Femtosecond Laser-Induced Self-Assembly of Ce3+-Doped YAG Nanocrystals
Abstract
1. Introduction
2. Materials and Methods
3. Results
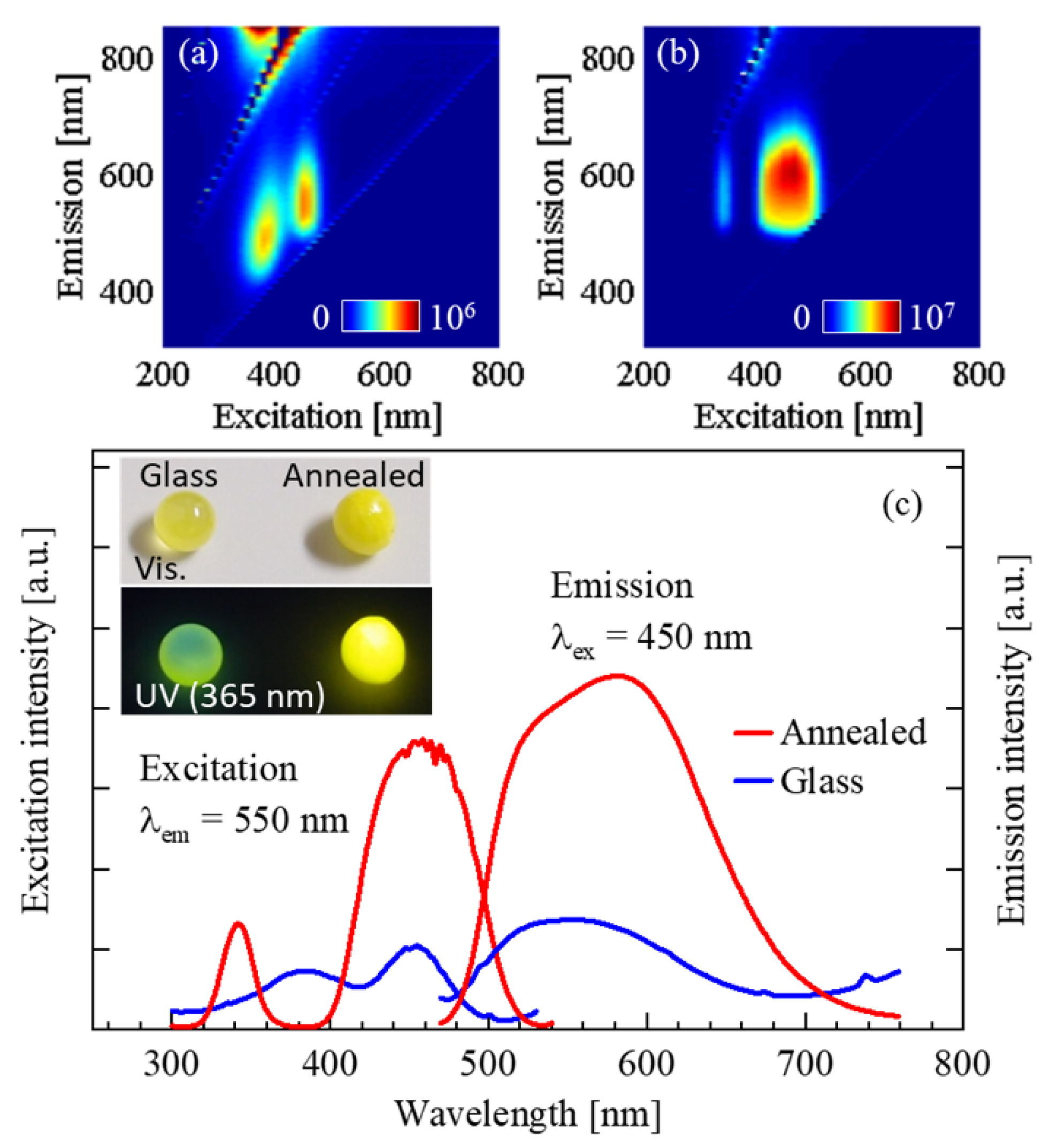
3.1. Photoluminescence Properties of Ce3+-Doped Yttrium Aluminum Garnet (YAG) Crystal and Glass
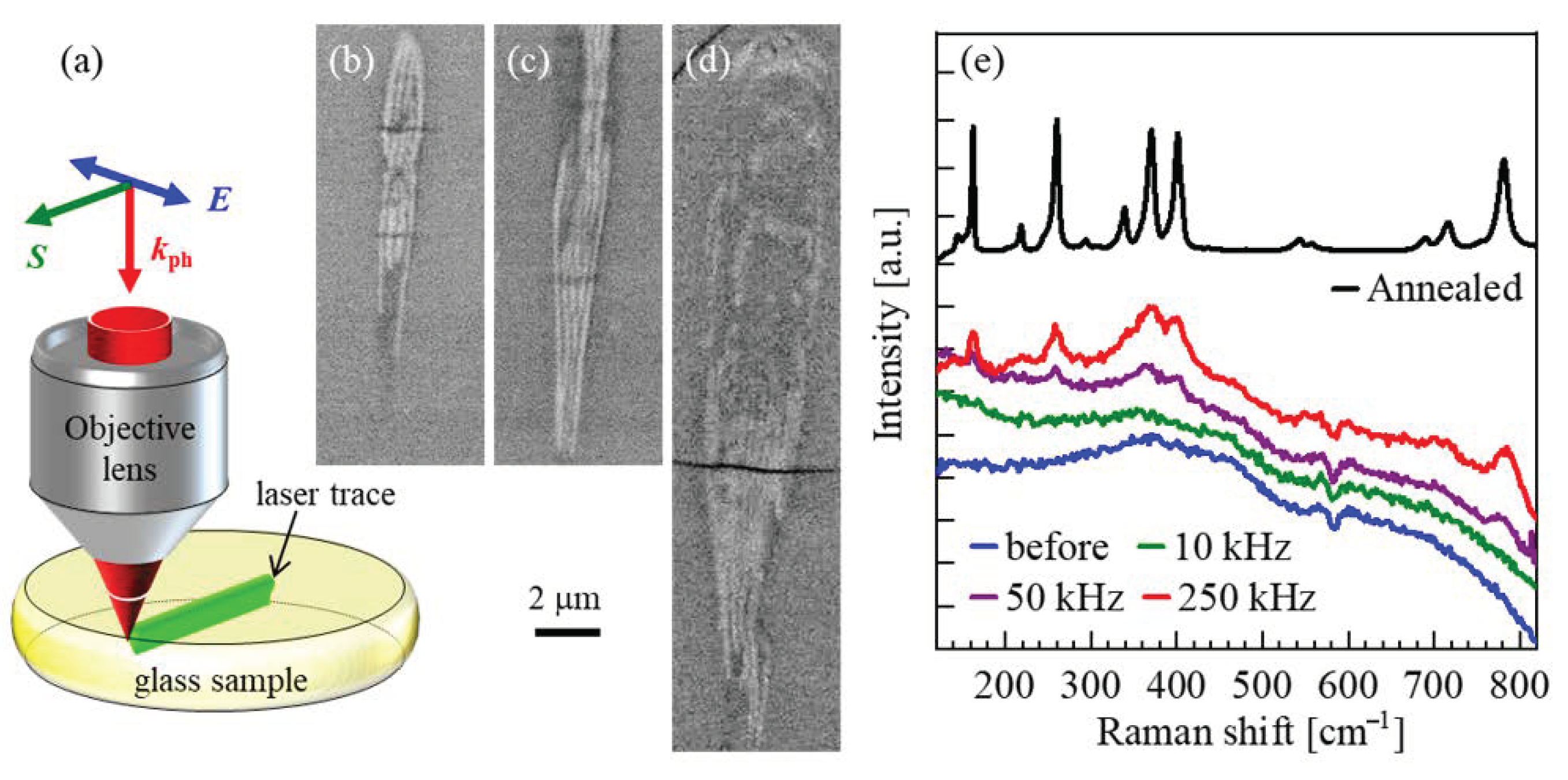
3.2. Photo-Induced Local Crystallization
3.3. Cathode Luminescence from Nanograting Structure
3.4. Progress of Photoinduced Crystallization
3.5. Nucleation and Growth of YAG Nanocrystals
4. Discussion
4.1. Polarization-Dependent Phenomena
4.2. Nucleation and Growth Mechanism for Photoinduced Crystallization
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zanotto, E.D. Metastable phases in lithium disilicate glasses. J. Non Cryst. Solids 1997, 219, 42–48. [Google Scholar] [CrossRef]
- Stookey, S.D. Photosensitive Glass. Ind. Eng. Chem. 1949, 41, 856–861. [Google Scholar] [CrossRef]
- Lumeau, J.; Glebova, L.; Golubkov, V.; Zanotto, E.D.; Glebov, L.B. Origin of crystallization-induced refractive index changes in photo-thermo-refractive glass. Opt. Mater. 2009, 32, 139–146. [Google Scholar] [CrossRef]
- Stookey, S.D.; Beall, G.H.; Pierson, J.E. Full-color photosensitive glass. J. Appl. Phys. 1978, 49, 5114–5123. [Google Scholar] [CrossRef]
- Strickland, D.; Mourou, G. Compression of amplified chirped optical pulses. Opt. Commun. 1985, 56, 219–221. [Google Scholar] [CrossRef]
- Gattass, R.R.; Mazur, E. Femtosecond laser micromachining in transparent materials. Nat. Photon. 2008, 2, 219–225. [Google Scholar] [CrossRef]
- Tirlapur, U.K.; Konig, K. Cell biology—Targeted transfection by femtosecond laser. Nature 2002, 418, 290–291. [Google Scholar] [CrossRef]
- Davis, K.M.; Miura, K.; Sugimoto, N.; Hirao, K. Writing waveguides in glass with a femtosecond laser. Opt. Lett. 1996, 21, 1729–1731. [Google Scholar] [CrossRef]
- Haque, M.; Lee, K.K.C.; Ho, S.; Fernandes, L.A.; Herman, P.R. Chemical-assisted femtosecond laser writing of lab-in-fibers. Lab Chip 2014, 14, 3817–3829. [Google Scholar] [CrossRef]
- Glezer, E.N.; Milosavljevic, M.; Huang, L.; Finlay, R.J.; Her, T.-H.; Callan, J.P.; Mazur, E. Three-dimensional optical storage inside transparent materials. Opt. Lett. 1996, 21, 2023–2025. [Google Scholar] [CrossRef]
- Shimotsuma, Y.; Kazansky, P.G.; Qiu, J.; Hirao, K. Self-Organized Nanogratings in Glass Irradiated by Ultrashort Light Pulses. Phys. Rev. Lett. 2003, 91, 247405. [Google Scholar] [CrossRef] [PubMed]
- Lancry, M.; Poumellec, B.; Canning, J.; Cook, K.; Poulin, J.-C.; Brisset, F. Ultrafast nanoporous silica formation driven by femtosecond laser irradiation. Laser Photon. Rev. 2013, 7, 953–962. [Google Scholar] [CrossRef]
- Sakakura, M.; Kurita, T.; Shimizu, M.; Yoshimura, K.; Shimotsuma, Y.; Fukuda, N.; Hirao, K.; Miura, K. Shape control of elemental distributions inside a glass by simultaneous femtosecond laser irradiation at multiple spots. Opt. Lett. 2013, 38, 4939–4942. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Jiang, X.; Zhu, C.; Shirai, M.; Si, J.; Jiang, N.; Hirao, P.K. Manipulation of Gold Nanoparticles inside Transparent Materials. Angew. Chem. Int. Ed. 2004, 43, 2230–2234. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kazansky, P.G.; Svirko, Y.P. Non-reciprocal ultrafast laser writing. Nat. Photon. 2008, 2, 99–104. [Google Scholar] [CrossRef]
- Komatsu, T.; Ihara, R.; Honma, T.; Benino, Y.; Sato, R.; Kim, H.; Fujiwara, T. Patterning of Non-Linear Optical Crystals in Glass by Laser-Induced Crystallization. J. Am. Ceram. Soc. 2007, 90, 699–705. [Google Scholar] [CrossRef]
- Komatsu, T.; Honma, T. Nucleation and Crystal Growth in Laser-Patterned Lines in Glasses. Front. Mater. 2016, 3, 32. [Google Scholar] [CrossRef]
- Stone, A.; Sakakura, M.; Shimotsuma, Y.; Miura, K.; Hirao, K.; Dierolf, V.; Jain, H. Femtosecond laser-writing of 3D crystal architecture in glass: Growth dynamics and morphological control. Mater. Des. 2018, 146, 228–238. [Google Scholar] [CrossRef]
- Miura, K.; Qiu, J.; Mitsuyu, T.; Hirao, K. Space-selective growth of frequency-conversion crystals in glasses with ultrashort infrared laser pulses. Opt. Lett. 2000, 25, 408–410. [Google Scholar] [CrossRef]
- Stone, A.; Jain, H.; Dierolf, V.; Sakakura, M.; Shimotsuma, Y.; Miura, K.; Hirao, K.; Lapointe, J.; Kashyap, R. Direct laser-writing of ferroelectric single-crystal waveguide architectures in glass for 3D integrated optics. Sci. Rep. 2015, 5, 10391. [Google Scholar] [CrossRef]
- Ogawa, K.; Honma, T.; Komatsu, T. Birefringence imaging and orientation of laser patterned β-BaB2O4 crystals with bending and curved shapes in glass. J. Solid State Chem. 2013, 207, 6–12. [Google Scholar] [CrossRef]
- Xuan, H.; Fan, C.; Bertrand, P.; Liu, Q.; Zeng, H.; Brisset, F.; Chen, G.; Zhao, X.; Lancry, M. Size-controlled oriented crystallization in SiO2-based glasses by femtosecond laser irradiation. J. Opt. Soc. Am. B 2014, 31, 376–381. [Google Scholar] [CrossRef]
- Sakakura, M.; Shimizu, M.; Shimotsuma, Y.; Miura, K.; Hirao, K. Temperature distribution and modification mechanism inside glass with heat accumulation during 250 kHz irradiation of femtosecond laser pulses. Appl. Phys. Lett. 2008, 93, 231112. [Google Scholar] [CrossRef]
- Mori, S. Nanogratings Embedded in Al2O3-Dy2O3 Glass by Femtosecond Laser Irradiation. J. Laser Micro Nanoeng. 2016, 11, 87–90. [Google Scholar] [CrossRef]
- Asai, T.; Shimotsuma, Y.; Kurita, T.; Murata, A.; Kubota, S.; Sakakura, M.; Miura, K.; Brisset, F.; Bertrand, P.; Lancry, M. Systematic Control of Structural Changes in GeO2 Glass Induced by Femtosecond Laser Direct Writing. J. Am. Ceram. Soc. 2015, 98, 1471–1477. [Google Scholar] [CrossRef]
- Mori, M.; Shimotsuma, Y.; Sei, T.; Sakakura, M.; Miura, K.; Udono, H. Tailoring thermoelectric properties of nanostructured crystal silicon fabricated by infrared femtosecond laser direct writing. Phys. Status Solidi A 2015, 212, 715–721. [Google Scholar] [CrossRef]
- Shimotsuma, Y.; Mori, S.; Nakanishii, Y.; Kim, E.; Sakakura, M.; Miura, K. Self-assembled glass/crystal periodic nanostructure in Al2O3-Dy2O3 binary glass. Appl. Phys. A 2018, 124, 82. [Google Scholar] [CrossRef]
- Cao, J.; Bertrand, P.; François, B.; Helbert, A.-L.; Lancry, M. Angular Dependence of the Second Harmonic Generation Induced by Femtosecond Laser Irradiation in Silica-Based Glasses: Variation with Writing Speed and Pulse Energy. World J. Nano Sci. Eng. 2015, 5, 96–106. [Google Scholar] [CrossRef]
- Bertrand, P.; Lancry, M.; Desmarchelier, R.; Hervé, E.; Brisset, F.; Poulin, J. Asymmetric Orientational Writing in glass with femtosecond laser irradiation. Opt. Mater. Express 2013, 3, 1586. [Google Scholar] [CrossRef]
- Cao, J.; Poumellec, B.; Mazerolles, L.; Brisset, F.; Helbert, A.-L.; Surble, S.; He, X.; Lancry, M. Nanoscale phase separation in lithium niobium silicate glass by femtosecond laser irradiation. J. Am. Ceram. Soc. 2017, 100, 115–124. [Google Scholar] [CrossRef]
- Kushibiki, J.; Takanaga, I.; Arakawa, M.; Sannomiya, T. Accurate measurements of the acoustical physical constants of LiNbO3 and LiTaO3 single crystals. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1999, 46, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Bertrand, P.; Brisset, F.; Helbert, A.-L.; Lancry, M. Tunable angular-dependent second-harmonic generation in glass by controlling femtosecond laser polarization. J. Opt. Soc. Am. B 2016, 33, 741. [Google Scholar] [CrossRef]
- Cao, J.; Lancry, M.; Brisset, F.; Mazerolles, L.; Saint-Martin, R.; Bertrand, P. Femtosecond Laser-Induced Crystallization in Glasses: Growth Dynamics for Orientable Nanostructure and Nanocrystallization. Cryst. Growth Des. 2019, 19, 2189–2205. [Google Scholar] [CrossRef]
- Papagelis, K.; Ves, S. Vibrational properties of the rare earth aluminum garnets. J. Appl. Phys. 2003, 94, 6491. [Google Scholar] [CrossRef]
- Yoshimoto, K.; Masuno, A.; Ueda, M.; Inoue, H.; Yamamoto, H.; Kawashima, T. Low phonon energies and wideband optical windows of La2O3-Ga2O3 glasses prepared using an aerodynamic levitation technique. Sci. Rep. 2017, 7, srep45600. [Google Scholar] [CrossRef] [PubMed]
- Alahraché, S.; Deschamps, M.; Lambert, J.; Suchomel, M.R.; Meneses, D.D.S.; Matzen, G.; Massiot, D.; Véron, E.; Allix, M. Crystallization of Y2O3–Al2O3 Rich Glasses: Synthesis of YAG Glass-Ceramics. J. Phys. Chem. C 2011, 115, 20499–20506. [Google Scholar] [CrossRef]
- Lee, C.-H.; Jung, S.-K.; Yoda, S.; Cho, W.-S. Microstructure of rapidly quenched YAG-based glass–ceramics prepared by aerodynamic levitation. Ceram. Int. 2015, 41, 14475–14481. [Google Scholar] [CrossRef]
- Chen, L.; Chen, X.; Liu, F.; Chen, H.; Wang, H.; Zhao, E.; Jiang, Y.; Chan, T.-S.; Wang, C.-H.; Zhang, W.; et al. Charge deformation and orbital hybridization: Intrinsic mechanisms on tunable chromaticity of Y3Al5O12:Ce3+ luminescence by doping Gd3+ for warm white LEDs. Sci. Rep. 2015, 5, 11514. [Google Scholar] [CrossRef]
- Robbins, D.J. The Effects of Crystal Field and Temperature on the Photoluminescence Excitation Efficiency of Ce3+ in YAG. J. Electrochem. Soc. 1979, 126, 1550–1555. [Google Scholar] [CrossRef]
- Ueda, J.; Tanabe, S. (INVITED) Review of luminescent properties of Ce3+-doped garnet phosphors: New insight into the effect of crystal and electronic structure. Opt. Mater. X 2019, 1, 100018. [Google Scholar] [CrossRef]
- Jacobs, R.R.; Krupke, W.F.; Weber, M.J. Measurement of excited-state-absorption loss for Ce3+ in Y3Al5O12 and implications for tunable 5d→4f rare-earth lasers. Appl. Phys. Lett. 1978, 33, 410–412. [Google Scholar] [CrossRef]
- Sato, Y.; Taira, T. The studies of thermal conductivity in GdVO4, YVO4, and Y3Al5O12 measured by quasi-one-dimensional flash method. Opt. Express 2006, 14, 10528. [Google Scholar] [CrossRef] [PubMed]
- Sontakke, A.D.; Ueda, J.; Xu, J.; Asami, K.; Katayama, M.; Inada, Y.; Tanabe, S. A Comparison on Ce3+ Luminescence in Borate Glass and YAG Ceramic: Understanding the Role of Host’s Characteristics. J. Phys. Chem. C 2016, 120, 17683–17691. [Google Scholar] [CrossRef]
- Wilhelm, S.; Gröbler, B.; Gluch, M.; Heinz, H. Confocal Laser Scanning Microscopy Principles; ZEISS: Jena, German, 2003; Available online: http://zeiss-campus.magnet.fsu.edu/referencelibrary/pdfs/ZeissConfocalPrinciples.pdf (accessed on 15 December 2020).
- Wilson, T. Resolution and optical sectioning in the confocal microscope. J. Microsc. 2011, 244, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, I.; Cvecek, K.; Schmidt, M. Evaluation of Nonlinear Absorptivity and Absorption Region in Fusion Welding of Glass using Ultrashort Laser Pulses. Phys. Procedia 2011, 12, 378–386. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, D.; Liu, X.; Tong, L.; Kazansky, P.G.; Qiu, J. Self-Organized Periodic Crystallization in Unconventional Glass Created by an Ultrafast Laser for Optical Attenuation in the Broadband Near-Infrared Region. Adv. Opt. Mater. 2019, 7, 19900593. [Google Scholar] [CrossRef]
- Audier, M.; Chenevier, B.; Roussel, H.; Vincent, L.; Pena, A.; Salaün, A.L. A very promising piezoelectric property of Ta2O5 thin films. II: Birefringence and piezoelectricity. J. Solid State Chem. 2011, 184, 2033–2040. [Google Scholar] [CrossRef]
- Krupka, J.; Derzakowski, K.; Tobar, M.; Hartnett, J.G.; Geyer, R.G. Complex permittivity of some ultralow loss dielectric crystals at cryogenic temperatures. Meas. Sci. Technol. 1999, 10, 387–392. [Google Scholar] [CrossRef]
- Kaminska, A.; Duzynska, A.; Berkowski, M.; Trushkin, S.; Suchocki, A. Pressure-induced luminescence of cerium-doped gadolinium gallium garnet crystal. Phys. Rev. B 2012, 85, 155111. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, J.; Zhang, J.; Zeng, S.; Zhou, H.; Smith, A.T.; Wang, W.; Sun, L.; Wang, Z. Mechanics-induced triple-mode anticounterfeiting and moving tactile sensing by simultaneously utilizing instantaneous and persistent mechanoluminescence. Mater. Horizons 2019, 6, 2003–2008. [Google Scholar] [CrossRef]
- Gildenburg, V.B.; Pavlichenko, I.A. High contrast periodic plasma pattern formation during the laser-induced breakdown in transparent dielectric. Phys. Plasmas 2017, 24, 122306. [Google Scholar] [CrossRef]
- Kelton, K. Crystal Nucleation in Liquids and Glasses. Solid State Physics 1991, 45, 75–177. [Google Scholar] [CrossRef]
- Balibar, S.; Caupin, F. Nucleation of crystals from their liquid phase. Comptes Rendus Phys. 2006, 7, 988–999. [Google Scholar] [CrossRef]
- Lifshitz, I.; Slyozov, V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Johnson, B.R.; Kriven, W.M. Crystallization kinetics of yttrium aluminum garnet (Y3Al5O12). J. Mater. Res. 2001, 16, 1795–1805. [Google Scholar] [CrossRef]
- Savytskii, D.; Knorr, B.; Dierolf, V.; Jain, H. Demonstration of single crystal growth via solid-solid transformation of a glass. Sci. Rep. 2016, 6, 23324. [Google Scholar] [CrossRef] [PubMed]



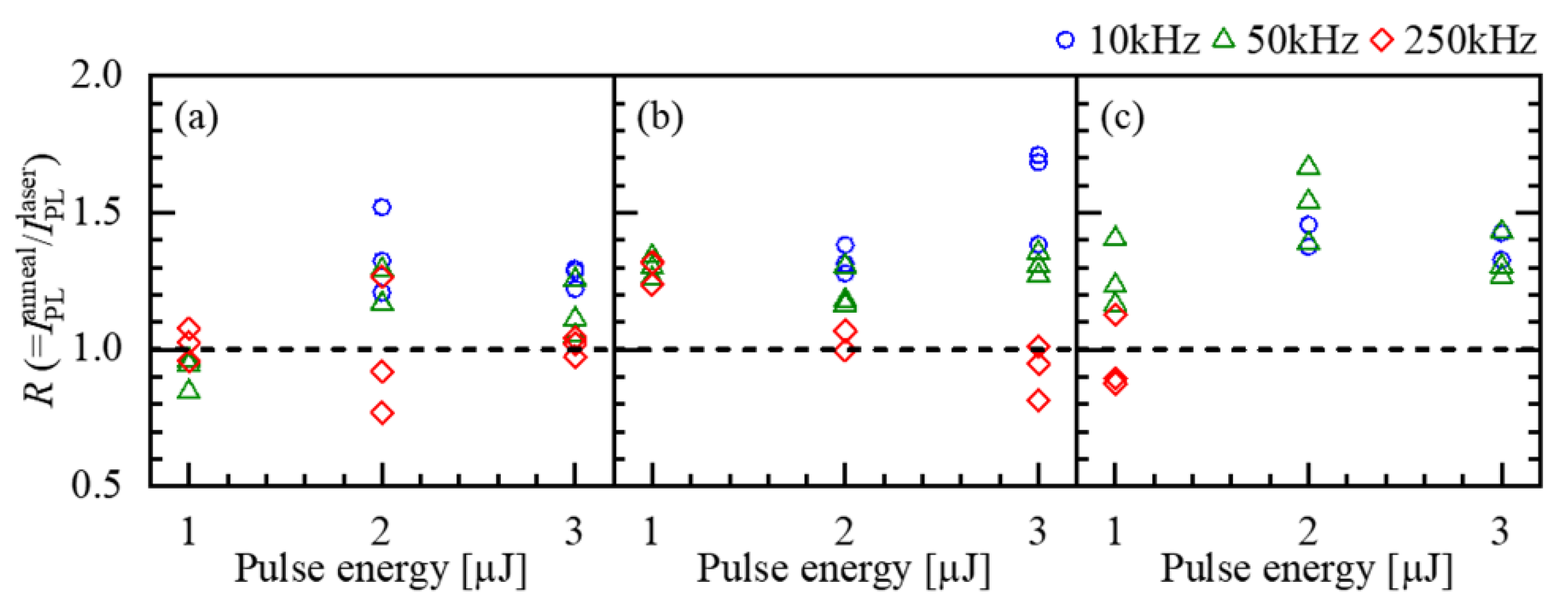
| Repetition Rate (kHz) | PL Intensity Ratio 1 |
|---|---|
| 10 | 6 |
| 50 | 38 |
| 250 | 198 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimotsuma, Y.; Tomura, K.; Okuno, T.; Shimizu, M.; Miura, K. Femtosecond Laser-Induced Self-Assembly of Ce3+-Doped YAG Nanocrystals. Crystals 2020, 10, 1142. https://doi.org/10.3390/cryst10121142
Shimotsuma Y, Tomura K, Okuno T, Shimizu M, Miura K. Femtosecond Laser-Induced Self-Assembly of Ce3+-Doped YAG Nanocrystals. Crystals. 2020; 10(12):1142. https://doi.org/10.3390/cryst10121142
Chicago/Turabian StyleShimotsuma, Yasuhiko, Kotaro Tomura, Tatsuya Okuno, Masahiro Shimizu, and Kiyotaka Miura. 2020. "Femtosecond Laser-Induced Self-Assembly of Ce3+-Doped YAG Nanocrystals" Crystals 10, no. 12: 1142. https://doi.org/10.3390/cryst10121142
APA StyleShimotsuma, Y., Tomura, K., Okuno, T., Shimizu, M., & Miura, K. (2020). Femtosecond Laser-Induced Self-Assembly of Ce3+-Doped YAG Nanocrystals. Crystals, 10(12), 1142. https://doi.org/10.3390/cryst10121142





