Fabrication of Lettuce-Like ZnO Gas Sensor with Enhanced H2S Gas Sensitivity
Abstract
1. Introduction
2. Experiment
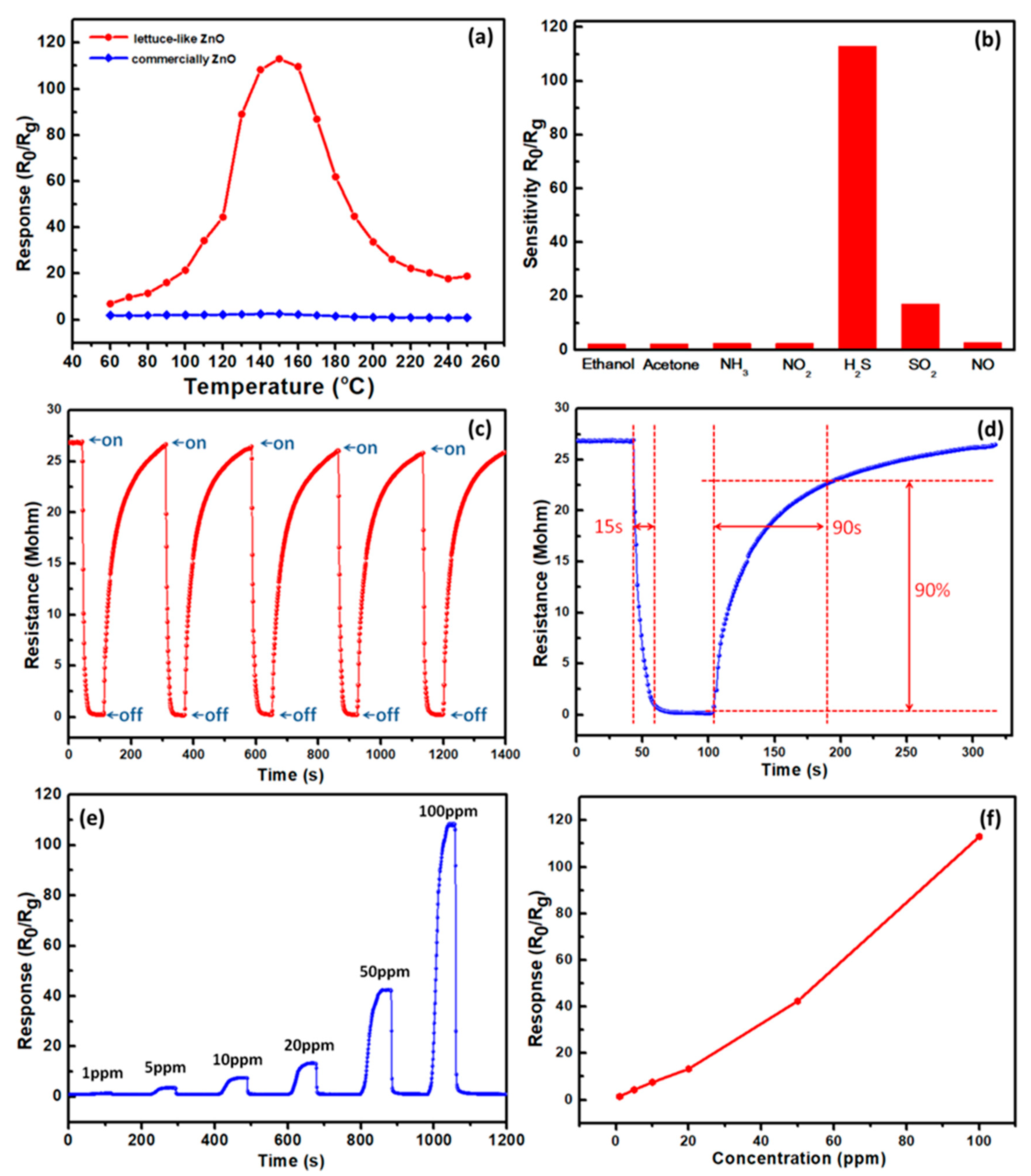
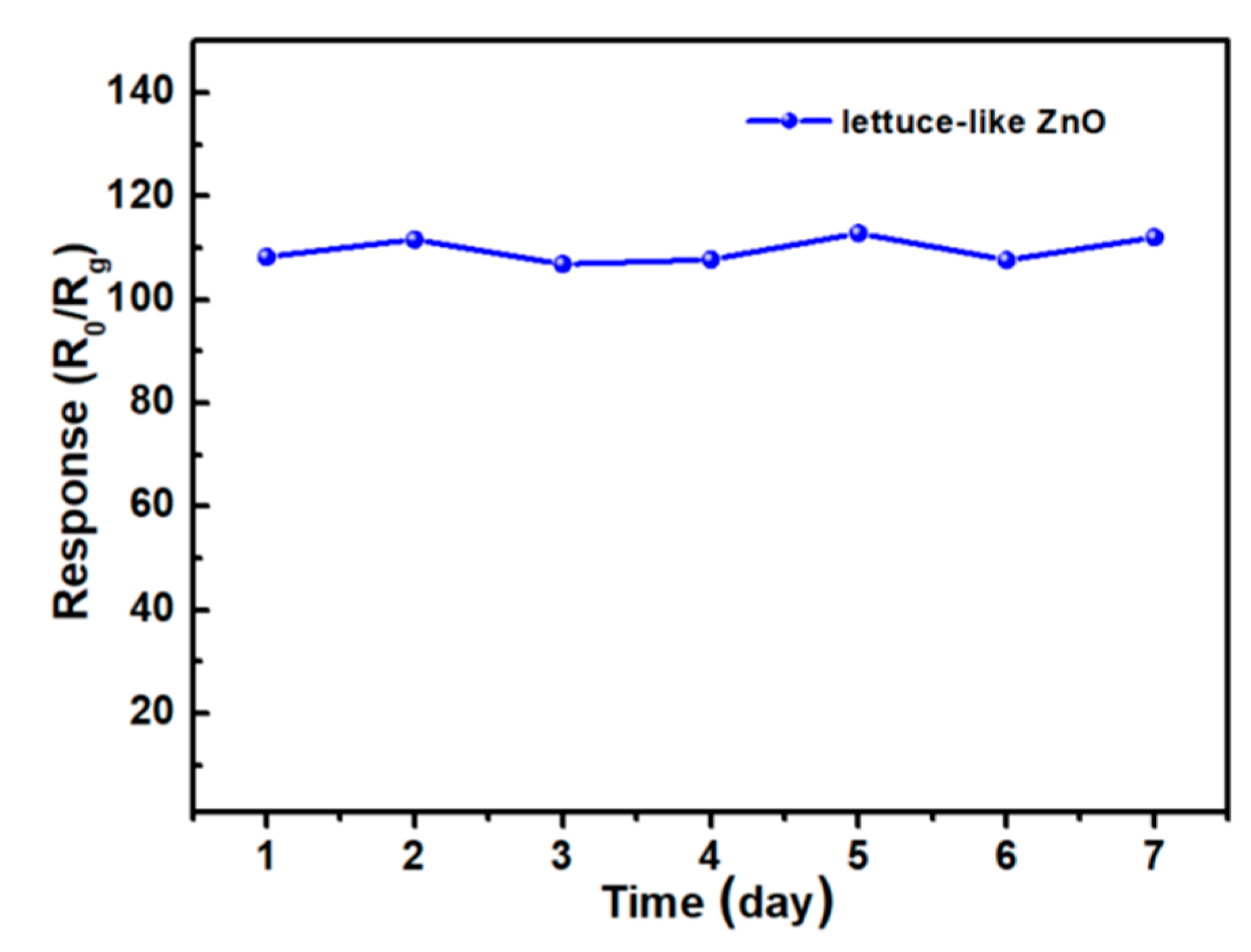
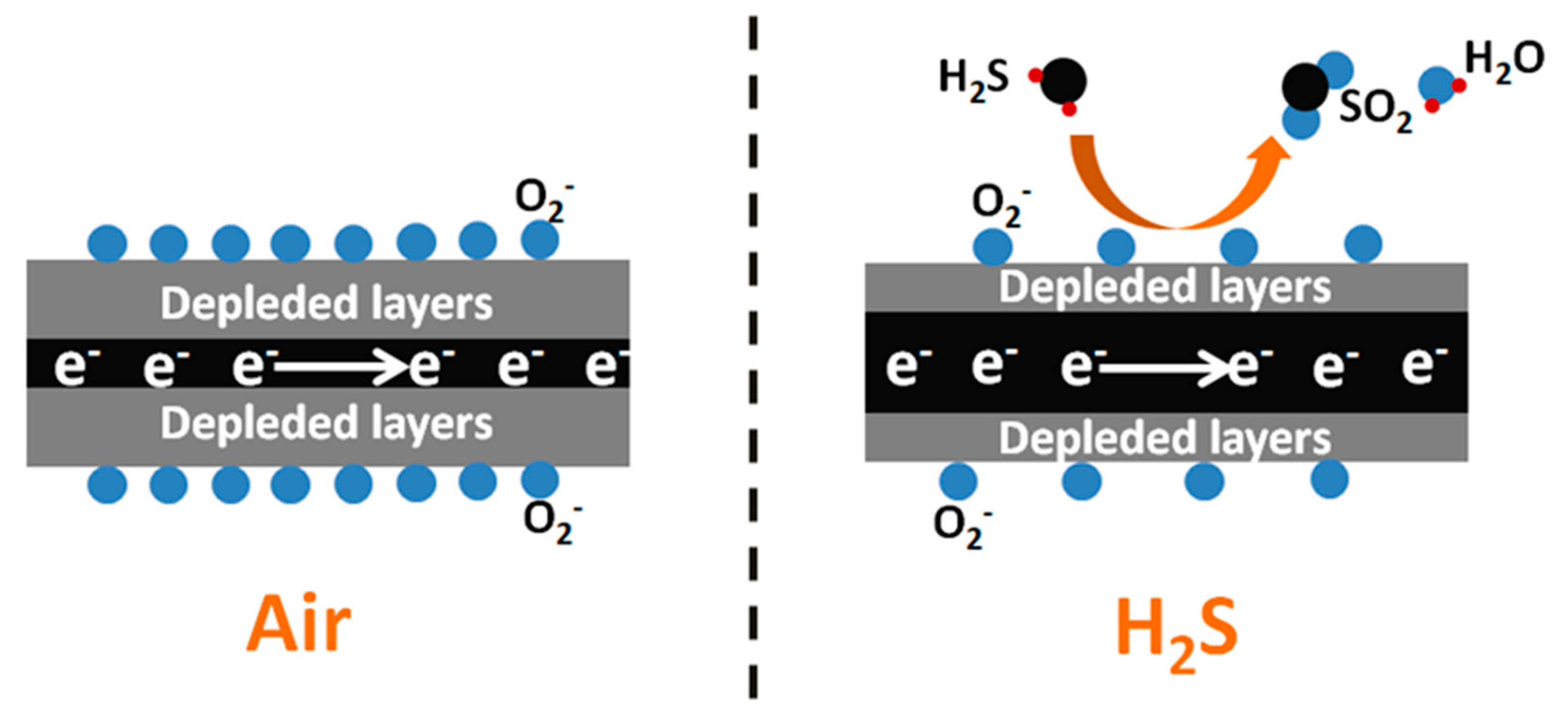
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wagner, T.; Haffer, S.; Weinberger, C.; Klaus, D.; Tiemann, M. Mesporous materials as gas sensors. Chem. Soc. Rev. 2013, 42, 4036–4053. [Google Scholar] [CrossRef]
- Geng, B.; Liu, J.; Wang, C. Multi-layer ZnO architectures: Polymer induced synthesis and their application as gas sensors. Sens. Actuators B 2010, 150, 742–748. [Google Scholar] [CrossRef]
- He, Y.; Zhang, W.; Zhang, S.; Kang, X.; Peng, W.; Xu, Y. Study of the photoconductive ZnO UV detector based on the electrically floated nanowire array. Sens. Actuators A 2012, 181, 6–12. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H. Hydrothermal synthesis of ZnO nanorods in the diameter regime of 50nm. J. Am. Chem. Soc. 2003, 125, 4430–4431. [Google Scholar] [CrossRef] [PubMed]
- Dhahri, R.; Hjiri, M.; ElMir, L.; Alamri, H.; Bonavita, A.; Iannazzo, D.; Leonardi, S.G.; Neri, G. CO sensing characteristics of In-doped ZnO semiconductor nanoparticles. J. Sci. Adv. Mater. Devices 2017, 2, 34–40. [Google Scholar] [CrossRef]
- Shukla, G.; Bhatnagar, M. H2S gas sensor based on Cu doped SnO2 nanostructure. J. Mater. Sci. Eng. A 2014, 4, 99–104. [Google Scholar]
- Katti, V.; Debnath, A.; Muthe, K.; Kaur, M.; Dua, A.; Gadkari, S.; Gupta, S.; Sahni, V. Mechanism of drifts in H2S sensing properties of SnO2:CuO composite thin film sensors prepared by thermal evaporation. Sens. Actuators B 2003, 96, 245–252. [Google Scholar] [CrossRef]
- Glass, D.C. A revive of the hellth effect of offs of hydrogen sulphide exposure. Ann. Occupypatial Hyg. 1990, 34, 323–327. [Google Scholar]
- Lia, Y.; Deng, D.; Xing, X.; Chen, N.; Liu, X.; Xiao, X.; Wang, Y. A high performance methanol gas sensor based on palladium-platinum-In2O3 composited nanocrystalline SnO2. Sens. Actuators B 2016, 237, 133–141. [Google Scholar] [CrossRef]
- Guo, K.; Wen, J.; Hao, Y. Optimal Packing of a Rotating Packed Bed for H2S Removal. Environ. Sci. Technol. 2014, 48, 6844–6849. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, G.; Zeng, G.; Liu, L.; Zhang, C. Metal oxides and metal salt nanos-tructures for hydrogen sulfide sensing: Mechanism and sensing performance. RSC Adv. 2015, 5, 54793–54805. [Google Scholar] [CrossRef]
- Liao, L.; Lu, H.; Li, J.; He, H.; Wang, D.; Fu, D.; Liu, C. Size dependence of gas sensitivity of ZnO nanorods. J. Phys. Chem. C 2007, 111, 1900–1903. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, H.; Liu, Z.; Han, B.; Zhang, X. A highly efficient chemical sensor material for H2S: A-Fe2O3 nanotubes fabricated using carbon nanotube templates. Adv. Mater. 2005, 17, 2993–2997. [Google Scholar] [CrossRef]
- Liu, T.; Xu, L.; Wang, X.; Li, Q.; Cui, Q.; Suo, H.; Zhao, C. A Simple Dip-Coating Method of SnO2-NiO Composite Thin Film on a Ceramic Tube Substrate for Methanol Sensing. Crystals 2019, 9, 621. [Google Scholar] [CrossRef]
- Rout, C.; Hegde, M.; Rao, C. H2S sensors based on tungsten oxide nanostructures. Sens. Actuators B 2008, 128, 488–493. [Google Scholar] [CrossRef]
- Liu, J.; Huang, X.; Ye, G.; Liu, W.; Jiao, Z.; Ghao, W.; Zhou, Z.; Yu, Z. H2S detection sensing characteristic of CuO/SnO2 sensor. Sensors 2003, 3, 110–118. [Google Scholar] [CrossRef]
- Mai, L.; Xu, L.; Gao, Q.; Han, C.; Hu, B.; Pi, Y. Single β-AgVO3 nanowire H2S sensor. Nano Lett. 2010, 10, 2604–2608. [Google Scholar] [CrossRef]
- Huber, F.; Riegert, S.; Madel, M.; Thonke, K. H2S sensing in the ppb regime with zinc oxide nanowires. Sens. Actuators B 2017, 239, 358–363. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Z.; He, X.; Xie, W. Detection of H2S at Room Temperature Using ZnO Sensors Based on Hall Effect. Int. J. Electrochem. Sci. 2017, 12, 6465–6476. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Zhu, M.; Ji, G.; Zhao, L.; Yin, J.; Bie, L. Acetone sensing of ZnO nanosheets synthesized using room-temperature precipitation. Sens. Actuators B 2017, 249, 611–623. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, J.; Lu, H.; Li, L.; Zheng, J.; Li, H.; Zhu, Z. Facile synthesis and ultrahigh ethanol response of hierarchically porous ZnO nanosheets. Sens. Actuators B 2012, 161, 209–215. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Shao, M.; Huang, J.; Deng, X.; Hou, P.; Xu, X. Fabrication of ZnO/ZnFe2O4 hollow nanocages through metal organic Frameworks route with enhanced gas sensing properties. Sens. Actuators B 2017, 251, 27–33. [Google Scholar] [CrossRef]
- Kwon, Y.; Kang, S.; Mirzaei, A.; Choi, M.; Bang, J.; Kim, S.; Kim, H. Enhancement of gas sensing properties by the functionalization of ZnO-branched SnO2 nanowires with Cr2O3 nanoparticles. Sens. Actuators B 2017, 249, 656–666. [Google Scholar] [CrossRef]
- Su, X.; Duan, G.; Xu, Z.; Zhou, F.; Cai, W. Structure and thickness-dependent gas sensing responses to NO2 under UV irradiation for the multilayered ZnO micro/nanostructured porous thin films. J. Colloid Interface Sci. 2017, 503, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Sarkar, D. One-pot synthesis of zinc oxide-polyaniline nanocomposite for fabrication of efficient room temperature ammonia gas sensor. Ceram. Int. 2017, 43, 11123–11131. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Xie, D.; X, J.; Xia, Y.; Xiang, L. Enhanced p-type NO2-sensing properties of ZnO nanowires utilizing CNTs electrode. Mater. Lett. 2017, 206, 18–21. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, L.; Liu, Y.; Liu, F.; Liang, X.; Liu, F.; Gao, Y.; Yan, X.; Lu, G. UV-activated ultrasensitive and fast reversible ppb NO2 sensing based on ZnO nanorod modified by constructing interfacial electric field with In2O3 nanoparticles. Sens. Actuators B 2020, 305, 127498. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Bai, J.; Liu, T.; Yu, Z.; Dai, M. Direct growth of NiO films on Al2O3 ceramics by electrochemical deposition and its excellent H2S sensing properties. Sens. Actuators B 2019, 296, 126619. [Google Scholar] [CrossRef]
- Miller, D.; Akbar, S.; Morris, P. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Yu, J.; Ippolito, S.; Wlodarski, W.; Strano, M.; Kalantarzadeh, K. Nanorod based schottky contact gas sensors in reversed bias condition. Nanotechnology 2010, 2126, 265502. [Google Scholar] [CrossRef]
- Chang, S. Oxygen chemisorption on tin oxide: Correlation between electrical conductivity and EPR measurements. J. Vac. Sci. Technol. 1980, 144, 366–369. [Google Scholar] [CrossRef]
- Lupan, O.; Ursaki, V.V.; Chai, G.; Chow, L.; Emelchenko, G.A.; Tiginyanu, I.M.; Gruzintsev, A.N.; Redkin, A.N. Selective hydrogen gas nanosensor using individual ZnO nanowire with fast response at room temperature. Sens. Actuators B 2010, 17, 56–66. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of Tin Oxide Surface With O2, H2O and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Kohl, D. The role of noble metals in the chemistry of solid-state gas sensors. Sens. Actuators B 1990, 1, 158–165. [Google Scholar] [CrossRef]
- Huang, H.; Xu, P.; Zheng, D.; Chen, C.; Li, X. Sulfuration–desulfuration reaction sensing effect of intrinsic ZnO nanowires for high-performance H2S detection. J. Mater. Chem. A 2015, 3, 6330–6339. [Google Scholar] [CrossRef]
- Luo, W.; Deng, J.; Fu, Q.; Zhou, D.; Hu, Y.; Gong, S.; Zheng, Z. Nanocrystalline SnO2 film prepared by the aqueous sol–gel method and its application as sensing films of the resistance and SAW H2S sensor. Sens. Actuators B 2015, 217, 119–128. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Gao, J.; Xu, L.; Liu, T.; Liu, Y.; Wang, X.; Suo, H.; Zhao, C. Fabrication of Lettuce-Like ZnO Gas Sensor with Enhanced H2S Gas Sensitivity. Crystals 2020, 10, 145. https://doi.org/10.3390/cryst10030145
Yu Z, Gao J, Xu L, Liu T, Liu Y, Wang X, Suo H, Zhao C. Fabrication of Lettuce-Like ZnO Gas Sensor with Enhanced H2S Gas Sensitivity. Crystals. 2020; 10(3):145. https://doi.org/10.3390/cryst10030145
Chicago/Turabian StyleYu, Ziyang, Jie Gao, Longxiao Xu, Tianyu Liu, Yueying Liu, Xiangyue Wang, Hui Suo, and Chun Zhao. 2020. "Fabrication of Lettuce-Like ZnO Gas Sensor with Enhanced H2S Gas Sensitivity" Crystals 10, no. 3: 145. https://doi.org/10.3390/cryst10030145
APA StyleYu, Z., Gao, J., Xu, L., Liu, T., Liu, Y., Wang, X., Suo, H., & Zhao, C. (2020). Fabrication of Lettuce-Like ZnO Gas Sensor with Enhanced H2S Gas Sensitivity. Crystals, 10(3), 145. https://doi.org/10.3390/cryst10030145



