Double Palindrome Water Chain in Cu(II) Theophylline Complex. Synthesis, Characterization, Biological Activity of Cu(II), Zn(II) Complexes with Theophylline
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Complexes and Crystallization
2.2. Materials and Methods
3. Results and Discussion
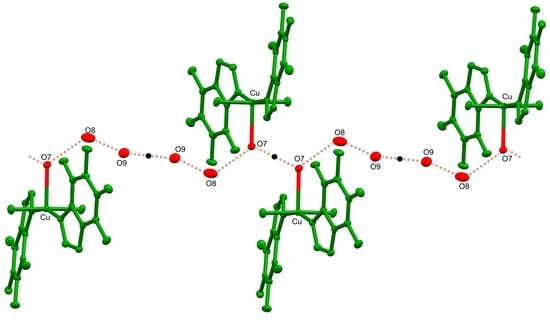
3.1. Crystal Structure
Crystal Packing Analysis
3.2. Infrared Spectra
3.3. TG-DTG-DTA Measurements
3.4. Antioxidant and Antimicrobial Activities
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gilbert, R.M. Caffeine Consumption. Prog. Clin. Biol. Res. 1984, 158, 185–213. [Google Scholar] [PubMed]
- Bujdosova, Z.; Gyoryova, K.; Ruzicka, A.; Melnik, M.; Koman, M. Crystal structures of two aromatiic zinc(II) caroboxylates: [Zn(4-chlorosalicylato)2(H2O)4]·2theophylline·(H2O)2 and unique [Zn(5-chlorosalicylato)2(isonicotinamine)2(H2O)]. J. Chem. Crystallogr. 2011, 41, 1077–1084. [Google Scholar] [CrossRef]
- Biradha, K.; Samai, S.; Maity, A.C.; Goswami, S. Supramolecular assembly of protonated xanthine alkaloids in their perchlorate salts. Cryst. Growth Des. 2009, 10, 937–942. [Google Scholar] [CrossRef]
- Fredholm, B.B. Methylxanthines; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Petrucci, R.; Chiarotto, I.; Mattiello, L.; Passeri, D.; Rossi, R.; Zollo, G.; Feroci, M. Graphene Oxide: A smart (starting) material for natural methylxanthines adsorption and detection. Molecules 2019, 24, 4247. [Google Scholar] [CrossRef]
- Monteiro, J.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Pharmacological potential of methylxanthines: Retrospective analysis and future expectations. Crit. Rev. Food Sci. Nutr. 2019, 59, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, R.; Zollo, G.; Curulli, A.; Marrosu, G. A new insight into the oxidative mechanism of caffeine and related methylxanthines in aprotic medium: May caffeine be really considered as an antioxidant? Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 1781–1789. [Google Scholar] [CrossRef]
- Panusa, A.; Petrucci, R.; Lavecchia, R.; Zuorro, A. UHPLC-PDA-ESI-TOF/MS metabolic profiling and antioxidant capacity of arabica and robusta coffee silverskin: Antioxidants vs phytotoxins. Food Res. Int. 2017, 99, 155–165. [Google Scholar] [CrossRef]
- Monteiro, J.P.; Alves, M.G.; Oliveira, P.F.; Silva, B.M. Structure-Bioactivity Relationships of Methylxanthines: Trying to Make Sense of All the Promises and the Drawbacks. Molecules 2016, 21, 974. [Google Scholar] [CrossRef]
- Franco, R.; Oñatibia-Astibia, A.; Martínez-Pinilla, E. Health Benefits of Methylxanthines in Cacao and Chocolate. Nutrients 2013, 5, 4159–4173. [Google Scholar] [CrossRef]
- Kossel, A. Über eine neue Base aus dem Pflanzenreich. Ber. Dtsch. Chem. Ges. 1888, 21, 2164–2167. [Google Scholar] [CrossRef]
- Fischer, E.; Ach, L. Synthese des Caffeins. Ber. Dtsch. Chem. Ges. 1985, 28, 3135–3139. [Google Scholar] [CrossRef]
- Sharron, H.F.; Konjeti, R.S.; Hengming, K.; Jackie, D.C. Inhibition of Cyclic Nucleotide Phosphodiesterases by Methylxanthines. In Methylxanthines, Handbook of Experimental Pharmacology; Fredholm, B.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 93–134. [Google Scholar]
- Santos, C.I.A.V.; Ramos, M.L.; Justino, L.L.G.; Burrows, H.D.; Valente, A.J.M.; Esteso, M.A.; Leaist, D.G.; Ribeiro, A.F.C. Effect of pH in the structure and mass transport by diffusion of theophylline. J. Chem. Thermodyn. 2017, 110, 162–170. [Google Scholar] [CrossRef]
- Barnes, P.J. Pulmonary Perspectives. Theophylline. Am. J. Respir. Crit. Care Med. 2013, 188, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Moratalla, R. Neurobiología de las metilxantinas. Trastor. Adict. 2008, 10, 201–207. [Google Scholar] [CrossRef]
- Peck, C.C.; Nichols, A.I.; Baker, J.; Lenert, L.L.; Ezra, D. Clinical pharmacodynamics of theophylline. J. Allergy Clin. Immunol. 1985, 76, 292–297. [Google Scholar] [CrossRef]
- Ashihara, H.; Crozier, A. Biosynthesis and metabolism of caffeine and related purine alkaloids in plants. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 1999; Volume 30, pp. 117–205. [Google Scholar]
- Nishijo, J.; Yonetani, I. The interaction of theophylline with benzylamine in aqueous solution. Chem. Pharm. Bull. 1982, 30, 4507–4511. [Google Scholar] [CrossRef][Green Version]
- Santos, C.I.A.V.; Ribeiro, A.C.F.; Esteso, M.A. Drug delivery systems: Study of inclusion complex formation between methylxanthines and cyclodextrins and their thermodynamic and transport properties. Biomolecules 2019, 9, 196. [Google Scholar] [CrossRef]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E. The Merck Index, 14th ed.; Merck: Rahway, NJ, USA, 2006. [Google Scholar]
- Cavalieri, L.F.; Fox, J.J.; Stone, A.; Chang, N. On the nature of xantine and substituted xanthenes in solution. J. Am. Chem. Soc. 1954, 76, 1119–1122. [Google Scholar] [CrossRef]
- Desoize, B. Metals and metal compounds in cancer treatment. Anticancer Res. 2004, 24, 1529–1544. [Google Scholar]
- Shaw, C.F., III. Gold-based medicinal agents. Chem. Rev. 1999, 99, 2589–2600. [Google Scholar] [CrossRef]
- Graf, N.; Lippard, S.J. Redox activation of metal-based prodrugs as a strategy for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Van Rijt, S.H.; Sadler, P.J. Current applications and future potential for bioinorganic chemistry in the development of anticancer drugs. Drug Discov. Today 2009, 14, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liang, X. Metal-mediated targeting in the body. Chem. Biol. Drug Des. 2013, 81, 311–322. [Google Scholar] [CrossRef]
- Gacki, M.; Kafarska, K.; Pietrzak, A.; Korona-Glowniak, I.; Wolf, W.M. Synthesis, characterization, crystal structure and biological activity of metal(II) complexes with theophylline. J. Saudi Chem. Soc. 2019, 23, 346–354. [Google Scholar] [CrossRef]
- El Hamdani, H.; El Amane, M.; Duhayon, C. Crystal structure of tetraaquabis (1,3-dimethyl-2,6-dioxo-7H- purin-7-ido-κ N7)cobalt(II). Acta Crystallogr. Sect. E Crystallogr. Commun. 2017, 73, 1302–1304. [Google Scholar] [CrossRef]
- Begum, N.S.; Monohar, H. Synthesis and X-ray crystal structure of a CuII-theophylline complex: [Cu(theo) (H2O)]·2H2O. Polyhedron 1994, 13, 307–312. [Google Scholar] [CrossRef]
- Wang, H.; Hu, T.; Wen, R.; Wang, Q.; Bu, X. In vitro controlled release of theophylline from metal-drug complexes. J. Mater. Chem. B 2013, 1, 3879–3882. [Google Scholar] [CrossRef]
- Hao, X.M.; Zhao, S.; Wang, H.; Wu, Y.B.; Yang, D.; Zhang, X.F.; Xu, Z.L. In vitro release of theophylline and cytotoxicity of two new metal–drug complexes. Polyhedron 2018, 142, 38–42. [Google Scholar] [CrossRef]
- Forizs, E.; Debreczeni, A.; Patrut, A. Synthesis, structure and DFT calculations on complexes of palladium (II) with theophylline. Rev. Roum. 2010, 55, 697–704. [Google Scholar]
- Caldwell, K.; Deacon, G.B.; Gatehouse, B.M.; Lee, S.C.; Canty, A.J. Organomercury medicinal chemistry. Synthesis and structure of a (β-methoxyethyl)mercury(II) derivative of N(7)-deprotonated theophylline, [Hg(C3H7O)(C7H7N4O2)]. Acta Cryst. C 1984, 40, 1533–1536. [Google Scholar] [CrossRef]
- Aoki, K.; Yamazaki, H. Interaction of tetrakis(-µ-carboxylato)dirhodiurn(II), an antitumour agent, with nucleic acid bases. X-ray crystal structures of [Rh2(acetato)4(theophylline)2] and [Rh2(acetato)4(caffeine)2]. J. Chem. Soc. Chem. Commun. 1980, 926, 186–188. [Google Scholar] [CrossRef]
- Griffith, E.H.; Amma, E.L. Reaction of PtCl42− with theophylline: X-ray crystal structures of bis(theophyllinum) tetrachloroplatine(II) and theophyllinum trichlorotheophyllineplatinate(II). Inorg. Chem. 1979, 7, 322–324. [Google Scholar]
- Feng, J.; Du, X.; Liu, H.; Sui, X.; Zhang, C.; Tang, Y.; Zhang, J. Manganeseese-mefenamic acid complexes exhibit high lipoxygenase inhibitory activity. Dalton Trans. 2014, 43, 10930–10939. [Google Scholar] [CrossRef] [PubMed]
- Rigaku Oxford Diffraction; CrysAlisPro 1.171.39.33c; Agilent Technologies UK Ltd.: Yarnton, UK, 2017.
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- HüBschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Cryst. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; Mccabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Van De Streek, J.; Wood, P.A. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Żesławska, E.; Korona-Głowniak, I.; Szczesio, M.; Olczak, A.; Żylewska, A.; Tejchman, W.; Malm, A. Structural analysis and antimicrobial activity of 2[1H]-pyrimidinethione/selenone derivatives. J. Mol. Struct. 2017, 1142, 261–266. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.; Shimoni, L.; Chang, N. Patterns in hydrogen bonding: Functionality and graph set analysis in crystals. Angew. Chem. Int. Ed. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]











| 1 | |
|---|---|
| Empirical formula | C14H24CuN8O9 |
| Formula weight | 511.95 |
| T (K) | 100.0 |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a (Å) | 12.3749(2) |
| b (Å) | 11.9281(3) |
| c (Å) | 13.7162(2) |
| α (°) | 90 |
| β (°) | 98.133(10) |
| γ (°) | 90 |
| V (Å3) | 2004.27(7) |
| Z | 4 |
| ρcalc (g/cm3) | 1.697 |
| F(000) | 1060 |
| Radiation | CuKα (λ = 1.54184) |
| θ range (°) | 4.506–70.052 |
| Reflections collected | 43730 |
| Independent reflections | 3811 |
| Goodness-of-fit (GOF) | 1.061 |
| R [I >= 2σ (I)] | 0.0295 |
| wR2 [I >= 2σ (I)] | 0.0810 |
| D-H⋯A | D-H | H⋯A | D⋯A | D-H⋯A |
|---|---|---|---|---|
| O5-H5A⋯O2 a | 0.77(3) | 1.94(3) | 2.701(2) | 175(3) |
| O5-H5B⋯O3 b | 0.81(2) | 1.94(3) | 2.722(2) | 162(2) |
| O6-H6A⋯N3 c | 0.73(3) | 2.00(3) | 2.721(2) | 174(3) |
| O6-H6B⋯N7 d | 0.77(2) | 1.99(2) | 2.756(2) | 179(3) |
| O7-H7A⋯O8 | 0.80(4) | 1.95(4) | 2.748(2) | 170(5) |
| O7-H7B⋯O7 e | 0.81(4) | 1.94(4) | 2.749(2) | 175(5) |
| O7-H7C⋯O1 | 0.82(2) | 1.87(2) | 2.686(2) | 172(2) |
| O8-H8A⋯O3 f | 0.82(3) | 2.14(3) | 2.956(2) | 173(5) |
| O8-H8B⋯O9 | 0.83(2) | 1.89(4) | 2.712(3) | 170(2) |
| O8-H8C⋯O7 | 0.78(4) | 2.20(4) | 2.748(2) | 127(4) |
| O9-H9A⋯O8 | 0.80(3) | 1.92(3) | 2.712(3) | 169(3) |
| O9-H9B⋯O9 g | 0.81(2) | 1.94(5) | 2.753(2) | 177(7) |
| O9-H9C⋯O4 | 0.80(2) | 2.07(2) | 2.849(2) | 163(3) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gacki, M.; Kafarska, K.; Pietrzak, A.; Korona-Głowniak, I.; Wolf, W.M. Double Palindrome Water Chain in Cu(II) Theophylline Complex. Synthesis, Characterization, Biological Activity of Cu(II), Zn(II) Complexes with Theophylline. Crystals 2020, 10, 97. https://doi.org/10.3390/cryst10020097
Gacki M, Kafarska K, Pietrzak A, Korona-Głowniak I, Wolf WM. Double Palindrome Water Chain in Cu(II) Theophylline Complex. Synthesis, Characterization, Biological Activity of Cu(II), Zn(II) Complexes with Theophylline. Crystals. 2020; 10(2):97. https://doi.org/10.3390/cryst10020097
Chicago/Turabian StyleGacki, Michał, Karolina Kafarska, Anna Pietrzak, Izabela Korona-Głowniak, and Wojciech M. Wolf. 2020. "Double Palindrome Water Chain in Cu(II) Theophylline Complex. Synthesis, Characterization, Biological Activity of Cu(II), Zn(II) Complexes with Theophylline" Crystals 10, no. 2: 97. https://doi.org/10.3390/cryst10020097
APA StyleGacki, M., Kafarska, K., Pietrzak, A., Korona-Głowniak, I., & Wolf, W. M. (2020). Double Palindrome Water Chain in Cu(II) Theophylline Complex. Synthesis, Characterization, Biological Activity of Cu(II), Zn(II) Complexes with Theophylline. Crystals, 10(2), 97. https://doi.org/10.3390/cryst10020097