-I2)2TCNQF Mixed-Stack Charge–Transfer Salt. J. Phys. Chem. C 2016, 120, 23740–23747. [Google Scholar] [CrossRef]
- Painelli, A.; Girlando, A. Electron–molecular vibration (e–mv) coupling in charge-transfer compounds and its consequences on the optical spectra: A theoretical framework. J. Chem. Phys. 1986, 84, 5655–5671. [Google Scholar] [CrossRef]
- Masino, M.; Girlando, A.; Farina, L.; Brillante, A. A new type of neutral ionic interface in mixed stack organic charge transfer crystals: Temperature induced ionicity change in ClMePD DMeDCNQI. Phys. Chem. Chem. Phys. 2001, 3, 1904–1910. [Google Scholar] [CrossRef]
- Maienschein-Cline, M.G.; Londergan, C.H. The CN stretching band of aliphatic thiocyanate is sensitive to solvent dynamics and specific solvation. J. Phys. Chem. A 2007, 111, 10020–10025. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yi, Y.; Zhen, Y.; Hu, W. Precisely tailoring the stoichiometric stacking of perylene–TCNQ co-crystals towards different nano and microstructures with varied optoelectronic performances. Small 2015, 11, 2150–2156. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Ma, L.; Tan, K.J.; Jiang, H.; Wei, F.; Yu, C.; Goetz, K.P.; Jurchescu, O.D.; McNeil, L.E.; Gurzadyan, G.G.; et al. Solvent-dependent stoichiometry in perylene-7,7,8,8-tetracyanoquinodimethane charge transfer compound single crystals. Cryst. Growth Des. 2014, 14, 6376–6382. [Google Scholar] [CrossRef]
- Salzillo, T.; Della Valle, R.G.; Venuti, E.; Kociok-Köhn, G.; Masino, M.; Girlando, A.; Brillante, A. Solution equilibrium between two structures of Perylene-F2 TCNQ charge transfer co-crystals. J. Cryst. Growth 2019, 516, 45–50. [Google Scholar] [CrossRef]
- Levina, E.O.; Chernyshov, I.Y.; Voronin, A.P.; Alekseiko, L.N.; Stash, A.I.; Vener, M.V. Solving the enigma of weak fluorine contacts in the solid state: A periodic DFT study of fluorinated organic crystals. RSC Adv. 2019, 9, 12520–12537. [Google Scholar] [CrossRef]
- D’Avino, G.; Della Valle, R.G.; Heimel, G.; Oehzelt, M.; Kera, S.; Ueno, N.; Beljonne, D.; Salzmann, I. Electrostatic Interactions Shape Molecular Organization and Electronic Structure of Organic Semiconductor Blends. Chem. Mater. 2020. [Google Scholar] [CrossRef]

| TCNQF1 1 | TCNQF2 2 | TCNQF4 2 | |
|---|---|---|---|
| Stoichiometry | 3:2 | 3:2 | 3:2 |
| Space group | P-1 | P-1 | P-1 |
| Temperature (K) | 150(2) | 150(2) | 150(2) |
| a (Å) | 7.2254(2) | 7.2307(1) | 7.2066(2) |
| b (Å) | 19.1275(4) | 19.1407(3) | 19.0974(6) |
| c (Å) | 22.3203(6) | 22.2723(5) | 22.4050(7) |
| α (°) | 112.2658(14) | 112.2834(8) | 111.8014(14) |
| β (°) | 90.2346(10) | 90.1900(8) | 90.0472(17) |
| γ (°) | 94.0322(15) | 93.7599(12) | 94.1283(17) |
| V (Å3) | 2846.09(13) | 2844.85(9) | 2854.3(15) |
| Z | 2 | 2 | 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salzillo, T.; Valle, R.G.D.; Venuti, E.; Brillante, A.; Kociok-Köhn, G.; Di Nuzzo, D.; Masino, M.; Girlando, A. (Perylene)3-(TCNQF1)2: Yet Another Member in the Series of Perylene–TCNQFx Polymorphic Charge Transfer Crystals. Crystals 2020, 10, 177. https://doi.org/10.3390/cryst10030177
Salzillo T, Valle RGD, Venuti E, Brillante A, Kociok-Köhn G, Di Nuzzo D, Masino M, Girlando A. (Perylene)3-(TCNQF1)2: Yet Another Member in the Series of Perylene–TCNQFx Polymorphic Charge Transfer Crystals. Crystals. 2020; 10(3):177. https://doi.org/10.3390/cryst10030177
Chicago/Turabian StyleSalzillo, Tommaso, Raffaele G. Della Valle, Elisabetta Venuti, Aldo Brillante, Gabriele Kociok-Köhn, Daniele Di Nuzzo, Matteo Masino, and Alberto Girlando. 2020. "(Perylene)3-(TCNQF1)2: Yet Another Member in the Series of Perylene–TCNQFx Polymorphic Charge Transfer Crystals" Crystals 10, no. 3: 177. https://doi.org/10.3390/cryst10030177
APA StyleSalzillo, T., Valle, R. G. D., Venuti, E., Brillante, A., Kociok-Köhn, G., Di Nuzzo, D., Masino, M., & Girlando, A. (2020). (Perylene)3-(TCNQF1)2: Yet Another Member in the Series of Perylene–TCNQFx Polymorphic Charge Transfer Crystals. Crystals, 10(3), 177. https://doi.org/10.3390/cryst10030177








