Exploring the Wet Mechanochemical Synthesis of Mg-Al, Ca-Al, Zn-Al and Cu-Al Layered Double Hydroxides from Oxides, Hydroxides and Basic Carbonates
Abstract
1. Introduction
2. Materials and Methods
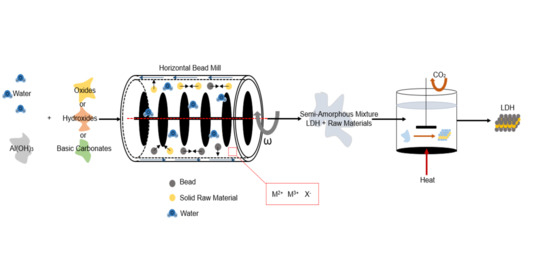
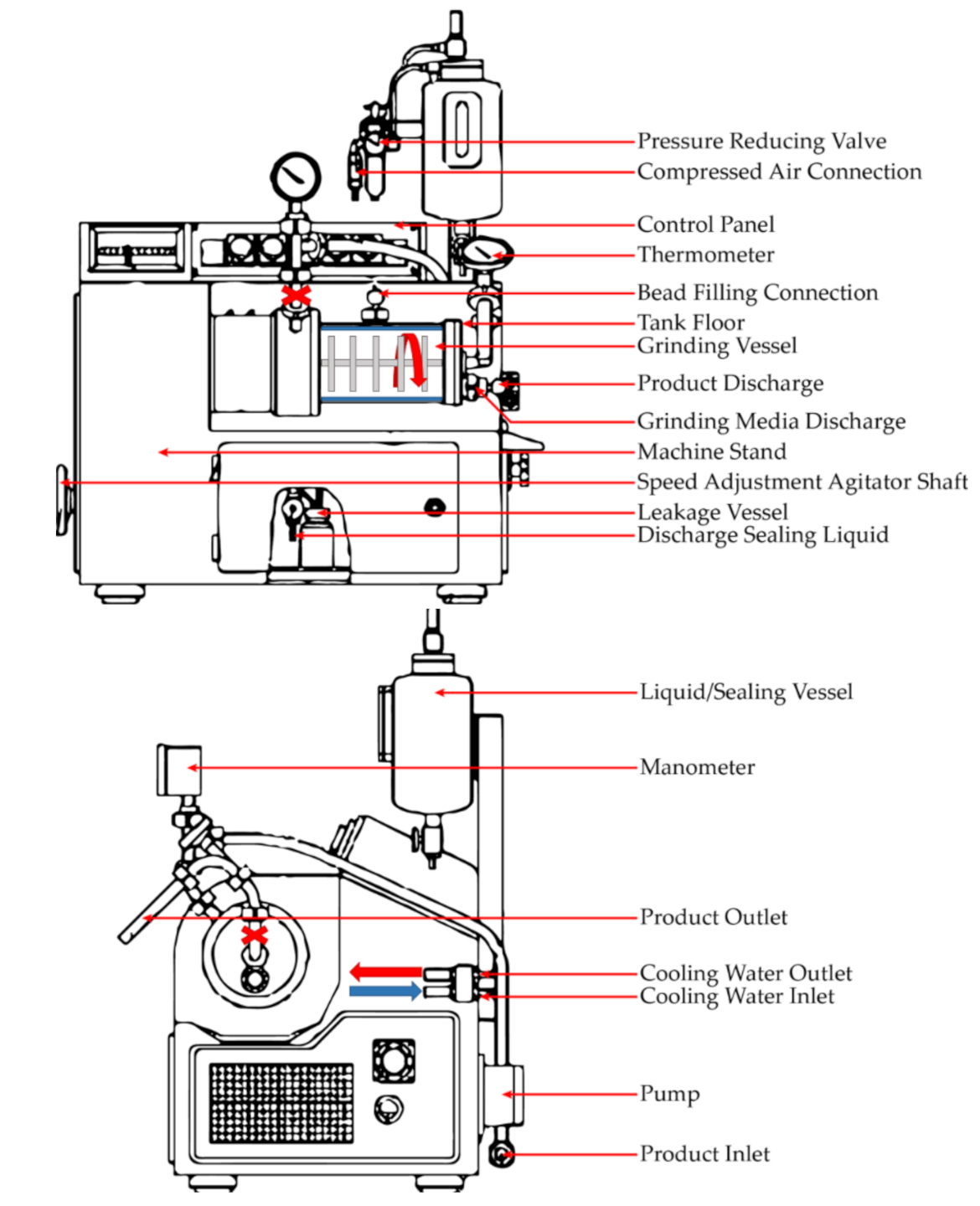
2.1. Milling Operation
2.2. Ageing Process
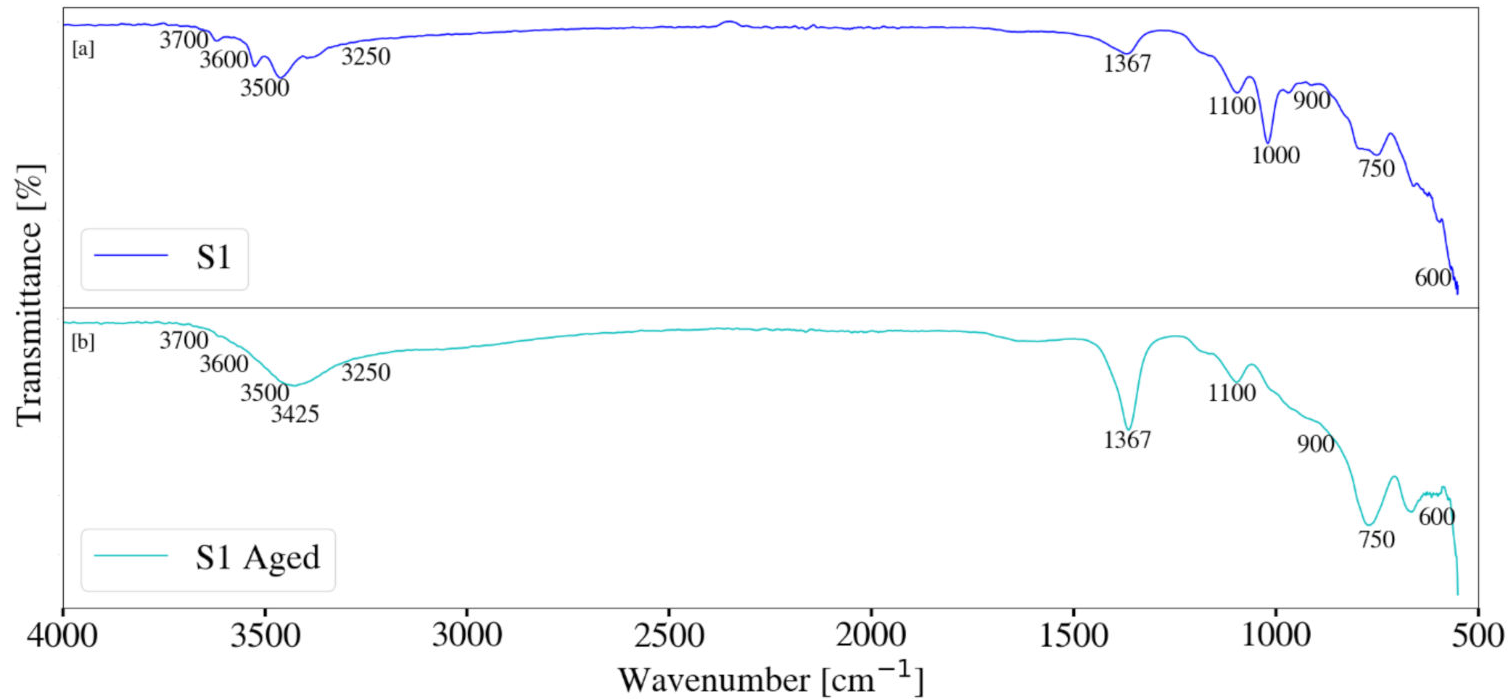
2.3. Mg-Al LDH
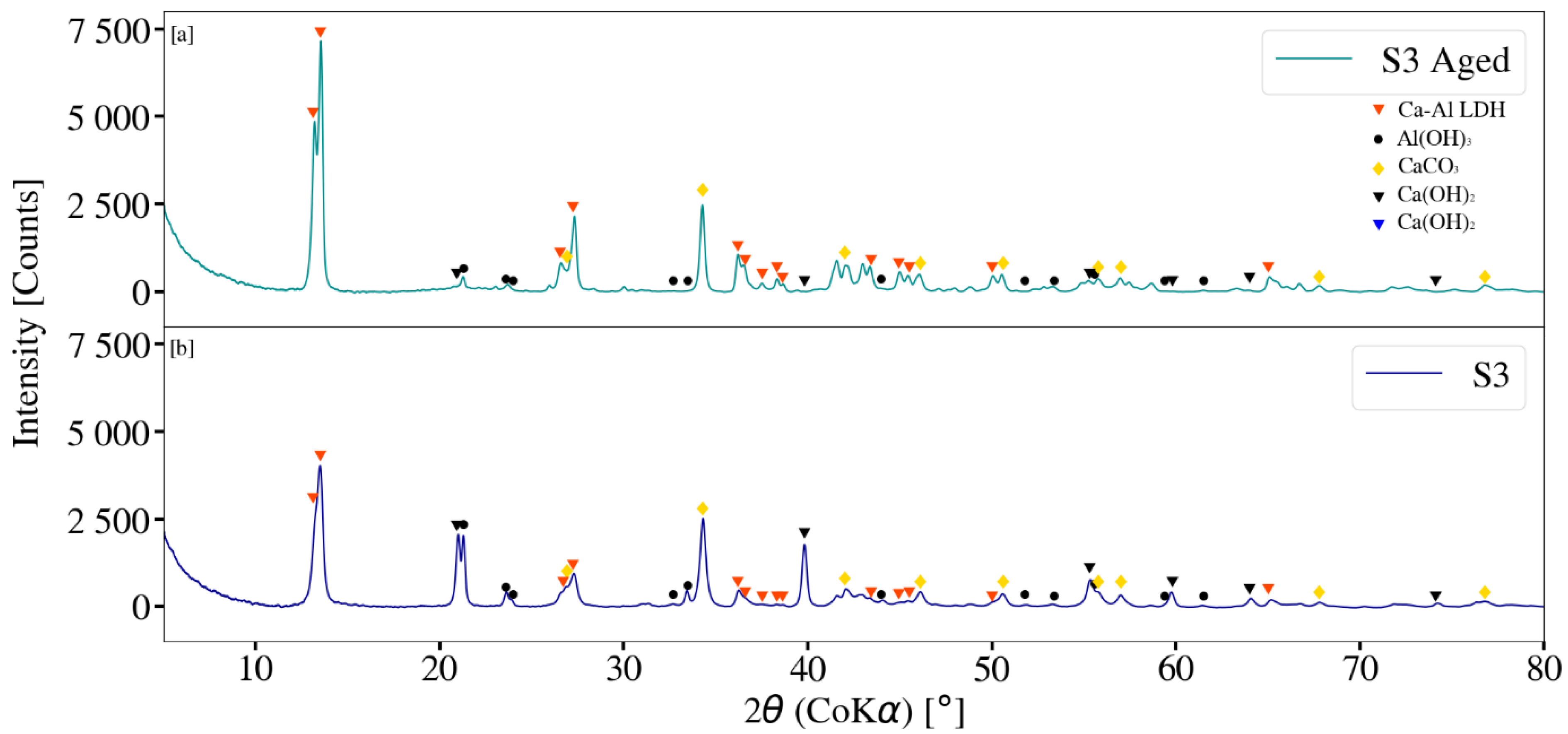
2.4. Ca-Al LDH
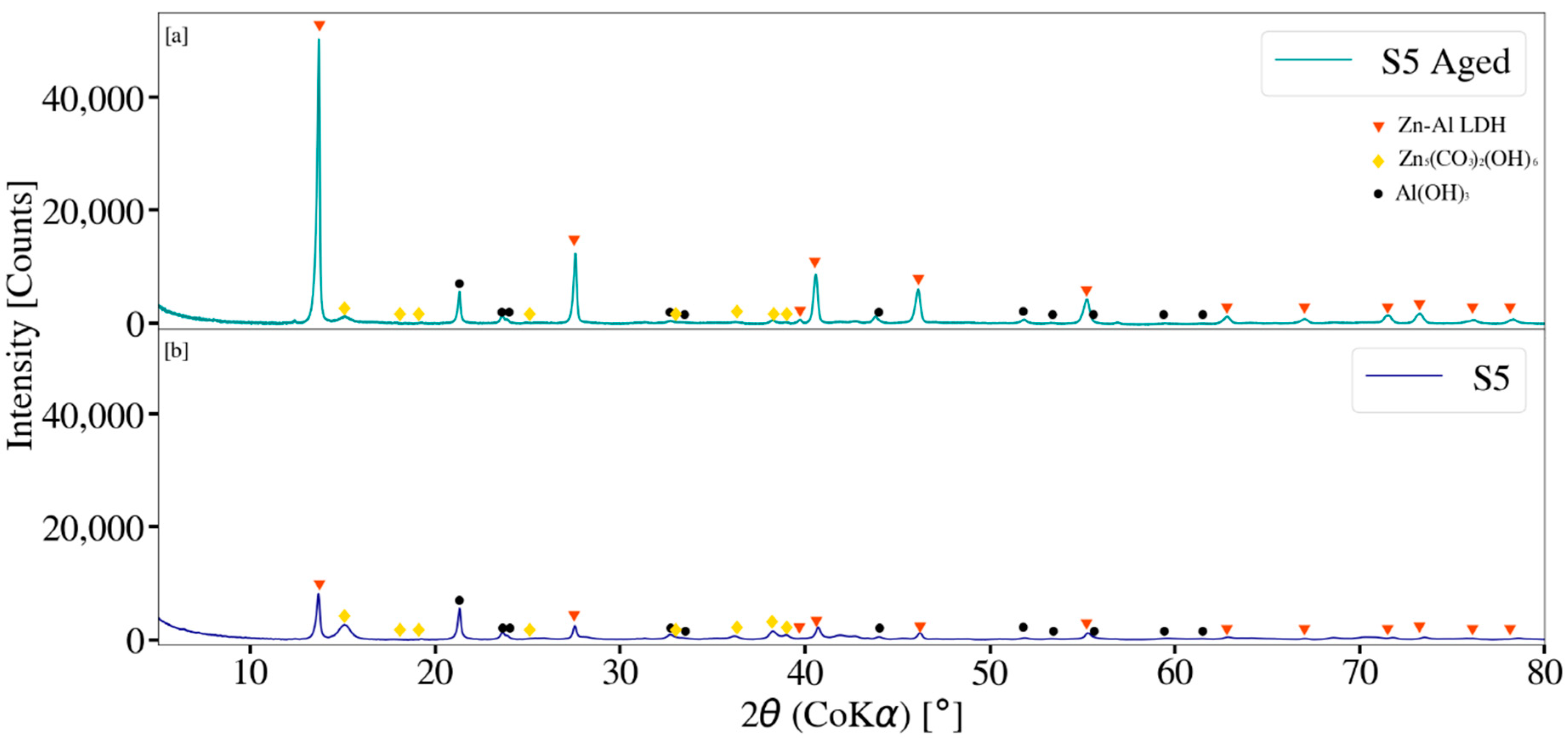
2.5. Zn-Al LDH
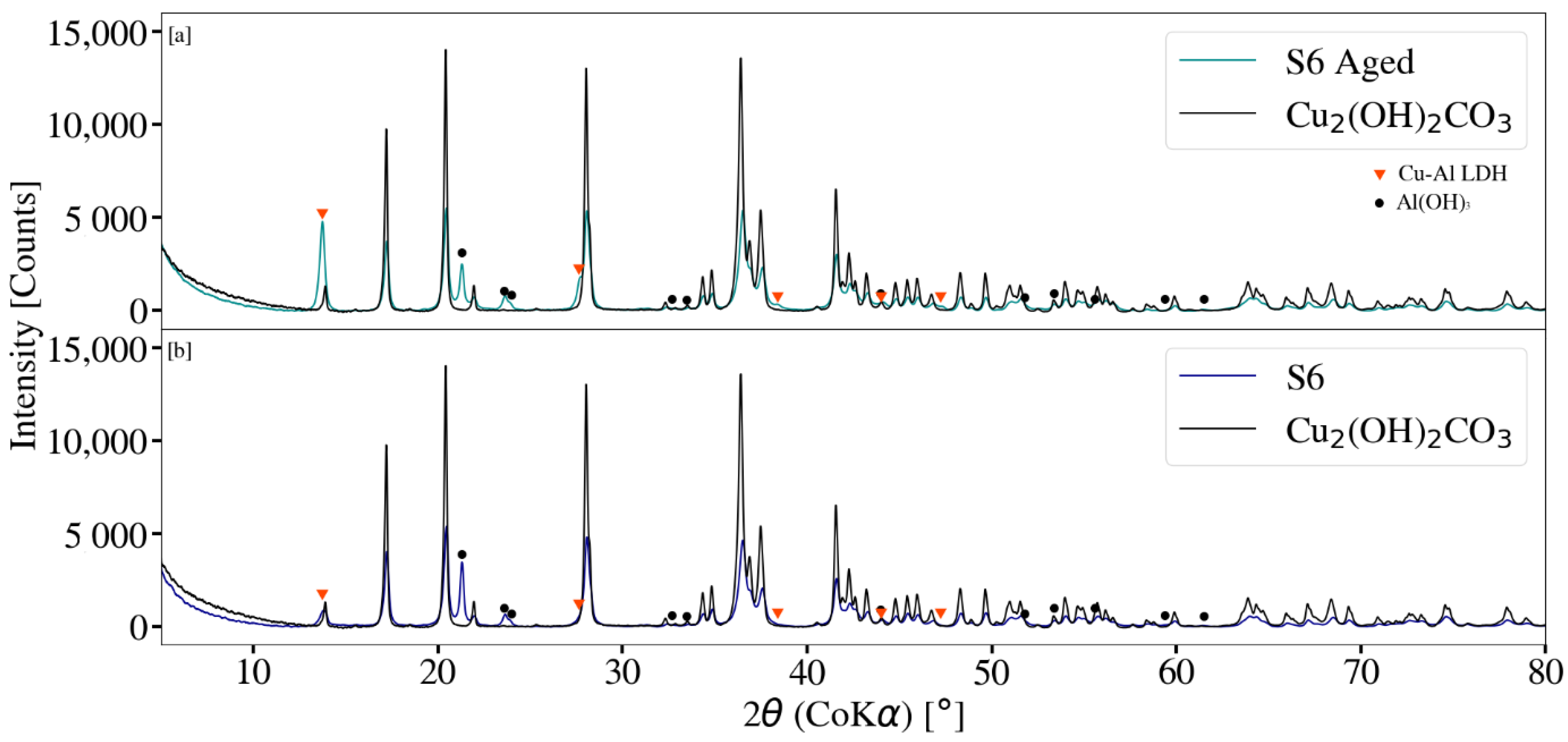
2.6. Cu-Al LDH
2.7. Material Characterisation
2.7.1. Particle Size Analysis (PSA)
2.7.2. Scanning Electron Microscopy (SEM)
2.7.3. X-ray Diffraction Analysis (XRD)
2.7.4. Fourier Transform Infrared Spectroscopy (FT-IR)
2.7.5. X-ray Fluorescence (XRF)
3. Results and Discussion
3.1. Particle Size Analysis
3.2. X-ray Fluorescence
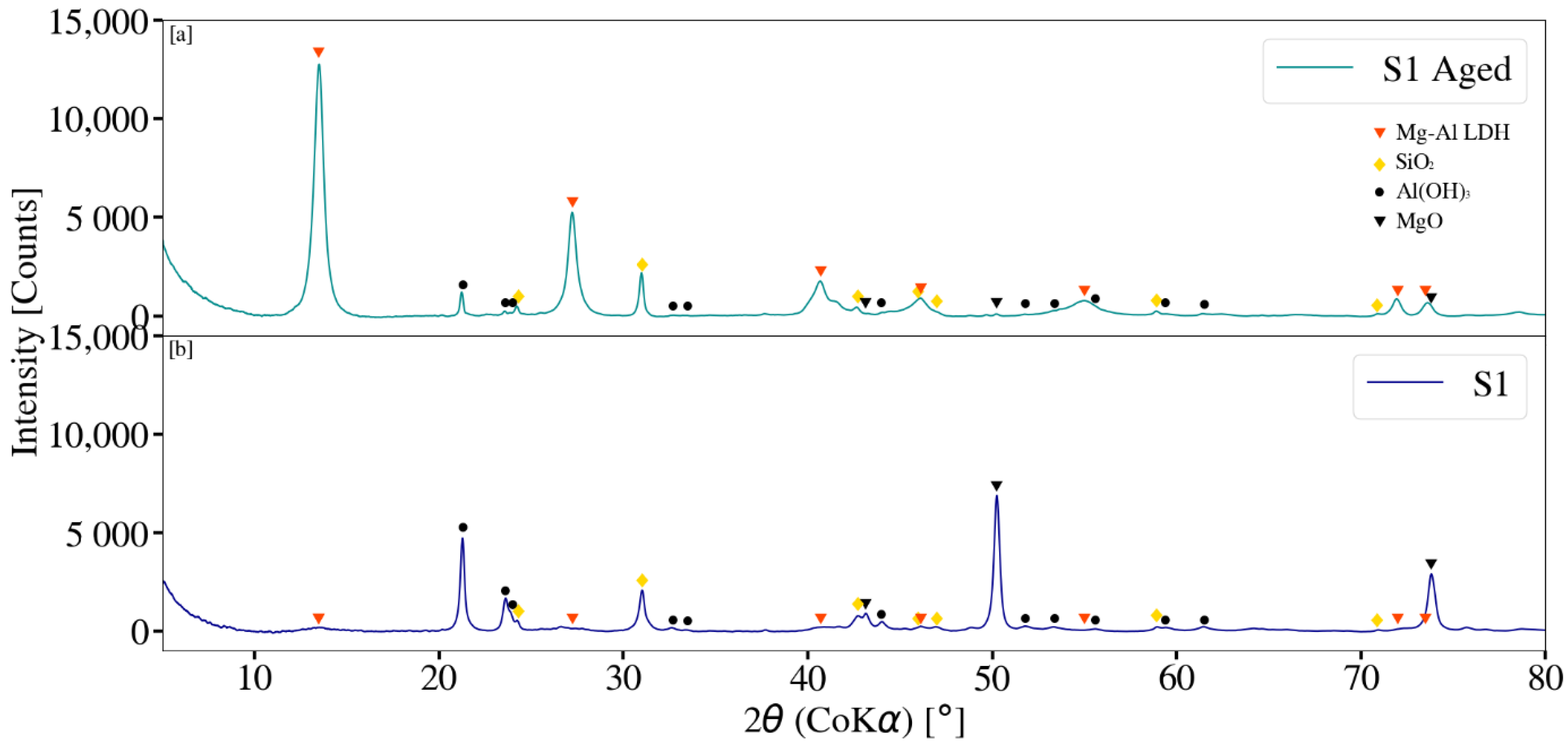
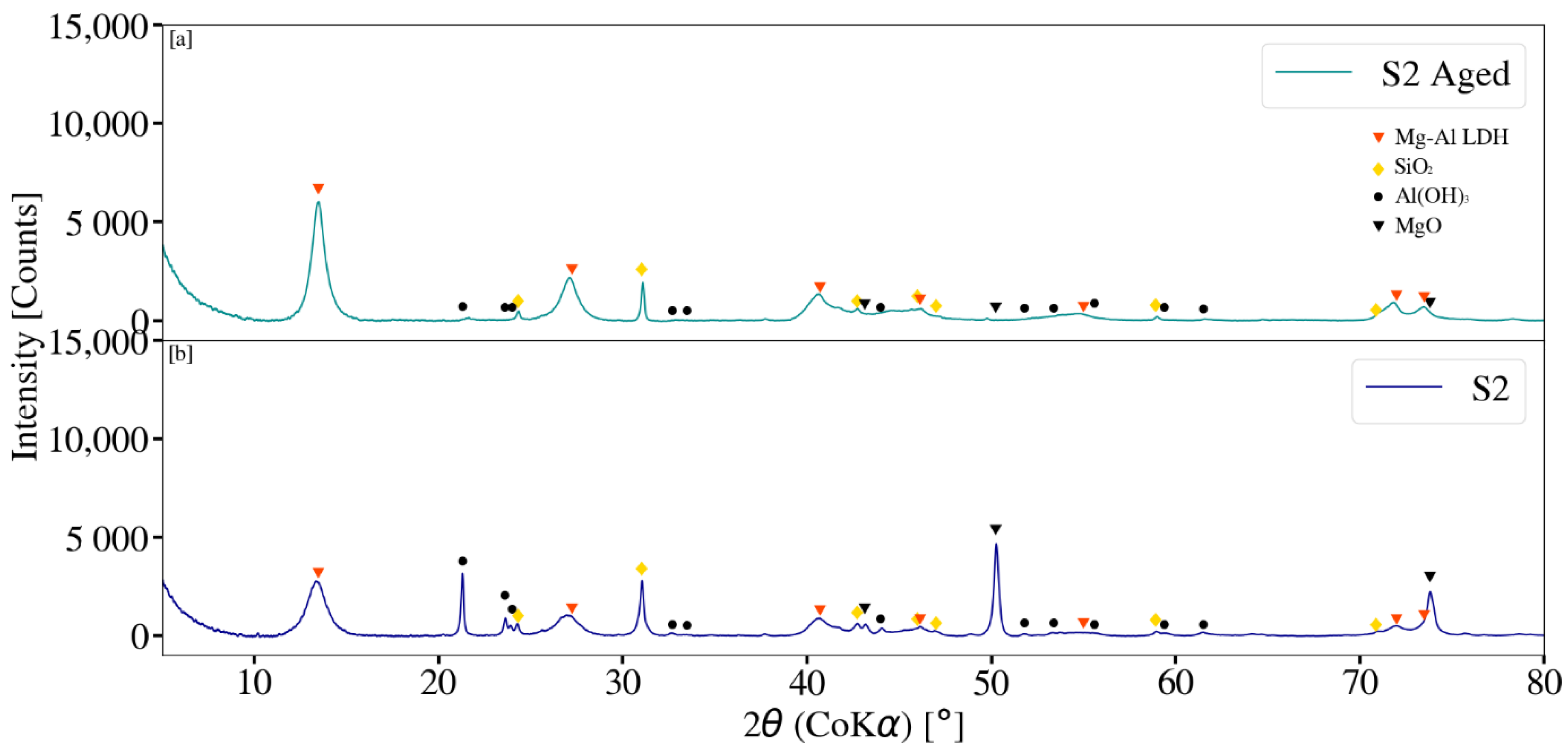
3.3. X-ray Diffraction Analysis
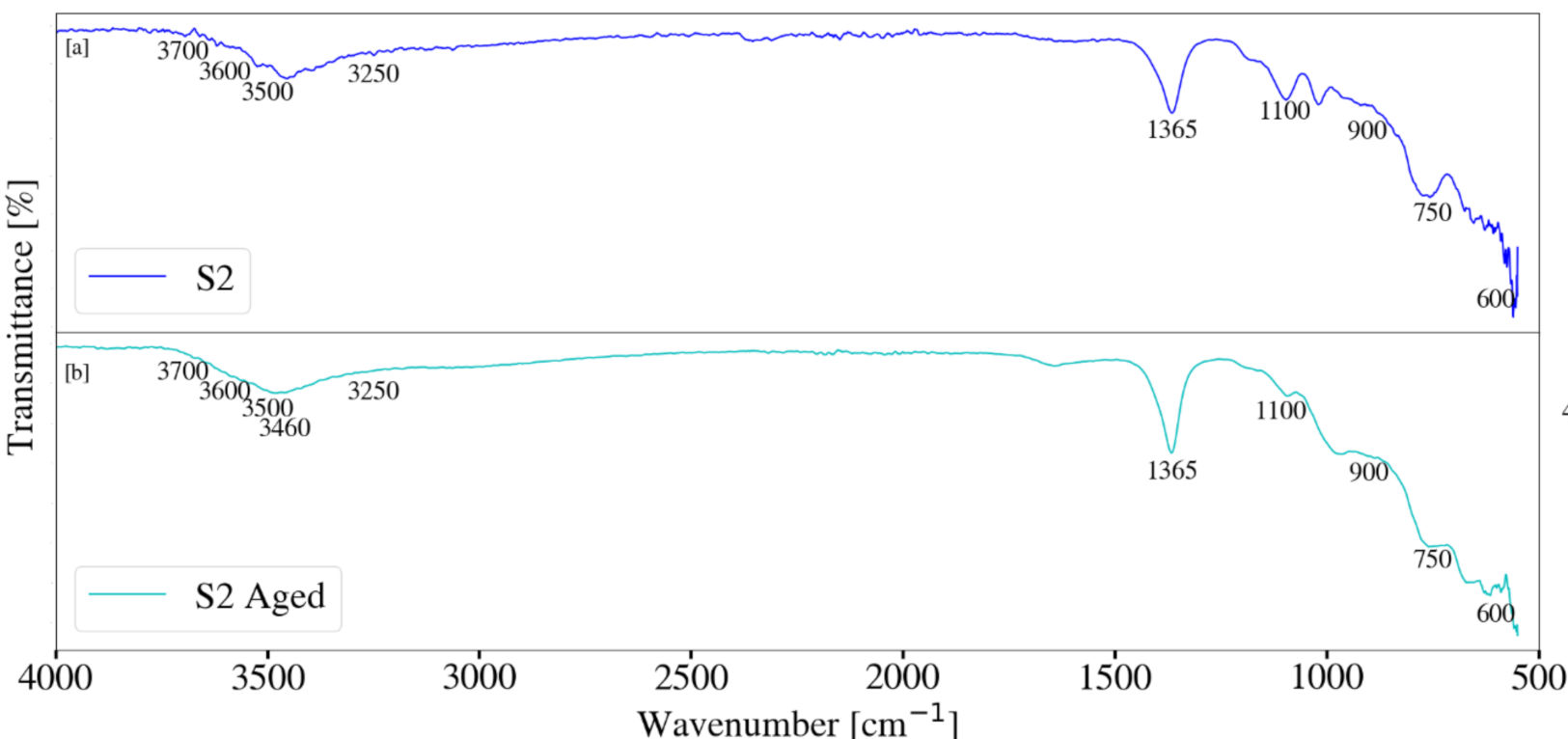
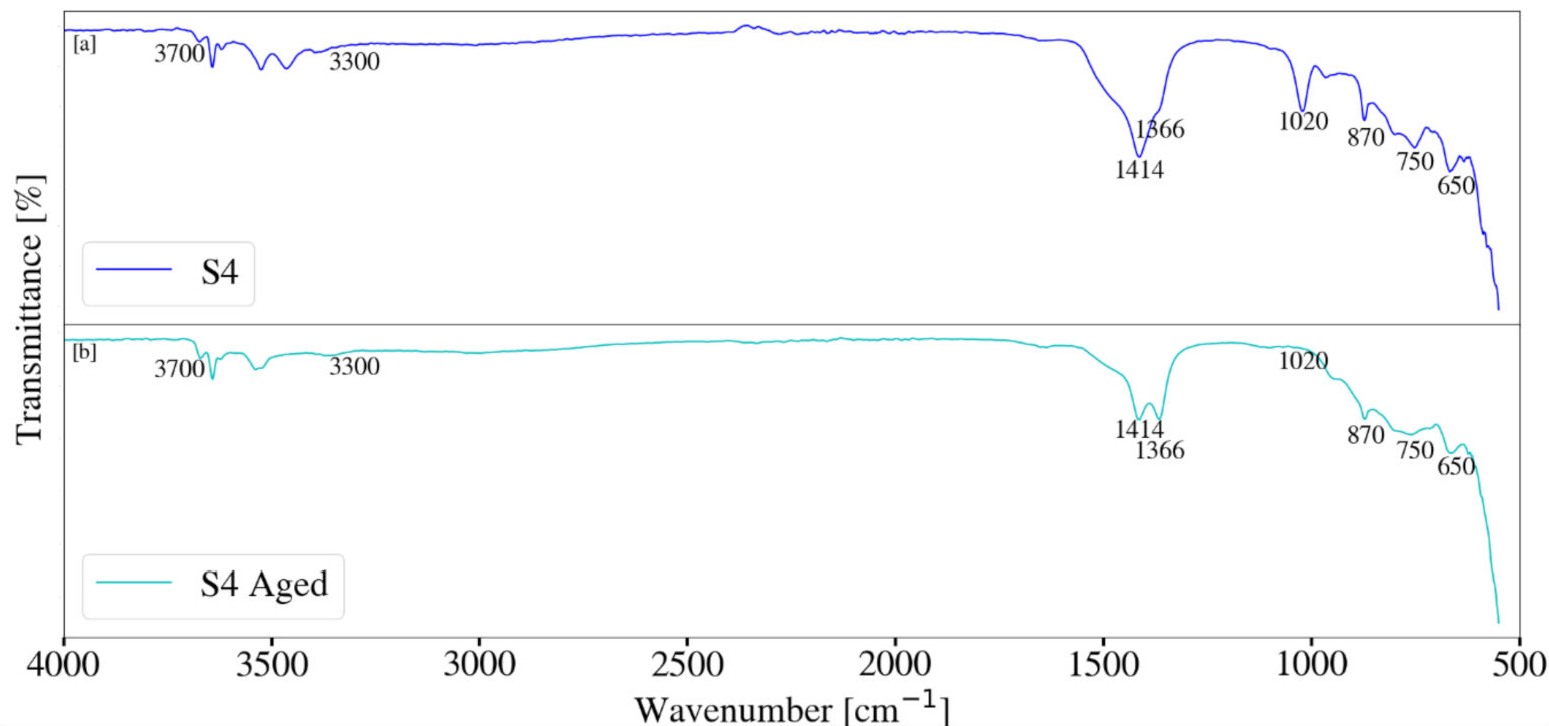
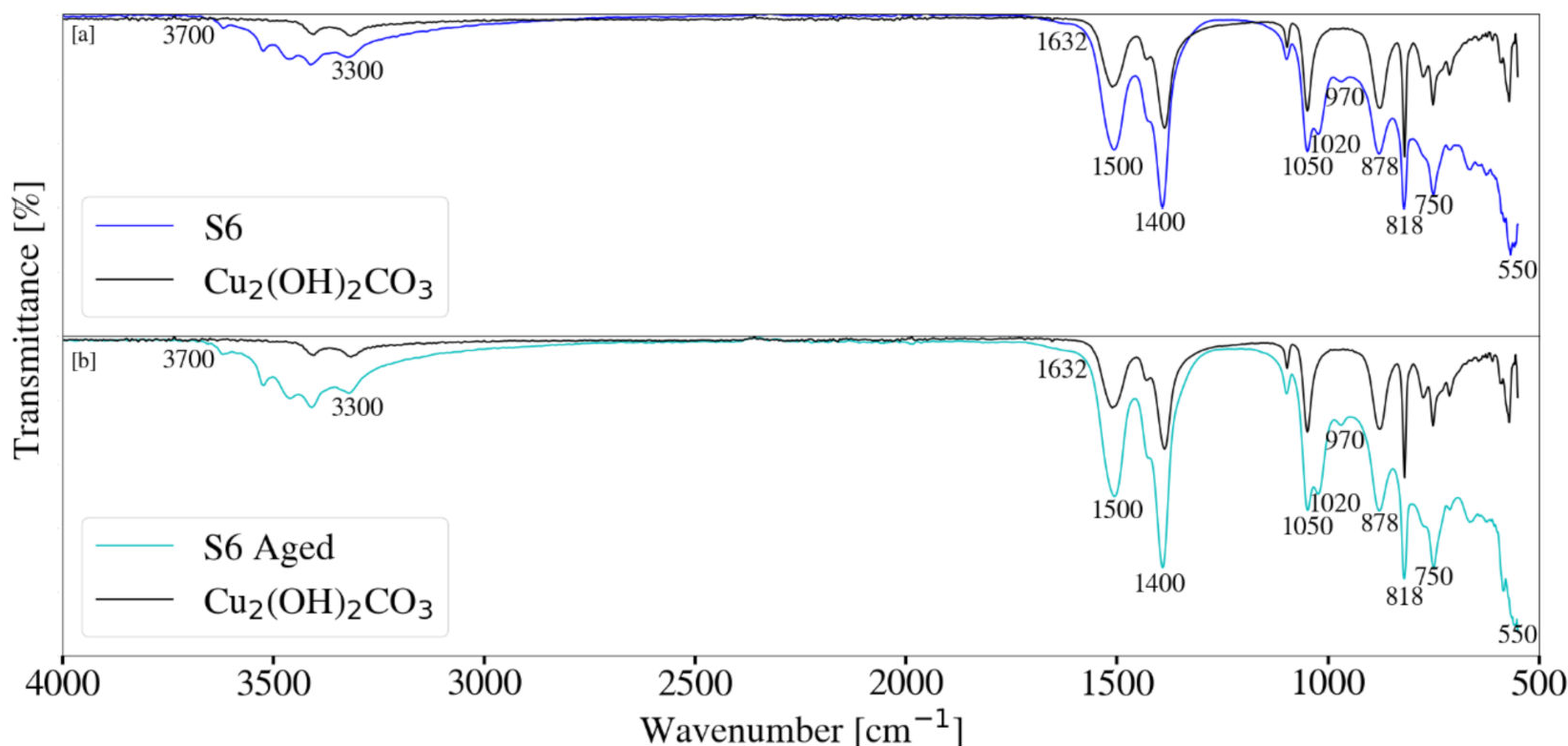
3.4. Fourier Transform Infrared Spectroscopy
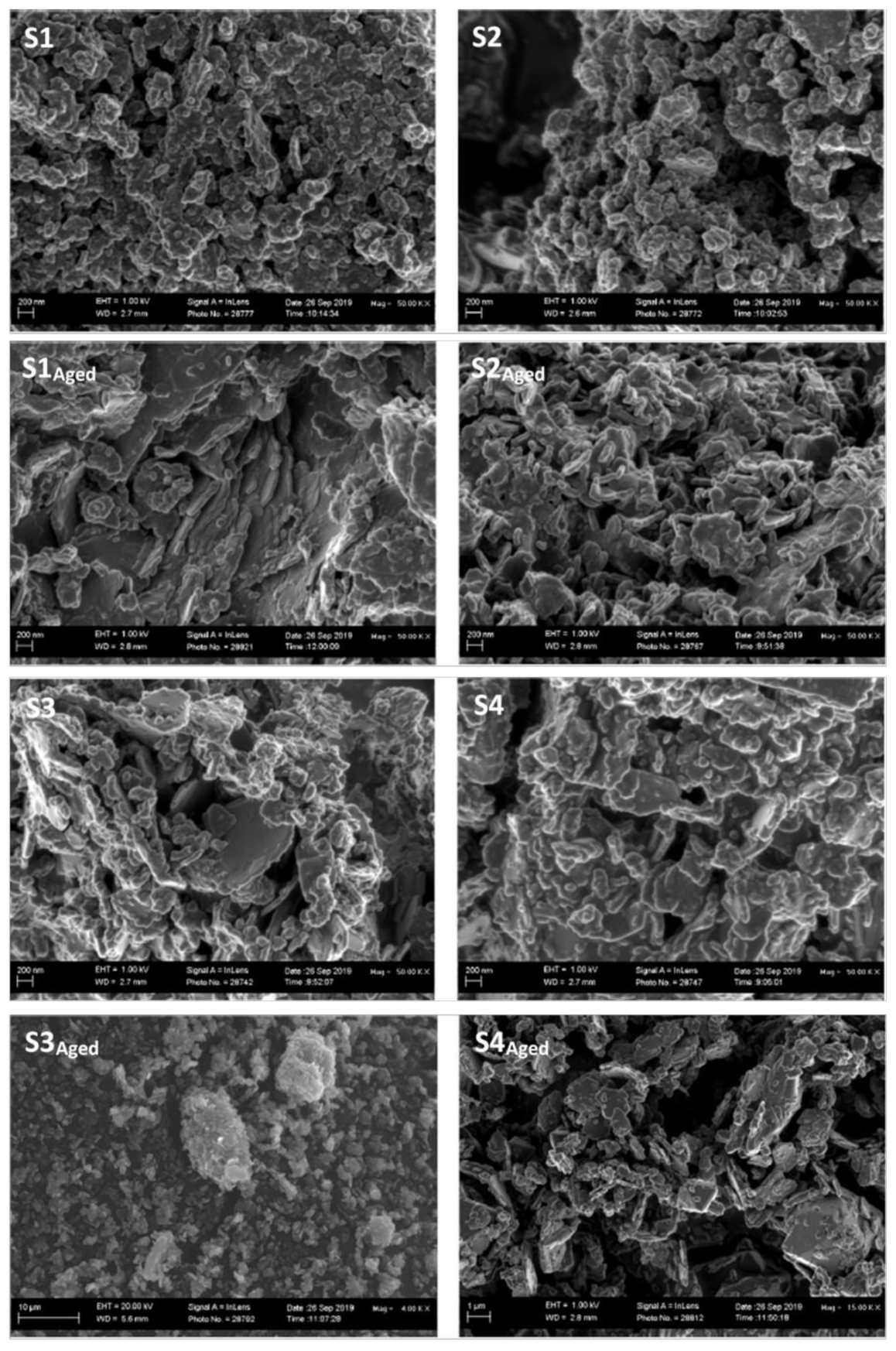
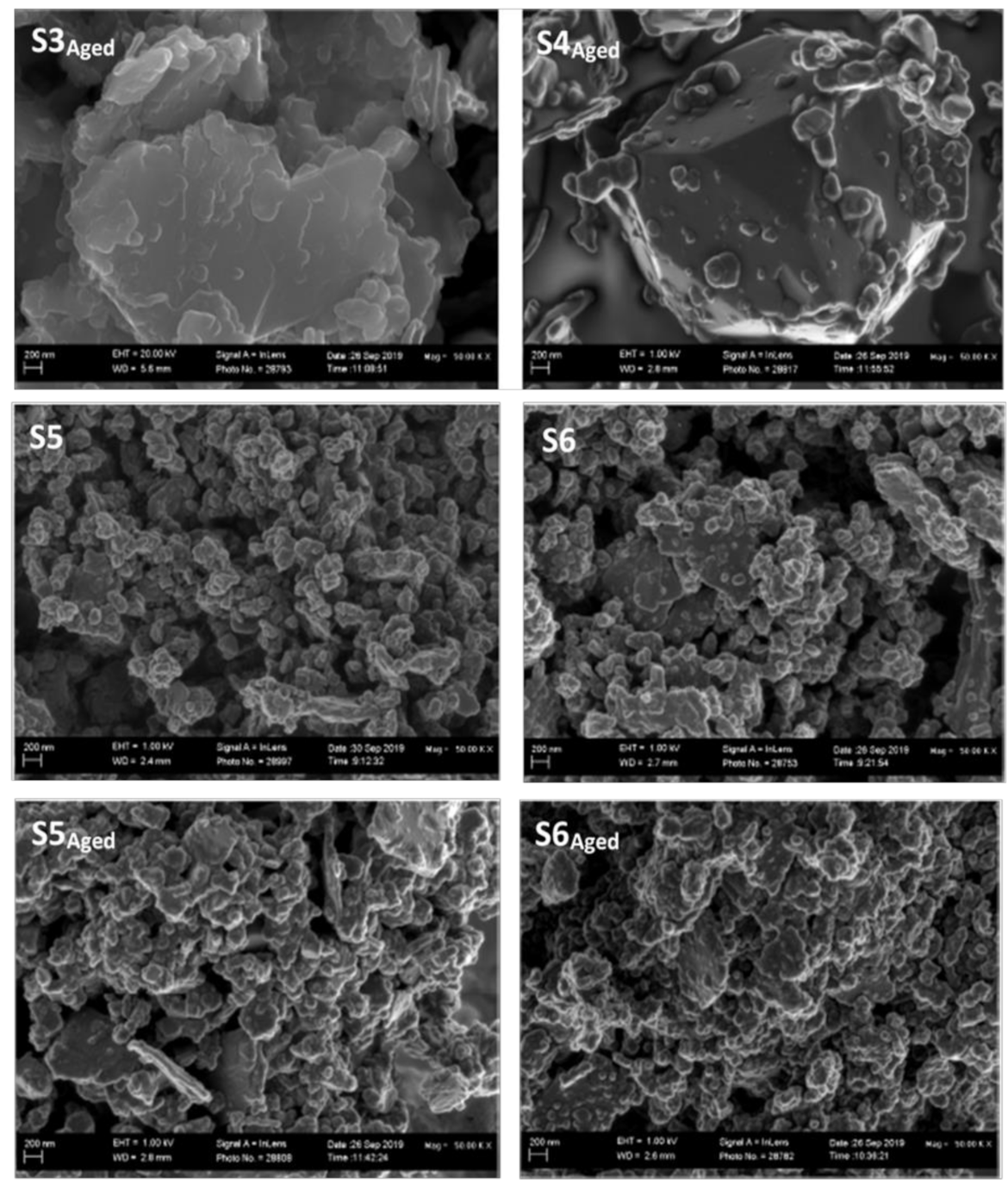
3.5. Scanning Electron Microscopy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bergaya, F.; Lagaly, G. Handbook of Clay Science, 1st ed.; Elsevier Science & Technology Books: San Diego, CA, USA, 2013; pp. 1021–1069. [Google Scholar]
- Qu, J.; Zhang, Q.; Li, X.; He, X.; Song, S. Mechanochemical approaches to synthesize layered double hydroxides: A review. Appl. Clay Sci. 2015, 119, 185–192. [Google Scholar] [CrossRef]
- Ferencz, Z.; Szanados, M.; Adok-Sipiczki, M.; Kukovecz, A.; Konya, Z.; Sipos, P.; Palinko, I. Mechanochemical assisted synthesis of pristine Ca(II)Sn(IV)-layered double hydroxides and their amino acid intercalated composites. Mater. Sci. 2014, 49, 8479–8486. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Liu, X.; Chen, M.; Wu, L.; Ai, Z. Mechanochemical synthesis of novel heterostructured Bi2S3/Zn-Al layered double hydroxide nano-particles as efficient visible light reactive Z-scheme photocatalysts. Appl. Surf. Sci. 2018, 452, 123–133. [Google Scholar] [CrossRef]
- Fahami, A.; Beall, G.W.; Enayatpour, S.; Tavangarian, T.; Fahami, M. Rapid preparation of nano hexagonal-shaped hydrocalumite via one-pot mechanochemistry method. Appl. Clay Sci. 2017, 136, 90–95. [Google Scholar] [CrossRef]
- Fahmi, A.; Duraia, E.M.; Beall, G.W.; Fahami, M. Facile synthesis and structural insight of chloride intercalated Ca-Al Layered double hydroxide nanopowders. J. Alloys Compd. 2017, 727, 970–977. [Google Scholar] [CrossRef]
- Du, W.; Zheng, L.; Li, X.; Fu, J.; Lu, X.; Hou, Z. Plate like Ni-Mg-Al layered double hydroxides synthesised via a solvent free approach and its application in hydrogenolysis of D sorbitol. App. Clay Sci. 2016, 123, 166–172. [Google Scholar] [CrossRef]
- Qu, J.; Li, X.; Lei, Z.; Li, Z.; Chen, M.; Zhang, Q. Mechano-Hydrothermal synthesis of tetraborate pillared Li-Al layered double hydroxide. Am. Ceram. Soc. 2016, 99, 1151–1154. [Google Scholar] [CrossRef]
- Zhang, F.; Du, N.; Song, S.; Liu, J.; Hou, W. Mechano-hydrothermal synthesis of Mg2Al-NO3 layered double hydroxides. Solid State Chem. 2013, 206, 45–50. [Google Scholar] [CrossRef]
- Zhang, F.; Hou, W. Mechano-hydrothermal preparation of Li-Al-OH layered double hydroxides. Sol. State Sci. 2018, 79, 93–98. [Google Scholar] [CrossRef]
- Ferencz, Z.; Kukovecz, A.; Konya, A.; Sipos, P.; Palinko, I. Optimisation of the synthesis parameters of mechanochemically prepared Ca-Al-layered double hydroxide. Appl. Clay Sci. 2015, 112, 94–99. [Google Scholar] [CrossRef]
- Kuramoto, K.; Intasa-ard, S.; Bureekaew, S.; Ogawa, M. Mechanochemical synthesis of finite particles of layered double hydroxide-acetate intercalation compound: Swelling, Thin film ion exchange. Sol. State Chem. 2017, 253, 147. [Google Scholar] [CrossRef]
- Tongamp, W.; Zhang, Q.; Saito, F. Mechanochemical route for synthesizing nitrate form layered double hydroxide. Powder Technol. 2008, 185, 43–48. [Google Scholar] [CrossRef]
- Pagano, C.; Marmottini, F.; Nocchetti, M.; Ramella, D.; Perioli, L. Effects of different milling techniques on the layered double hydroxides final properties. Appl. Clay Sci. 2018, 151, 124–133. [Google Scholar] [CrossRef]
- Tongamp, W.; Zhang, Q.; Saito, F. Preparation of meixnerite (Mg–Al–OH) type layered double hydroxide by a mechanochemical route. Mater. Sci. 2007, 42, 9210–9215. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Ai, Z.; Wu, L.; Zhang, Q. Mechanochemical synthesis of CdS/MgAl LDH-precursor as improved visible light driven photocatalyst for organic dye. App. Clay Sci. 2018, 163, 265–272. [Google Scholar] [CrossRef]
- Qu, J.; He, X.; Chen, M.; Hu, H.; Zhang, Q.; Liu, X. Mechanochemical synthesis of Cu-Al and methyl orange intercalated Cu-Al layered double hydroxides. Mater. Chem. Phys. 2017, 191, 173–180. [Google Scholar] [CrossRef]
- Qu, J.; He, X.; Chen, M.; Huang, P.; Zhang, Q.; Liu, X. A facile mechanochemical approach to synthesize Zn-Al layered double hydroxide. Solid State Chem. 2017, 250, 1–5. [Google Scholar] [CrossRef]
- Zhong, L.; He, X.; Qu, J.; Li, X.; Lei, Z.; Zhang, Q.; Liu, X. Precursor preparation for Ca-Al layered double hydroxide to remove hexavalent chromium co-existing with calcium and magnesium chloride. Solid State Chem. 2017, 245, 200–206. [Google Scholar] [CrossRef]
- Fahami, A.; Beall, G.W. Mechanosynthesis and characterization of Hydrotalcite like Mg–Al–SO4-LDH. Mater. Lett. 2016, 165, 192–195. [Google Scholar]
- Qu, J.; Zhong, L.; Li, Z.; Chen, M.; Zhang, Q.; Liu, X. Effect of anion addition on the syntheses of Ca–Al layered double hydroxide via a two-step mechanochemical process. Appl. Clay Sci. 2016, 124, 267–270. [Google Scholar] [CrossRef]
- Szabados, M.; Mészáros, R.; Erdei, S.; Kónya, Z.; Kukovecz, Á.; Sipos, P.; Pálinkó, I. Ultrasonically enhanced mechanochemical synthesis of Ca-Al-layered double hydroxides intercalated by a variety of inorganic anions. Ultrason. Sonochem. 2016, 31, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Szabados, M.; Pásztor, K.; Csendes, Z.; Muráth, S.; Kónya, Z.; Kukovecz, Á.; Carlson, S.; Sipos, P.; Pálinkó, I. Synthesis of high-quality, well-characterised CaAlFe layered triple hydroxide with the combination of dry-milling and ultrasonic irradiation in aqueous solution at elevated temperature. Ultrason. Sonochem. 2016, 32, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Szabados, M.; Varga, G.; Kónya, Z.; Kukovecz, Á.; Carlson, S.; Sipos, P.; Pálinkó, I. Ultrasonically-enhanced preparation, characterization of Ca-Fe-layered double hydroxides with various interlayer halide, azide and oxo anions (CO32−,NO3−,ClO4−). Ultrason. Sonochem. 2018, 40, 853–860. [Google Scholar] [CrossRef]
- Iwasaki, T.; Yoshii, H.; Nakamura, H.; Watano, S. Simple and rapid synthesis of Ni-Fe Layered double hydroxide by a new mechanochemical method. Appl. Clay Sci. 2012, 58, 120–124. [Google Scholar] [CrossRef]
- Zhang, X.; Qi, F.; Li, S.; Wei, S.; Zhou, K. A mechanochemical approach to get stunningly uniform magnesium aluminium layered double hydroxides. Appl. Surf. Sci. 2012, 259, 245–251. [Google Scholar] [CrossRef]
- Labuschagne, F.J.W.J.; Molefe, D.M.; Focke, W.W.; van der Westhuizen, I.; Wright, H.C.; Royeppen, M.D. Heat stabilising flexible PVC with layered double hydroxide derivatives. Polym. Degrad. 2015, 113, 49–54. [Google Scholar] [CrossRef]
- Rosenberg, S.P.; Wilson, D.J.; Heath, C.A. Some Aspects of Calcium Chemistry in the Bayer Process. Light Met. 2001, 1, 210–216. [Google Scholar]
- Kano, J.; Yamashita, J.; Saito, F. Effect of heat-assisted grinding of calcium hydroxide-gibbsite mixture on formation of hydrated calcium aluminate and its hydration behavior. Powder Technol. 1998, 98, 279–280. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Ramen Characteristic Group Frequencies: Tables and Charts, 1st ed.; John Wiley and Sons: Hoboken, NJ, USA, 2001; pp. 287–295. [Google Scholar]
- Qu, J.; He, X.; Li, X.; Ai, Z.; Li, Y.; Zhang, Q.; Liu, X. Precursor preparation of Zn–Al layered double hydroxide by ball milling for enhancing adsorption and photocatalytic decoloration of methyl orange. RSC Adv. 2017, 7, 31466–31474. [Google Scholar] [CrossRef]
- Qu, J.; He, Z.; Lei, Q.; Zhang, Q.; Liu, X. Mechanochemical synthesis of dodecyl sulfate anion intercalated Cu-Al layered double hydroxide. Solid State Sci. 2017, 74, 125–130. [Google Scholar] [CrossRef]



| Sample | D10 [μm] | D50 [μm] | D90 [μm] |
|---|---|---|---|
| S1 | 2.35 | 7.79 | 17.6 |
| S2 | 1.66 | 7.22 | 18.8 |
| S3 | 1.98 | 7.84 | 23.6 |
| S4 | 2.29 | 9.17 | 686 |
| S5 | 1.71 | 4.13 | 10.5 |
| S6 | 1.34 | 5.55 | 15.6 |
| Sample | D10 [μm] | D50 [μm] | D90 [μm] |
|---|---|---|---|
| S1 | 0.962 | 3.39 | 6.21 |
| S2 | 0.693 | 2.33 | 4.94 |
| S3 | 0.594 | 1.70 | 36.9 |
| S4 | 0.661 | 2.71 | 86.0 |
| S5 | 0.77 | 2.51 | 4.83 |
| S6 | 0.764 | 2.43 | 4.93 |
| Sample | Expected MII:MIII Ratio | Calculated MII:MIII Ratio |
|---|---|---|
| S1 | 2.00: 1.00 | 2.00:1.04 |
| S2 | 3.00:1.00 | 3.00:1.04 |
| S3 | 2.00:1.00 | 2.00:1.09 |
| S4 | 2.00:1.00 | 2.00:0.94 |
| S5 | 1.00:1.00 | 1.00:1.01 |
| S6 | 2.00:1.00 | 2.00:10.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barnard, B.A.; Labuschagné, F.J.W.J. Exploring the Wet Mechanochemical Synthesis of Mg-Al, Ca-Al, Zn-Al and Cu-Al Layered Double Hydroxides from Oxides, Hydroxides and Basic Carbonates. Crystals 2020, 10, 954. https://doi.org/10.3390/cryst10100954
Barnard BA, Labuschagné FJWJ. Exploring the Wet Mechanochemical Synthesis of Mg-Al, Ca-Al, Zn-Al and Cu-Al Layered Double Hydroxides from Oxides, Hydroxides and Basic Carbonates. Crystals. 2020; 10(10):954. https://doi.org/10.3390/cryst10100954
Chicago/Turabian StyleBarnard, Brenda Antoinette, and Frederick Johannes Willem Jacobus Labuschagné. 2020. "Exploring the Wet Mechanochemical Synthesis of Mg-Al, Ca-Al, Zn-Al and Cu-Al Layered Double Hydroxides from Oxides, Hydroxides and Basic Carbonates" Crystals 10, no. 10: 954. https://doi.org/10.3390/cryst10100954
APA StyleBarnard, B. A., & Labuschagné, F. J. W. J. (2020). Exploring the Wet Mechanochemical Synthesis of Mg-Al, Ca-Al, Zn-Al and Cu-Al Layered Double Hydroxides from Oxides, Hydroxides and Basic Carbonates. Crystals, 10(10), 954. https://doi.org/10.3390/cryst10100954