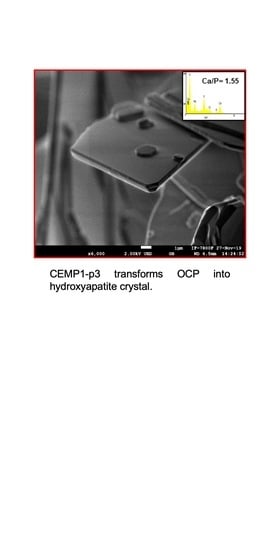
Cemp1-p3 Peptide Promotes the Transformation of Octacalcium Phosphate into Hydroxyapatite Crystals
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis
2.2. Synthesis of Octacalcium Phosphate Crystals by Co-Precipitation
2.3. Binding of CEMP1-p3 to OCP Crystals
2.4. Octacalcium Phosphate Constant Composition Seeded Growth (CCSG) Assays
2.5. Characterization of the Mineral Deposits
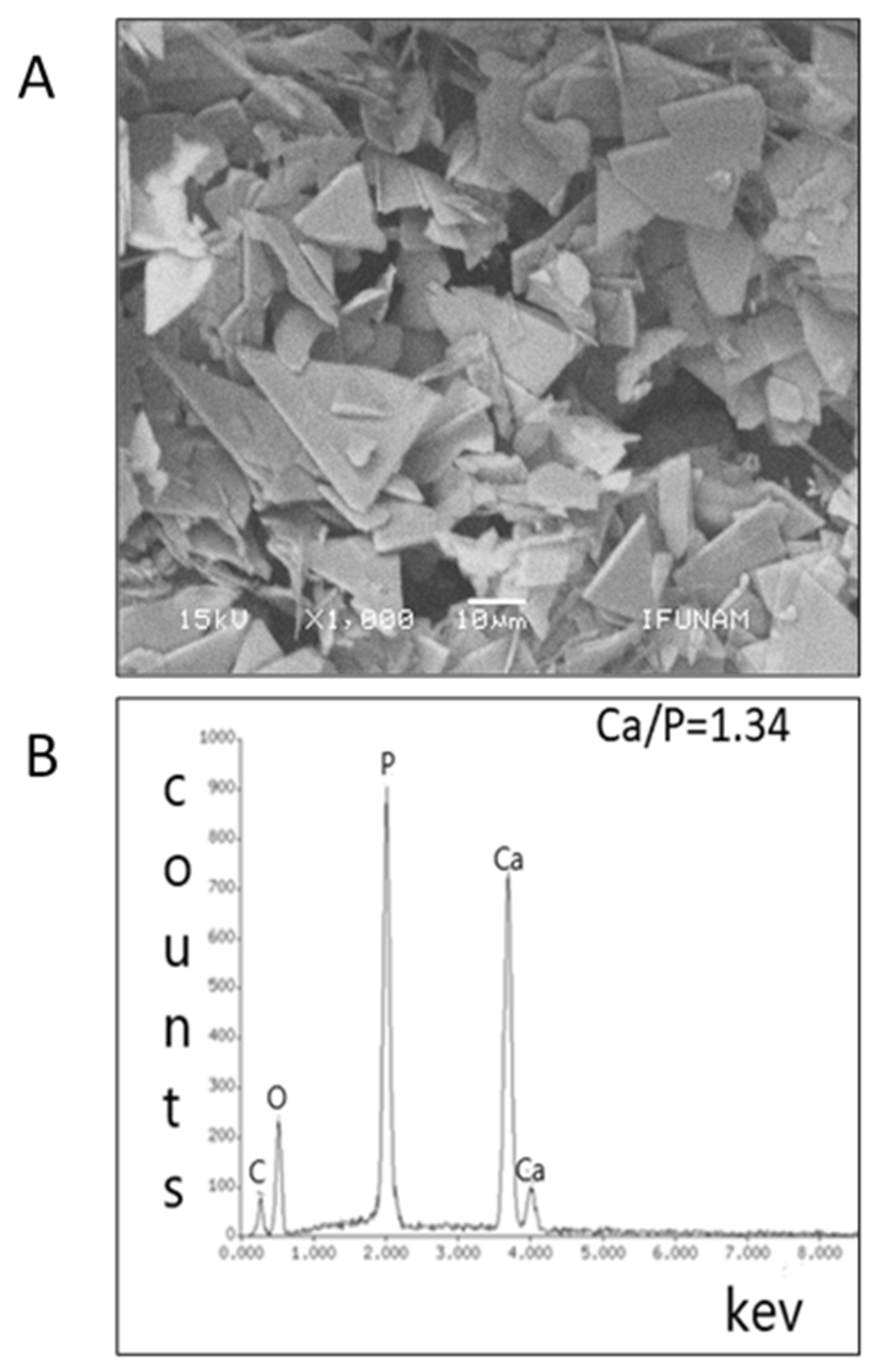
2.5.1. Scanning Electron Microscopy
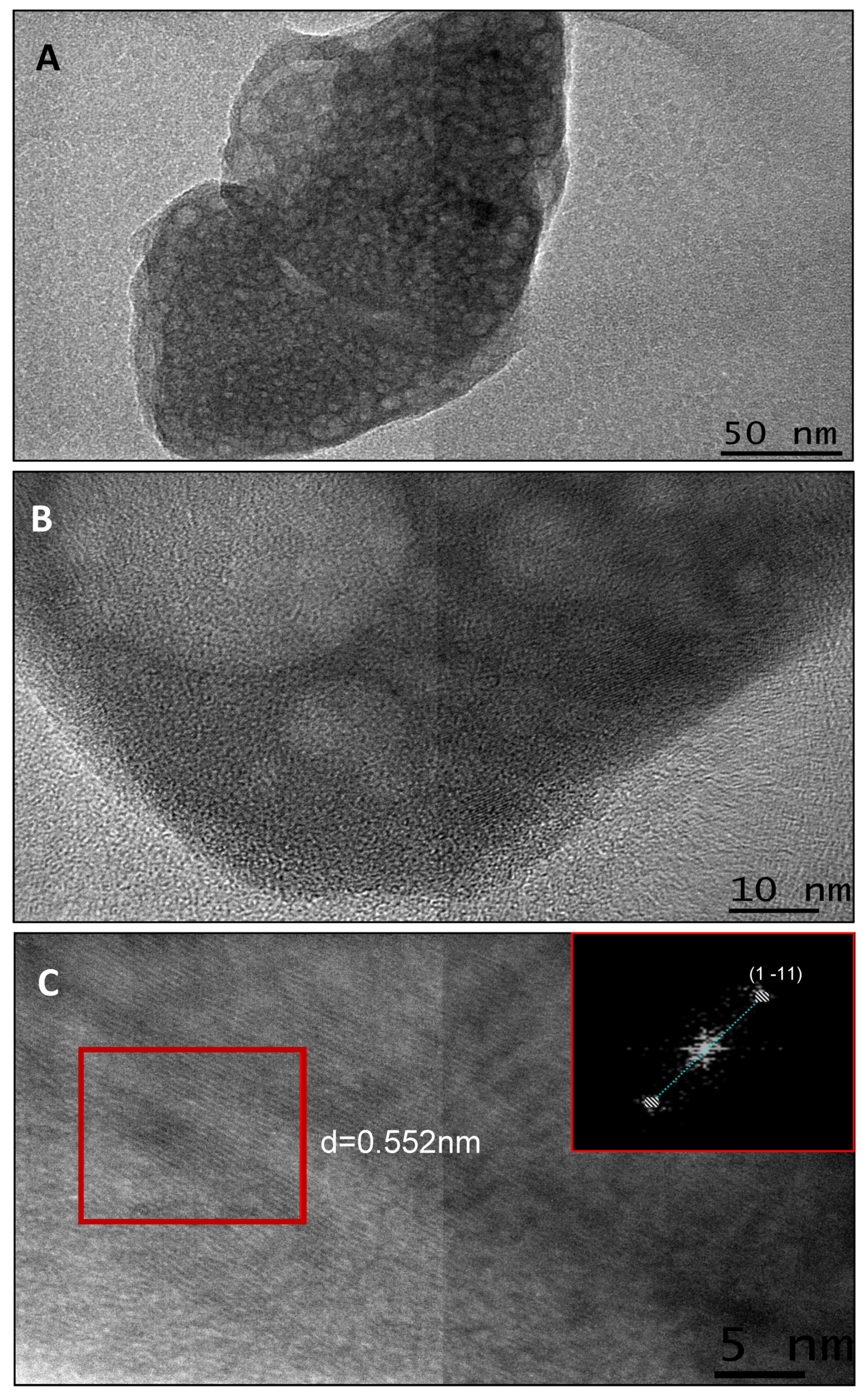
2.5.2. Transmission Electron Microscopy
2.5.3. High Resolution Transmission Electron Microscopy (HRTEM)
2.5.4. Micro Raman Spectra
3. Results
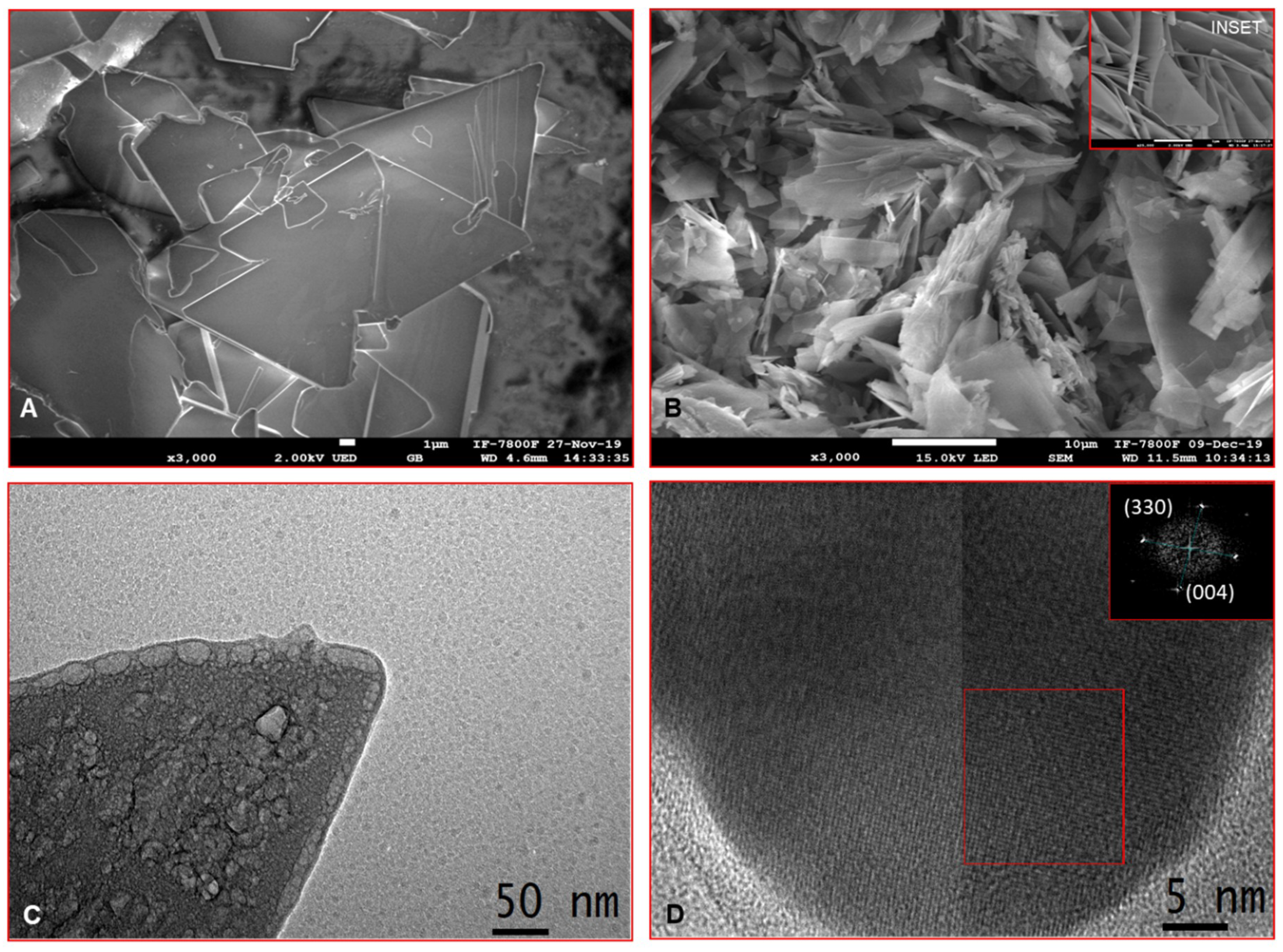
3.1. Synthesis and Characterization of Octacalcium Phosphate Crystals
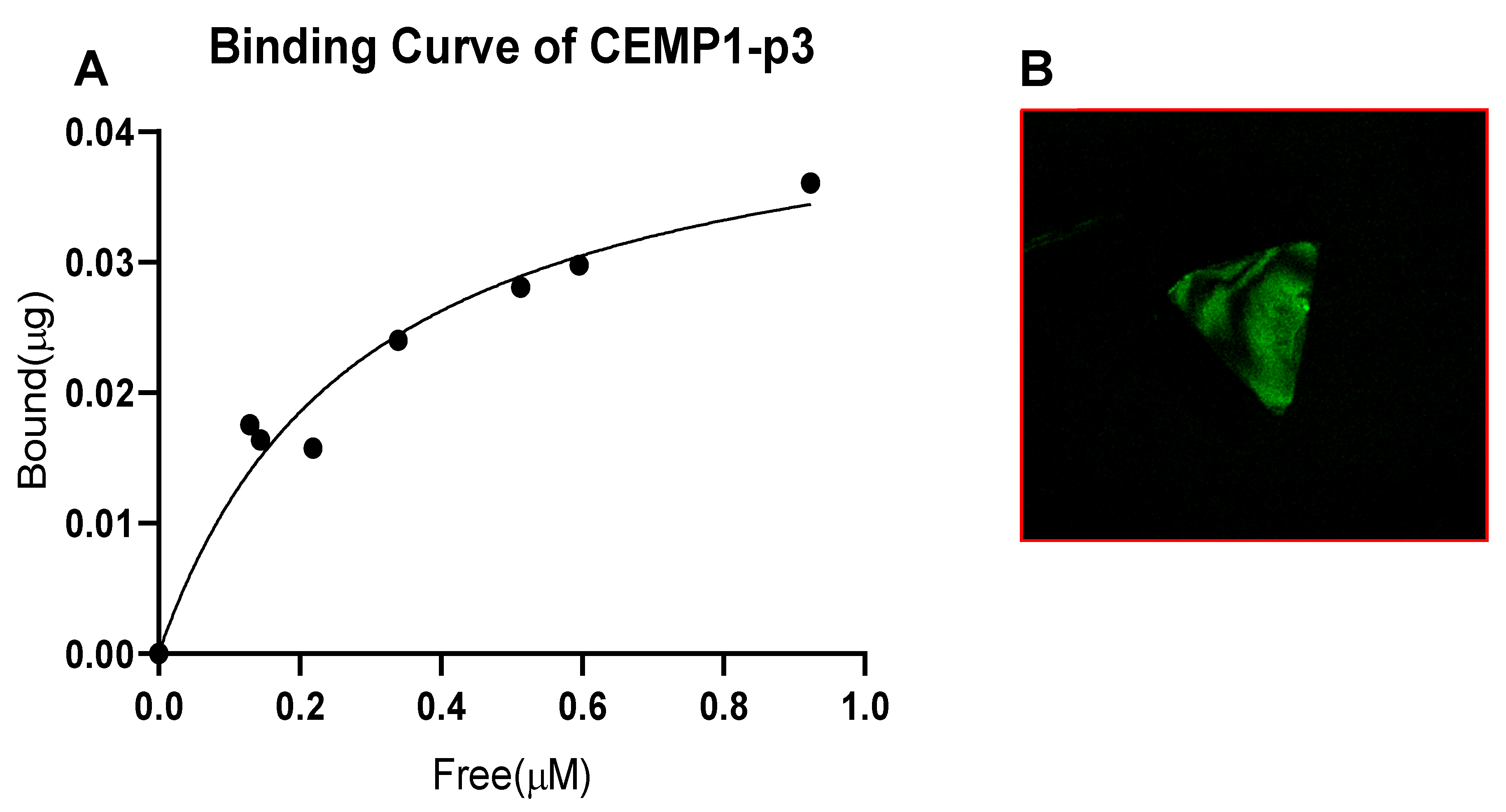
3.2. Binding of CEMP1-p3 to OCP Crystals
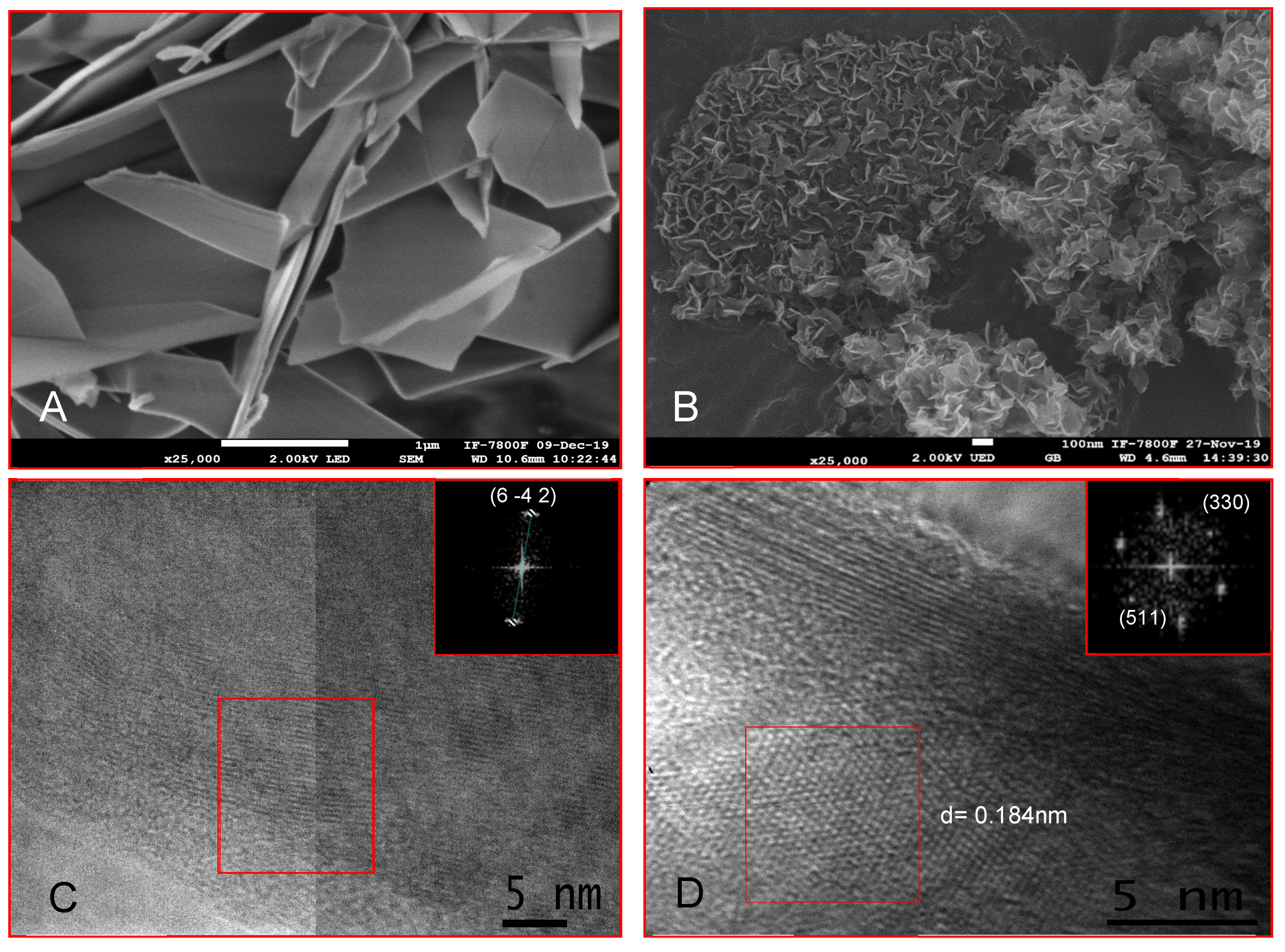
3.3. Octacalcium Phosphate Constant Composition Seeded Growth (CCSG)
3.4. OCP Growth in Simulated Physiological Buffer
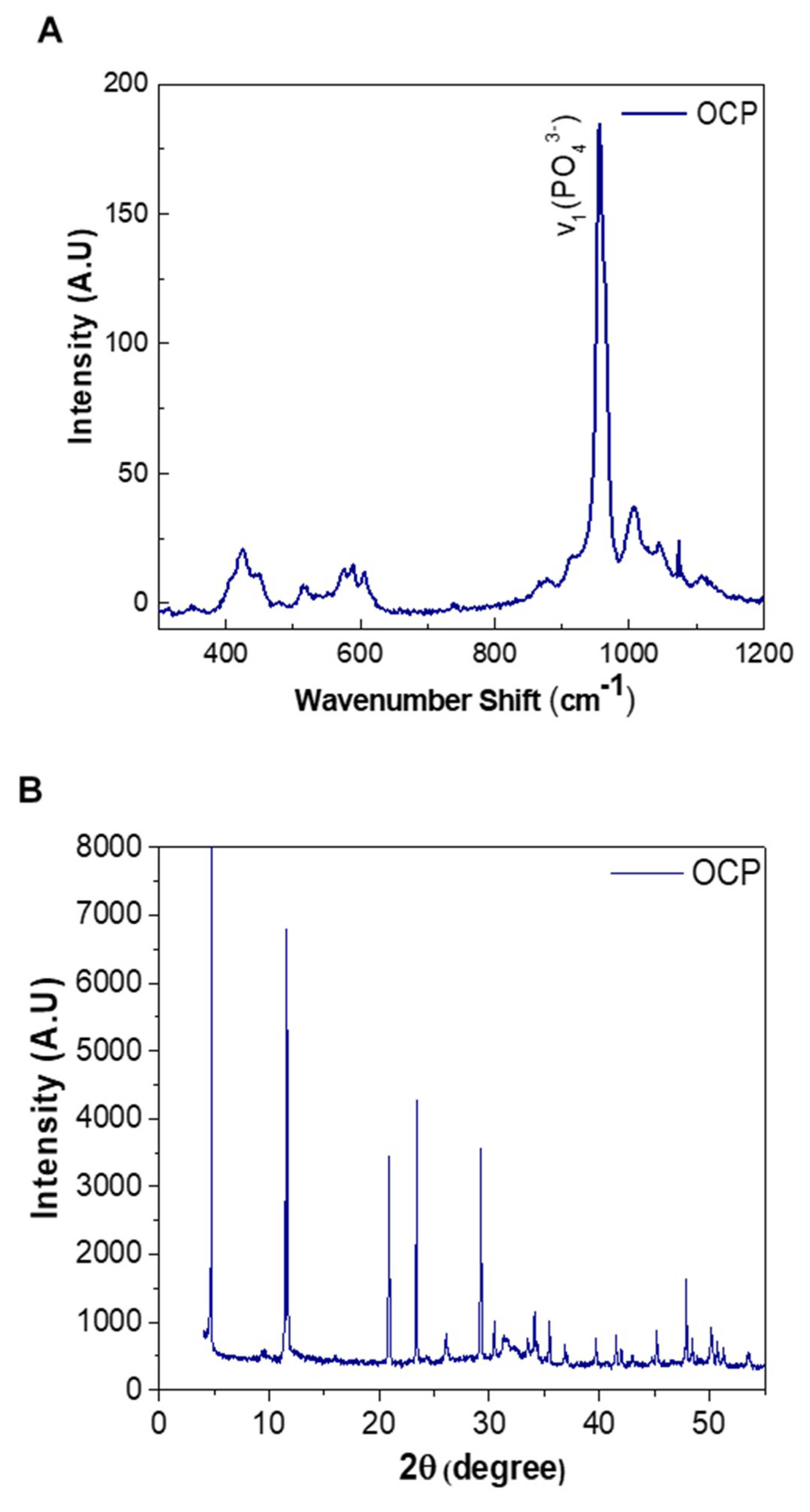
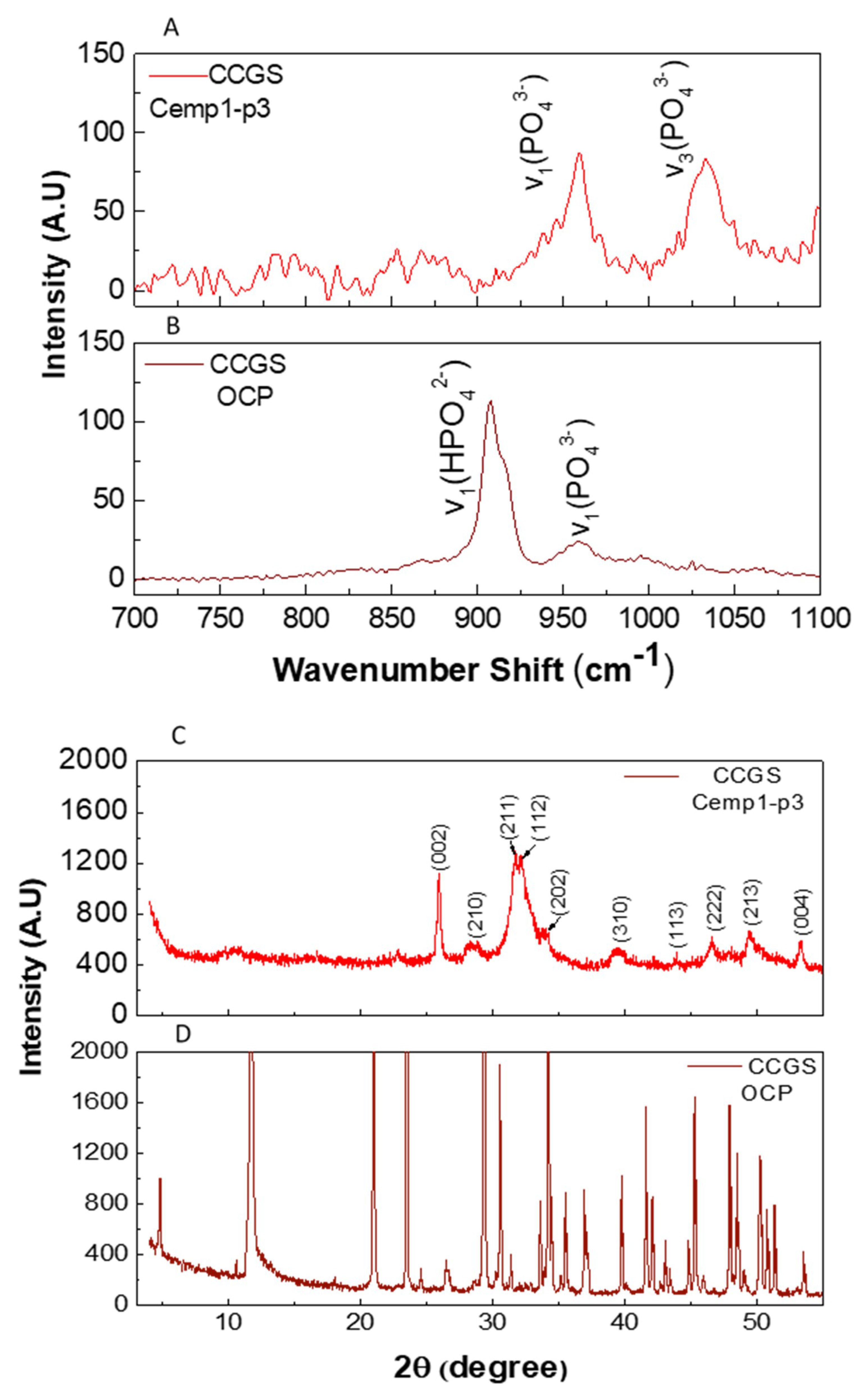
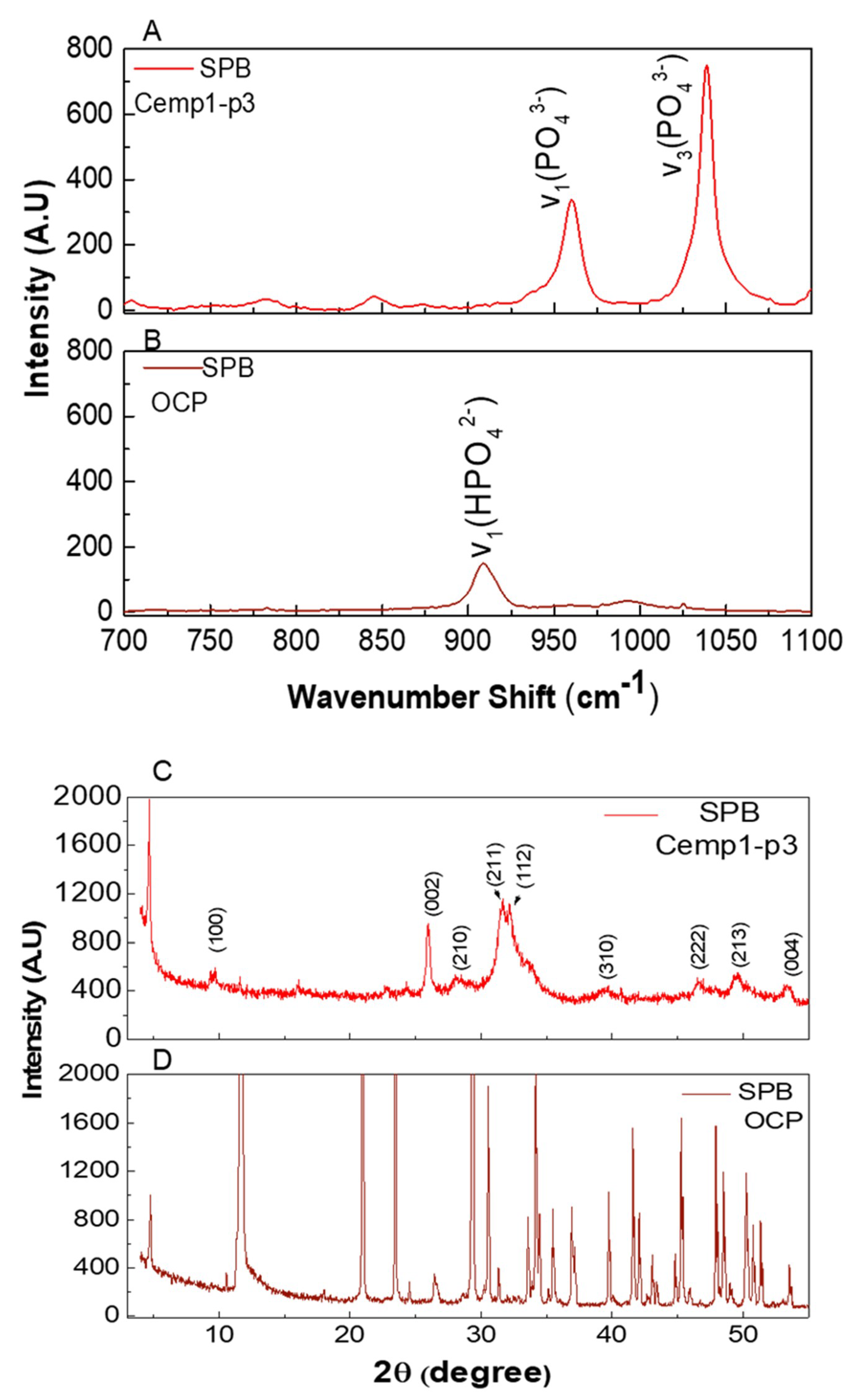
3.5. Micro Raman Analysis
3.6. Powder X-ray Diffraction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoz, L.; Romo, E.; Sanz, M.; Nunez, J.; Gaitan, L.; Mercado, G.; Arzate, H.; Zeichner-David, M. Cementum protein 1 (CEMP1) induces differentiation by human periodontal ligament cells under three-dimensional culture conditions. Cell Biol. Int. 2011, 36, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Y.; Yang, J.; Wu, W.; Miao, L.; Yu, Y.; Yang, X.; Sun, W.B. The synthesis of hydroxyapatite with different crystallinities by controlling the concentration of recombinant CEMP1 for biological application. Mater. Sci. Eng. C 2016, 59, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Romo, E.; Bermúdez, M.; Narayanan, A.S.; Zeichner-David, M.; Santos-Cuevas, C.L.; Arzate, H. Bone Regeneration in Rat Cranium Critical-Size Defects Induced by Cementum Protein 1 (CEMP1). PLoS ONE 2013, 8, e78807. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Montoya, G.; Correa, R.; Arenas, J.; Hoz, L.; Romo, E.; Arroyo, R.; Zeichner--David, M.; Arzate, H. Cementum protein 1--derived peptide (CEMP 1-p1) modulates hydroxyapatite crystal formation in vitro. J. Pept. Sci. 2019, 25, e3211. [Google Scholar] [CrossRef]
- Carmona-Rodríguez, B.; Álvarez-Pérez, M.A.; Narayanan, A.S.; Zeichner-David, M.; Reyes-Gasga, J.; Molina-Guarneros, J.A.; García-Hernández, A.L.; Suarez-Franco, J.L.; Gil Chavarría, I.; Villarreal-Ramirez, E.; et al. Human Cementum Protein 1 induces expression of bone and cementum proteins by human gingival fibroblasts. Biochem. Biophys. Res. Commun. 2007, 358, 763–769. [Google Scholar] [CrossRef]
- Komaki, M.; Iwasaki, K.; Arzate, H.; Narayanan, A.S.; Izumi, Y.; Morita, I. Cementum protein 1 (CEMP1) induces a cementoblastic phenotype and reduces osteoblastic differentiation in periodontal ligament cells. J. Cell. Physiol. 2012, 227, 649–657. [Google Scholar] [CrossRef]
- Villarreal-Ramirez, E.; Moreno, A.; Mas-Oliva, J.; Chávez-Pacheco, J.L.; Narayanan, A.S.; Gil-Chavarría, I.; Zeichner-David, M.; Arzate, H. Characterization of recombinant human cementum protein 1 (hrCEMP1): Primary role in biomineralization. Biochem. Biophys. Res. Commun. 2009, 384, 49–54. [Google Scholar] [CrossRef]
- Correa, R.; Arenas, J.; Montoya, G.; Hoz, L.; López, S.; Salgado, F.; Arroyo, R.; Salmeron, N.; Romo, E.; Zeichner-David, M.; et al. Synthetic cementum protein 1–derived peptide regulates mineralization in vitro and promotes bone regeneration in vivo. FASEB J. 2018, 33, 1167–1178. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 2005, 81, 1705–1728. [Google Scholar] [CrossRef]
- Zhang, P.; Hong, Z.; Yu, T.; Chen, X.; Jing, X. In vivo mineralization and osteogenesis of nanocomposite scaffold of poly(lactide-co-glycolide) and hydroxyapatite surface-grafted with poly(l-lactide). Biomaterials 2009, 30, 58–70. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Navarrete, D.A. Chapter 1: Biological Apatites in Bone and Teeth. In Nanoceramics in Clinical Use: From Materials to Applications, 2nd ed.; Nanoscience & Nanotechnology Series; Royal Society of Chemistry: London, UK, 2015; pp. 1–29. [Google Scholar] [CrossRef]
- Srivastava, S. Sorption of Divalent Metal Ions from Aqueous Solution by Oxidized Carbon Nanotubes and Nanocages: A Review. Adv. Mater. Lett. 2013, 4, 2–8. [Google Scholar] [CrossRef]
- Sugiura, Y.; Saito, Y.; Endo, T.; Makita, Y. Effect of the Ionic Radius of Alkali Metal Ions on Octacalcium Phosphate Formation via Different Substitution Modes. Cryst. Growth Des. 2019, 19, 4162–4171. [Google Scholar] [CrossRef]
- Kamitakahara, M.; Ohtoshi, N.; Kawashita, M.; Ioku, K. Spherical porous hydroxyapatite granules containing composites of magnetic and hydroxyapatite nanoparticles for the hyperthermia treatment of bone tumor. J. Mater. Sci. Mater. Med. 2016, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Oktar, F.N.; Gökçe, H.; Gunduz, O.; Sahin, Y.; Ağaoğullari, D.; Turner, I.; Ozyegin, L.; Ben-Nissan, B. Bioceramic Production from Giant Purple Barnacle (Megabalanus tintinnabulum). Key Eng. Mater. 2014, 631, 137–142. [Google Scholar] [CrossRef]
- Rao, A.; Roncal-Herrero, T.; Schmid, E.; Drechsler, M.; Scheffner, M.; Gebauer, D.; Kröger, R.; Cölfen, H. On Biomineralization: Enzymes Switch on Mesocrystal Assembly. ACS Central Sci. 2019, 5, 357–364. [Google Scholar] [CrossRef]
- Mann, S.; Archibald, D.D.; Didymus, J.M.; Douglas, T.; Heywood, B.R.; Meldrum, F.C.; Reeves, N.J. Crystallization at Inorganic-organic Interfaces: Biominerals and Biomimetic Synthesis. Science 1993, 261, 1286–1292. [Google Scholar] [CrossRef]
- Tomson, M.B.; Nancollas, G.H.; Tamm, G.R.; Cobb, J.S. Mineralization Kinetics: A Constant Composition Approach. Science 1978, 200, 1059–1060. [Google Scholar] [CrossRef]
- Fowler, B.O.; Markovic, M.; Brown, W.E. Octacalcium phosphate. 3. Infrared and Raman vibrational spectra. Chem. Mater. 1993, 5, 1417–1423. [Google Scholar] [CrossRef]
- Tao, J.; Battle, K.C.; Pan, H.; Salter, E.A.; Chien, Y.-C.; Wierzbicki, A.; De Yoreo, J.J. Energetic basis for the molecular-scale organization of bone. Proc. Natl. Acad. Sci. USA 2014, 112, 326–331. [Google Scholar] [CrossRef]
- Timchenko, P.E.; Timchenko, E.V.; Pisareva, E.V.; Vlasov, M.Y.; Volova, L.T.; Frolov, O.O.; Kalimullina, A.R. Experimental studies of hydroxyapatite by Raman spectroscopy. J. Opt. Technol. 2018, 85, 130–135. [Google Scholar] [CrossRef]
- Santana-Vázquez, M.; Estevez, O.; Ascencio-Aguirre, F.; Mendoza-Cruz, R.; Bazán-Díaz, L.; Zorrila, C.; Herrera-Becerra, R. Tannic acid assisted synthesis of flake-like hydroxyapatite nanostructures at room temperature. Appl. Phys. A 2016, 122, 868. [Google Scholar] [CrossRef]
- Azzopardi, P.V.; O’Young, J.; Lajoie, G.; Karttunen, M.; Goldberg, H.A.; Hunter, G.K. Roles of Electrostatics and Conformation in Protein-Crystal Interactions. PLoS ONE 2010, 5, e9330. [Google Scholar] [CrossRef] [PubMed]
- Farmanesh, S.; Ramamoorthy, S.; Chung, J.; Asplin, J.R.; Karande, P.; Rimer, J.D. Specificity of Growth Inhibitors and their Cooperative Effects in Calcium Oxalate Monohydrate Crystallization. J. Am. Chem. Soc. 2013, 136, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Khalili, M.; Saunders, J.A.; Liwo, A.; Ołdziej, S.; Scheraga, H.A. A united residue force-field for calcium–protein interactions. Protein Sci. 2009, 13, 2725–2735. [Google Scholar] [CrossRef] [PubMed]
- Antonakos, A.; Liarokapis, E.; Leventouri, T. Micro-Raman and FTIR studies of synthetic and natural apatites. Biomaterials 2007, 28, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Lotsari, A.; Rajasekharan, A.K.; Halvarsson, M.; Andersson, M. Transformation of amorphous calcium phosphate to bone-like apatite. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Jamalian, A.; Sneekes, E.-J.; Dekker, L.J.M.; Ursem, M.; Luider, T.M.; Burgers, P. (Peter) Dimerization of Peptides by Calcium Ions: Investigation of a Calcium-Binding Motif. Int. J. Proteom. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Habraken, W.J.E.M.; Tao, J.; Brylka, L.J.; Friedrich, H.; Bertinetti, L.; Schenk, A.S.; Verch, A.; Dmitrovic, V.; Bomans, P.H.H.; Frederik, P.M.; et al. Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate. Nat. Commun. 2013, 4, 1507. [Google Scholar] [CrossRef]
- Gower, L.B. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chem. Rev. 2008, 108, 4551–4627. [Google Scholar] [CrossRef]
- Dove, P.M.; Han, N.; Wallace, A.F. Systematic dependence of kinetic and thermodynamic barriers to homogeneous silica nucleation on NaCl and amino acids. J. Mater. Res. 2019, 34, 442–455. [Google Scholar] [CrossRef]
- Ito, N.; Kamitakahara, M.; Yoshimura, M.; Ioku, K. Importance of nucleation in transformation of octacalcium phosphate to hydroxyapatite. Mater. Sci. Eng. C 2014, 40, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C.; Belcher, A.M. Emulating biology: Building nanostructures from the bottom up. Proc. Natl. Acad. Sci. USA 2002, 99, 6451–6455. [Google Scholar] [CrossRef] [PubMed]
- Penel, G.; Leroy, G.; Rey, C.; Bres, E. MicroRaman Spectral Study of the PO4 and CO3 Vibrational Modes in Synthetic and Biological Apatites. Calcif. Tissue Int. 1998, 63, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Gazit, E. Self-assembled peptide nanostructures: The design of molecular building blocks and their technological utilization. Chem. Soc. Rev. 2007, 36, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.R.; Wierzbicki, A.S.; Orme, C.A.; Cody, A.M.; Hoyer, J.R.; Nancollas, G.H.; Zepeda, S.; De Yoreo, J.J. Molecular modulation of calcium oxalate crystallization by osteopontin and citrate. Proc. Natl. Acad. Sci. USA 2004, 101, 1811–1815. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Xing, R.; Yan, X. Peptide self-assembly: Thermodynamics and kinetics. Chem. Soc. Rev. 2016, 45, 5589–5604. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, M.; Montoya, G.; Herrera, R.; Hoz, L.; Romo, E.; Zamora, C.; Wintergerst, A.; Arzate, H. Cemp1-p3 Peptide Promotes the Transformation of Octacalcium Phosphate into Hydroxyapatite Crystals. Crystals 2020, 10, 1131. https://doi.org/10.3390/cryst10121131
Santana M, Montoya G, Herrera R, Hoz L, Romo E, Zamora C, Wintergerst A, Arzate H. Cemp1-p3 Peptide Promotes the Transformation of Octacalcium Phosphate into Hydroxyapatite Crystals. Crystals. 2020; 10(12):1131. https://doi.org/10.3390/cryst10121131
Chicago/Turabian StyleSantana, Maricela, Gonzalo Montoya, Raúl Herrera, Lía Hoz, Enrique Romo, Claudia Zamora, Ana Wintergerst, and Higinio Arzate. 2020. "Cemp1-p3 Peptide Promotes the Transformation of Octacalcium Phosphate into Hydroxyapatite Crystals" Crystals 10, no. 12: 1131. https://doi.org/10.3390/cryst10121131
APA StyleSantana, M., Montoya, G., Herrera, R., Hoz, L., Romo, E., Zamora, C., Wintergerst, A., & Arzate, H. (2020). Cemp1-p3 Peptide Promotes the Transformation of Octacalcium Phosphate into Hydroxyapatite Crystals. Crystals, 10(12), 1131. https://doi.org/10.3390/cryst10121131