Processing of Functional Composite Resins Using Deep Eutectic Solvent
Abstract
:1. Introduction
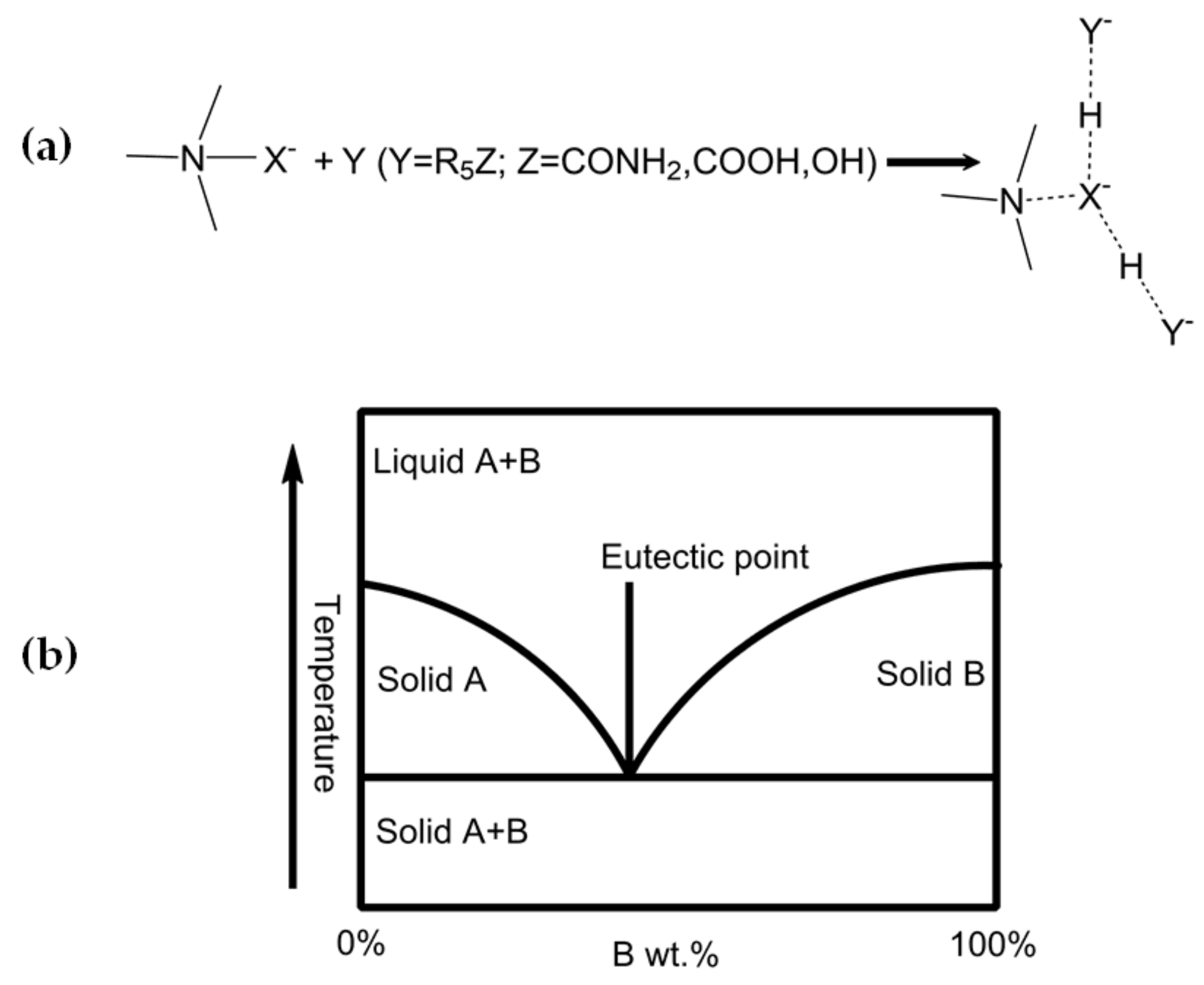
2. Deep Eutectic Solvent
3. Composite Resins Processing with DES
3.1. Epoxy Resin
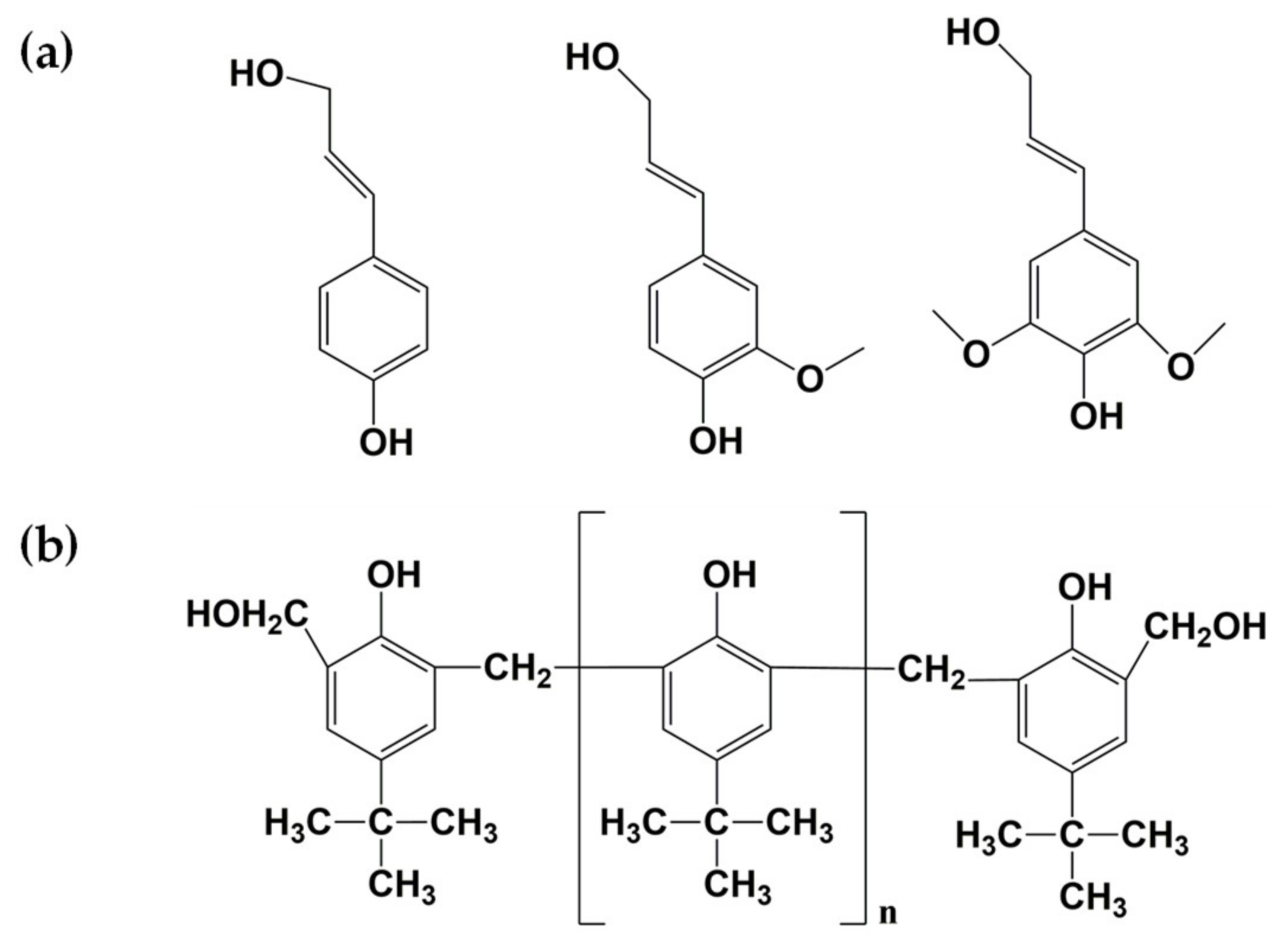
3.2. Phenolic Resin
3.3. Acrylic Resins
3.4. Polyester Resin
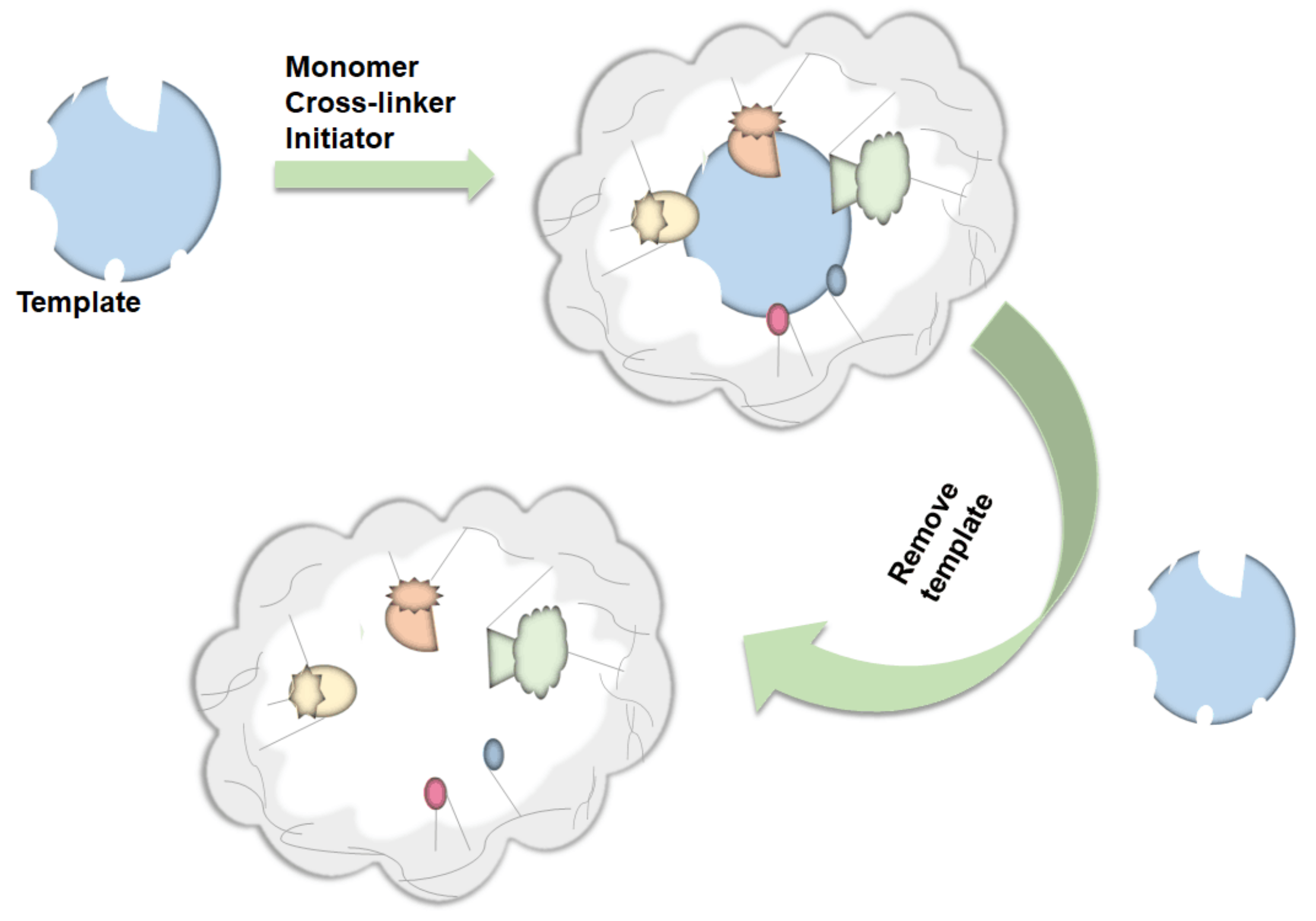
3.5. Imprinted Resin
4. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Post, W.; Susa, A.; Blaauw, R.; Molenveld, K.; Knoop, R.J.I. A Review on the potential and limitations of recyclable thermosets for structural applications. Polym. Rev. 2020, 60, 359–388. [Google Scholar] [CrossRef]
- Cabanes, A.; Valdés, F.J.; Fullana, A. A review on VOCs from recycled plastics. Sustain. Mater. Technol. 2020, 25, e00179. [Google Scholar] [CrossRef]
- Bhattacharya, A. Grafting: A versatile means to modify polymers techniques, factors and applications. Prog. Polym. Sci. 2004, 29, 767–814. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 9, 70–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, A.P.; Boothby, D.; Capper, G. Deep eutectic solvents formed between choline chloride and carboxylic acids: Versatile alternatives to ionic liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jerome, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Row, K.H. Hydrophobic ionic liquid modified thermoresponsive molecularly imprinted monolith for the selective recognition and separation of tanshinones. J. Sep. Sci. 2018, 41, 3372–3381. [Google Scholar] [CrossRef]
- Tang, W.; Ho Row, K. Evaluation of CO2-induced azole-based switchable ionic liquid with hydrophobic/hydrophilic reversible transition as single solvent system for coupling lipid extraction and separation from wet microalgae. Bioresour. Technol. 2020, 296, 122309. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L. Solubility of metal oxides in deep eutectic solvents based on choline chloride. J. Chem. Eng. Data 2006, 51, 1280–1282. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Shishov, A.; Pochivalov, A.; Nugbienyo, L.; Andruch, V.; Bulatov, A. Deep eutectic solvents are not only effective extractants. Trac Trend Anal. Chem. 2020, 129, 115956. [Google Scholar] [CrossRef]
- Vilková, M.; Płotka-Wasylka, J.; Andruch, V. The role of water in deep eutectic solvent-base extraction. J. Mol. Liq. 2020, 304, 112747. [Google Scholar] [CrossRef]
- Lou, R.; Ma, R.; Lin, K.; Ahamed, A.; Zhang, X. Facile extraction of wheat straw by deep eutectic solvent (DES) to produce lignin nanoparticles. ACS Sustain. Chem. Eng. 2019, 7, 10248–10256. [Google Scholar] [CrossRef]
- Tang, W.; Gao, F.; Duan, Y.; Zhu, T.; Ho Row, K. Exploration of deep eutectic solvent-based molecularly imprinted polymers as solid-phase extraction sorbents for screening chloramphenicol in milk. J. Chromatogr. Sci. 2017, 55, 654–661. [Google Scholar] [CrossRef] [Green Version]
- Carriazo, D.A.; Gutierrez, M.C.; Ferrer, M.L.; del Monte, F. Deep-eutectic solvents playing multiple roles in the synthesis of polymers and related materials. Chem. Soc. Rev. 2012, 41, 4996–5014. [Google Scholar] [CrossRef]
- Tang, B.; Row, K.H. Recent developments in deep eutectic solvents in chemical sciences. Mon. Für Chem. Chem. Mon. 2013, 144, 1427–1454. [Google Scholar] [CrossRef]
- Francisco, M.D.; van den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chem. Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Ge, X.; Gu, C.; Wang, X.; Tu, J. Deep eutectic solvents (DESs)-derived advanced functional materials for energy and environmental applications: Challenges, opportunities, and future vision. J. Mater. Chem. A 2017, 5, 8209–8229. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Frisch, G.; Garrett, H.; Hartley, J. Ionic liquids form ideal solutions. Chem. Commun. 2011, 47, 11876. [Google Scholar] [CrossRef]
- Perna, F.M.; Vitale, P.; Capriati, V. Deep eutectic solvents and their applications as green solvents. Curr. Opin. Green Sustain. Chem. 2020, 21, 27–33. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Wu, X.; Li, G. Theoretical study on the structures and properties of mixtures of urea and choline chloride. J. Mol. Modeling 2013, 19, 2433–2441. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xue, J.; Dong, X.; Yu, Q.; Baker, S.; Wang, M.; Huang, H. Antimicrobial properties of benzalkonium chloride derived polymerizable deep eutectic solvent. Int. J. Pharm. 2020, 575, 119005. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Kumar, N.; Lynam, J.G. Theoretical and experimental study of choline chloride-carboxylic acid deep eutectic solvents and their hydrogen bonds. J. Mol. Struct. 2020, 1222, 128849. [Google Scholar] [CrossRef]
- Troter, D.; Đokić-Stojanović, D.; Đordević, B.; Todorovic, V.; Konstantinović, S.; Veljković, V. The physicochemical and thermodynamic properties of the choline chloride-based deep eutectic solvents. J. Serb. Chem. Soc. 2017, 82, 1039–1052. [Google Scholar] [CrossRef] [Green Version]
- Aroso, I.M.; Craveiro, R.; Dionisio, M.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Fundamental studies on natural deep eutectic solvents: Physico-chemical, thermal and rheological properties. In Proceedings of the 6th Nordic Wood Biorefinery Conference, Helsinki, Finland, 20–22 October 2015; pp. 155–160. [Google Scholar]
- Tomai, P.; Lippiello, A.; D’Angelo, P.; Persson, I.; Martinelli, A.; Di Lisio, V.; Curini, R.; Fanali, C.; Gentili, A. A low transition temperature mixture for the dispersive liquid-liquid microextraction of pesticides from surface waters. J. Chromatogr. 2019, 1605, 360329. [Google Scholar] [CrossRef]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. New natural and renewable low transition temperature mixtures (LTTMs): Screening as solvents for lignocellulosic biomass processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [Green Version]
- Stefanovic, R.; Webber, G.B.; Page, A.J. Polymer solvation in choline chloride deep eutectic solvents modulated by the hydrogen bond donor. J. Mol. Liq. 2019, 279, 584–593. [Google Scholar] [CrossRef]
- Tiecco, M.; Cappellini, F.; Nicoletti, F.; Del Giacco, T.; Germani, R.; Di Profio, P. Role of the hydrogen bond donor component for a proper development of novel hydrophobic deep eutectic solvents. J. Mol. Liq. 2019, 281, 423–430. [Google Scholar] [CrossRef]
- Tang, W.; Dai, Y.; Row, K.H. Evaluation of fatty acid/alcohol-based hydrophobic deep eutectic solvents as media for extracting antibiotics from environmental water. Anal. Bioanal. Chem. 2018, 410, 7325–7336. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Row, K.H. Design and evaluation of polarity controlled and recyclable deep eutectic solvent based biphasic system for the polarity driven extraction and separation of compounds. J. Clean. Prod. 2020, 268, 122306. [Google Scholar] [CrossRef]
- Tang, W.; An, Y.; Row, K.H. Recoverable deep eutectic solvent-based aniline organic pollutant separation technology using choline salt as adsorbent. J. Mol. Liq. 2020, 306, 112910. [Google Scholar] [CrossRef]
- Sas, O.G.; Fidalgo, R.; Domínguez, I.; Macedo, E.A.; González, B. Physical properties of the pure deep eutectic solvent, [ChCl]: [Lev] (1:2) DES, and its binary mixtures with alcohols. J. Chem. Eng. Data 2016, 61, 4191–4202. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Gray, S. Design of improved deep eutectic solvents using hole theory. Chemphyschem 2006, 7, 803–806. [Google Scholar] [CrossRef]
- Chen, W.; Xue, Z.; Wang, J.; Jiang, J.; Zhao, X.; Mu, T. Investigation on the thermal stability of deep eutectic solvents. Acta Phys. Chim. Sin. 2018, 34, 904–911. [Google Scholar] [CrossRef]
- Ghaedi, H.; Ayoub, M.; Sufian, S.; Lal, B.; Uemura, Y. Thermal stability and FT-IR analysis of Phosphonium-based deep eutectic solvents with different hydrogen bond donors. J. Mol. Liq. 2017, 242, 395–403. [Google Scholar] [CrossRef]
- Saputra, R.; Walvekar, R.; Khalid, M.; Mubarak, N.M. Synthesis and thermophysical properties of ethylammonium chloride-glycerol-ZnCl2 ternary deep eutectic solvent. J. Mol. Liq. 2020, 310, 113232. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Al-Saadi, M.A.; Hayyan, A.; AlNashef, I.M.; Mirghani, M.E.S. Assessment of cytotoxicity and toxicity for phosphonium-based deep eutectic solvents. Chemosphere 2013, 93, 455–459. [Google Scholar] [CrossRef]
- Hayyan, M.; Looi, C.Y.; Hayyan, A.; Wong, W.F.; Hashim, M.A. In vitro and in vivo toxicity profiling of ammonium-based deep eutectic solvents. PLoS ONE 2015, 10, 0117934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torregrosa-Crespo, J.; Marset, X.; Guillena, G.; Ramón, D.J.; María Martínez-Espinosa, R. New guidelines for testing “Deep eutectic solvents” toxicity and their effects on the environment and living beings. Sci. Total Environ. 2020, 704, 135382. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, J.X.; Tang, Y.L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef]
- Das, S.; Mondal, A.; Balasubramanian, S. Recent advances in modeling green solvents. Curr. Opin. Green Sustain. Chem. 2017, 5, 37–43. [Google Scholar] [CrossRef]
- Lionetto, F.; Timo, A.; Frigione, M. Cold-cured epoxy-based organic-inorganic hybrid resins containing deep eutectic solvents. Polymers 2018, 11, 14. [Google Scholar] [CrossRef] [Green Version]
- Maka, H.; Spychaj, T. Epoxy resin crosslinked with conventional and deep eutectic ionic liquids. Polim. Polym. 2012, 57, 34–40. [Google Scholar] [CrossRef]
- Mąka, H.; Spychaj, T.; Adamus, J. Lewis acid type deep eutectic solvents as catalysts for epoxy resin crosslinking. RSC Adv. 2015, 5, 82813–82821. [Google Scholar] [CrossRef]
- Staciwa, P.; Spychaj, T. New aromatic diamine-based deep eutectic solvents designed for epoxy resin curing. Polimery 2018, 63, 453–457. [Google Scholar] [CrossRef]
- Gholami, H.; Arab, H.; Mokhtarifar, M.; Maghrebi, M.; Baniadam, M. The effect of choline-based ionic liquid on CNTs’ arrangement in epoxy resin matrix. Mater. Des. 2016, 91, 180–185. [Google Scholar] [CrossRef]
- Mąka, H.; Spychaj, T.; Kowalczyk, K. Imidazolium and deep eutectic ionic liquids as epoxy resin crosslinkers and graphite nanoplatelets dispersants. J. Appl. Polym. Sci. 2014, 131, 40401. [Google Scholar] [CrossRef]
- Guo, L.; Gu, C.; Feng, J.; Guo, Y.; Jin, Y.; Tu, J. Hydrophobic epoxy resin coating with ionic liquid conversion pretreatment on magnesium alloy for promoting corrosion resistance. J. Mater. Sci. Technol. 2020, 37, 9–18. [Google Scholar] [CrossRef]
- Lian, H.; Hong, S.; Carranza, A.; Mota-Morales, J.D.; Pojman, J.A. Processing of lignin in urea–zinc chloride deep-eutectic solvent and its use as a filler in a phenol-formaldehyde resin. RSC Adv. 2015, 5, 28778–28785. [Google Scholar] [CrossRef]
- Hong, S.; Sun, X.; Lian, H.; Pojman, J.A.; Mota-Morales, J.D. Zinc chloride/acetamide deep eutectic solvent-mediated fractionation of lignin produces high- and low-molecular-weight fillers for phenol-formaldehyde resins. J. Appl. Polym. Sci. 2019, 137, 48385. [Google Scholar] [CrossRef]
- Cao, X.; Hong, S.; Ding, Q.; Chen, F.; Zhu, M.; Lian, H. Lignin modified by DES with choline chloride/Zinc chloride to prepared phenol-formaldehyde resin adhesive. China For. Prod. Ind. 2016, 43, 25–29. [Google Scholar]
- Zhong, M.; Liu, H.; Wang, M.; Zhu, Y.W.; Chen, X.Y.; Zhang, Z.J. Hierarchically N/O-enriched nanoporous carbon for supercapacitor application: Simply adjusting the composition of deep eutectic solvent as well as the ratio with phenol-formaldehyde resin. J. Power Sources 2019, 438, 226982. [Google Scholar] [CrossRef]
- Deng, J.; Chen, L.; Hong, S.; Lian, H. UZnCl2-DES assisted synthesis of phenolic resin-based carbon aerogels for capacitors. J. Porous Mater. 2020, 27, 789–800. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Dai, Q.; Zhou, Y. Magnetic deep eutectic solvents molecularly imprinted polymers for the selective recognition and separation of protein. Analytica Chimica Acta 2016, 93, 168–178. [Google Scholar] [CrossRef]
- Fazende, K.F.; Phachansitthi, M.; Mota-Morales, J.D.; Pojman, J.A. Frontal polymerization of deep eutectic solvents composed of acrylic and methacrylic acids. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 4046–4050. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Gutiérrez, M.C.; Ferrer, M.L.; Jiménez, R.; Santiago, P.; Sanchez, I.C.; Terrones, M.; Del Monte, F.; Luna-Bárcenas, G. Synthesis of macroporous poly (acrylic acid)-carbon nanotube composites by frontal polymerization in deep-eutectic solvents. J. Mater. Chem. 2013, 1, 3970–3976. [Google Scholar] [CrossRef]
- Mota-Morales, J.D.; Gutiérrez, M.C.; Ferrer, M.L.; Sanchez, I.C.; Elizalde-Peña, E.A.; Pojman, J.A.; Monte, F.D.; Luna-Bárcenas, G. Deep eutectic solvents as both active fillers and monomers for frontal polymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 1767–1773. [Google Scholar] [CrossRef]
- Li, R.; Chen, G.; He, M.; Tian, J.; Su, B. Patternable transparent and conductive elastomers towards flexible tactile/strain sensors. J. Mater. Chem. C 2017, 5, 8475–8481. [Google Scholar] [CrossRef]
- Li, R.; Zhang, K.; Chen, G.; Su, B.; Tian, J.; He, M.; Lu, F. Green polymerizable deep eutectic solvent (PDES) type conductive paper for origami 3D circuits. Chem. Commun. 2018, 54, 2304–2307. [Google Scholar]
- Wang, M.; Li, R.; Chen, G.; Zhou, S.; Feng, X.; Chen, Y.; He, M.; Liu, D.; Song, T.; Qi, H. Highly Stretchable, Transparent, and Conductive Wood Fabricated by in Situ Photopolymerization with Polymerizable Deep Eutectic Solvents. ACS Appl. Mater. Interfaces 2019, 11, 14313–14321. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-M.; Cho, J.Y. Microwave-mediated rapid tailoring of PET fabric surface by using environmentally-benign, biodegradable Urea-Choline chloride Deep eutectic solvent. Fibers Polym. 2016, 17, 847–856. [Google Scholar] [CrossRef]
- Cho, J.Y.; Choi, H.-M.; Oh, K.W. Rapid hydrophilic modification of poly (ethylene terephthalate) surface by using deep eutectic solvent and microwave irradiation. Text. Res. J. 2016, 86, 1318–1327. [Google Scholar] [CrossRef]
- Choi, S.; Choi, H.-M. Eco-friendly, expeditious depolymerization of PET in the blend fabrics by using a bio-based deep eutectic solvent under microwave irradiation for composition identification. Fibers Polym. 2019, 20, 752–759. [Google Scholar] [CrossRef]
- Sert, E.; Yılmaz, E.; Atalay, F.S. Chemical recycling of polyethlylene terephthalate by glycolysis using deep eutectic solvents. J. Polym. Environ. 2019, 27, 2956–2962. [Google Scholar] [CrossRef]
- Liu, B.; Fu, W.; Lu, X.; Zhou, Q.; Zhang, S. Lewis acid-base synergistic catalysis for polyethylene terephthalate degradation by 1,3-dimethylurea/Zn (OAc)2 deep eutectic solvent. ACS Sustain. Chem. Eng. 2019, 7, 3292–3300. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, X.; Geng, Y.; Zhou, Q.; Lu, X.; Zhang, S. Deep eutectic solvents as highly active catalysts for the fast and mild glycolysis of poly (ethylene terephthalate) (PET). Green Chem. 2015, 17, 2473–2479. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, X.; Ju, Z.; Liu, B.; Yao, H.; Xu, J.; Zhou, Q.; Hu, Y.; Zhang, S. Alcoholysis of polyethylene terephthalate to produce dioctyl terephthalate using choline chloride-based deep eutectic solvents as efficient catalysts. Green Chem. 2019, 21, 897–906. [Google Scholar] [CrossRef]
- Musale, R.M.; Shukla, S.R. Deep eutectic solvent as effective catalyst for aminolysis of polyethylene terephthalate (PET) waste. Int. J. Plast. Technol. 2016, 20, 106–120. [Google Scholar] [CrossRef]
- García-Argüelles, S.; García, C.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Near-to-eutectic mixtures as bifunctional catalysts in the low-temperature-ring-opening-polymerization of ε-caprolactone. Green Chem. 2015, 17, 3632–3643. [Google Scholar] [CrossRef]
- Pradeepkumar, P.; Rajendran, N.K.; Alarfaj, A.A.; Munusamy, M.A.; Rajan, M. Deep eutectic solvent-mediated FA-g-β-alanine-co-PCL drug carrier for sustainable and site-specific drug delivery. ACS Appl. Bio. Mater. 2018, 1, 2094–2109. [Google Scholar] [CrossRef]
- Garcia-Arguelles, S.; Serrano, C.M.; Gutierrez, M.C.; Ferrer, L.M.; Yuste, L.; Rojo, F.; del Monte, F. Deep eutectic solvent-assisted synthesis of biodegradable polyesters with antibacterial properties. Langmuir 2013, 29, 9525–9534. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Han, J.; Khan, M.Y.; He, D.; Peng, H.; Chen, D.; Xie, X.; Xue, Z. Deep eutectic solvents for green and efficient iron-mediated ligand-free atom transfer radical polymerization. Polym. Chem. 2017, 8, 1616–1627. [Google Scholar] [CrossRef]
- Wang, J.; Dong, X.; Yu, Q.; Baker, S.N.; Li, H.; Larm, N.E.; Baker, G.A.; Chen, L.; Tan, J.; Chen, M. Incorporation of antibacterial agent derived deep eutectic solvent into an active dental composite. Dent. Mater. 2017, 33, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; Liu, X.; Li, L.; Tang, B.; Row, K.H. Ternary choline chloride/caffeic acid/ethylene glycol deep eutectic solvent as both a monomer and template in a molecularly imprinted polymer. J. Sep. Sci. 2017, 40, 2286–2291. [Google Scholar] [CrossRef]
- Li, G.; Tang, W.; Cao, W.; Wang, Q.; Zhu, T. Molecularly imprinted polymers combination with deep eutectic solvents for solid-phase extraction of caffeic acid from hawthorn. Chin. J. Chromatogr. 2015, 33, 792–798. [Google Scholar] [CrossRef]
- Tang, W.; Row, K.H. Fabrication of water-compatible molecularly imprinted resin in a hydrophilic deep eutectic solvent for the determination and purification of quinolones in wastewaters. Polymers 2019, 11, 871. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Yan, H.; Cao, J.; Han, Y.; Shen, S.; Bai, L. Molecularly imprinted phloroglucinol-formaldehyde-melamine resin prepared in a deep eutectic solvent for selective recognition of clorprenaline and bambuterol in urine. Anal. Chim. Acta 2017, 951, 68–77. [Google Scholar] [CrossRef]
- Xu, K.; Wang, Y.; Wei, X.; Chen, J.; Xu, P.; Zhou, Y. Preparation of magnetic molecularly imprinted polymers based on a deep eutectic solvent as the functional monomer for specific recognition of lysozyme. Mikrochim. Acta 2018, 185, 146. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Chruściel, J.J.; Leśniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Wu, J.; Yuan, L.; Wang, Y.; Du, X.; Wang, R.; Marwa, P.W.; Petlulu, P. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Chen, X.; Hou, J.; Gu, Q.; Wang, Q.; Gao, J.; Sun, J.; Fang, Q. A non-bisphenol-A epoxy resin with high Tg derived from the bio-based protocatechuic Acid:Synthesis and properties. Polymer 2020, 195, 122443. [Google Scholar] [CrossRef]
- Hsu, Y.-I.; Huang, L.; Asoh, T.-A.; Uyama, H. Anhydride-cured epoxy resin reinforcing with citric acid-modified cellulose. Polym. Degrad. Stab. 2020, 178, 109213. [Google Scholar] [CrossRef]
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Carvalho, A.P.A.; Santos, D.F.; Soares, B.G. Epoxy/imidazolium-based ionic liquid systems: The effect of the hardener on the curing behavior, thermal stability, and microwave absorbing properties. J. Appl. Polym. Sci. 2020, 137, 48326. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Livi, S.; Soares, B.G.; Pruvost, S.; Duchet-Rumeau, J.; Gérard, J.-F. Ionic liquids: A new route for the design of epoxy networks. ACS Sustain. Chem. Eng. 2015, 4, 481–490. [Google Scholar] [CrossRef]
- Sidorov, O.I.; Vygodskii, Y.S.; Lozinskaya, E.I.; Milekhin, Y.M.; Matveev, A.A.; Poisova, T.P.; Ferapontov, F.V.; Sokolov, V.V. Ionic liquids as curing catalysts for epoxide-containing compositions. Polym. Sci. Ser. D 2017, 10, 134–142. [Google Scholar] [CrossRef]
- Donato, R.K.; Matějka, L.; Schrekker, H.S.; Pleštil, J.; Jigounov, A.; Brus, J.; Šlouf, M. The multifunctional role of ionic liquids in the formation of epoxy-silica nanocomposites. J. Mater. Chem. 2011, 21, 13801–13810. [Google Scholar] [CrossRef]
- Doolan, P.C.; Gore, P.H.; Hollingworth, R.H.; Waters, D.N.; Al-Ka’Bi, J.F.; Farooqi, J.A. Kinetic studies of lewis acidity. Part 2. Catalysis by tin (IV) chloride, by some organotin (IV) chlorides, and by tin (II) chloride of the anionotropic rearrangement of henylpropnl in tetramethylene sulphone solution. J. Chem. Soc. 1986, 17, 501–506. [Google Scholar] [CrossRef]
- Mąka, H.; Spychaj, T.; Sikorski, W. Deep eutectic ionic liquids as epoxy resin curing agents. Int. J. Polym. Anal. Charact. 2014, 19, 682–692. [Google Scholar] [CrossRef]
- Lionetto, F.; Timo, A.; Frigione, M. Curing kinetics of epoxy-deep eutectic solvent mixtures. Thermochim. Acta 2015, 612, 70–78. [Google Scholar] [CrossRef]
- Chen, X.; Xiong, L.; Li, H.; Chen, X. Research progress of deep eutectic solvent in lignocellulose pretreatment to promote enzymatic hydrolysis efficiency. Adv. New Renew. Energy 2019, 7, 415–422. [Google Scholar]
- Xie, Y.; Guo, X.; Lv, Y.; Wang, H. Research progress of deep eutectic solvent in the dissolution and separation of fiber raw materials. Chem. Ind. For. Prod. 2019, 39, 11–18. [Google Scholar]
- Sun, X.; Ling, C.; Lian, H. The pyrolysis and pyrolysis kinetics of phenol-formaldehyde resin modified with lignin in deep eutectic solvent. China For. Prod. Ind. 2016, 43, 19–24. [Google Scholar]
- Muylaert, I.; Verberckmoes, A.; De Decker, J.; Van Der Voort, P. Ordered mesoporous phenolic resins: Highly versatile and ultra stable support materials. Adv. Colloid Interface Sci. 2012, 175, 39–51. [Google Scholar] [CrossRef]
- Effendi, A.; Gerhauser, H.; Bridgwater, A.V. Production of renewable phenolic resins by thermochemical conversion of biomass: A review. Renew. Sustain. Energy Rev. 2008, 12, 2092–2116. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.-P.; Zhou, P.; Liu, Z.-Q.; Luo, S.-Z.; Chu, W.; Guo, Z. Formation of poly (acrylic acid)/alumina composite via in situ polymerization of acrylic acid adsorbed within oxide pores. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Patil, Y.; Ameduri, B. Advances in the (co)polymerization of alkyl 2-trifluoromethacrylates and 2-(trifluoromethyl) acrylic acid. Prog. Polym. Sci. 2013, 38, 703–739. [Google Scholar] [CrossRef]
- Ballard, N.; Asua, J.M. Radical polymerization of acrylic monomers: An overview. Prog. Polym. Sci. 2018, 79, 40–60. [Google Scholar] [CrossRef]
- Sánchez-Leija, R.J.; Pojman, J.A.; Luna-Bárcenas, G.; Mota-Morales, J.D. Controlled release of lidocaine hydrochloride from polymerized drug-based deep-eutectic solvents. J. Mater. Chem. B 2014, 2, 7495–7501. [Google Scholar] [CrossRef] [PubMed]
- Tomé, L.I.N.; Baião, V.; da Silva, W.; Brett, C.M.A. Deep eutectic solvents for the production and application of new materials. Appl. Mater. Today 2018, 10, 30–50. [Google Scholar] [CrossRef]
- Pang, K.; Kotek, R.; Tonelli, A.E. Review of conventional and novel polymerization processes for polyesters. Prog. Polym. Sci. 2006, 31, 1009–1037. [Google Scholar] [CrossRef]
- Zhang, X. Hyperbranched aromatic polyesters: From synthesis to applications. Prog. Org. Coat. 2010, 69, 295–309. [Google Scholar] [CrossRef]
- Gao, Y.; Romero, P.; Zhang, H.; Huang, M.; Lai, F. Unsaturated polyester resin concrete: A review. Constr. Build. Mater. 2019, 228, 116709. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, X.; Yao, H.; Zhou, Q.; Xin, J.; Lu, X.; Zhang, S. Degradation of poly (ethylene terephthalate) catalyzed by metal-free choline-based ionic liquids. Green Chem. 2020, 22, 3122–3131. [Google Scholar] [CrossRef]
- Zhou, X.D.; Chen, K.; Guo, J.L.; Zhang, Y.F. Synthesis and application of quaternary ammonium-functionalized hyperbranched polyester Antibacterial Agent. Adv. Mater. Res. 2013, 796, 412–415. [Google Scholar] [CrossRef]
- Bures, F. Quaternary ammonium compounds: Simple in structure, complex in application. Top. Curr. Chem. 2019, 377, 14. [Google Scholar] [CrossRef]
- Vasapollo, G.; Sole, R.D.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly imprinted polymers: Present and future prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turiel, E.; Esteban, A.M. Molecularly imprinted polymers. Solid Phase Extr. 2020, 215–233. [Google Scholar]
- BelBruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Kartal, F.; Cimen, D.; Bereli, N.; Denizli, A. Molecularly imprinted polymer based quartz crystal microbalance sensor for the clinical detection of insulin. Mater. Sci. Eng. 2019, 97, 730–737. [Google Scholar] [CrossRef]
- Sibrian-Vazquez, M.; Spivak, D.A. Characterization of molecularly imprinted polymers employing crosslinkers with nonsymmetric polymerizable groups. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3668–3675. [Google Scholar] [CrossRef]
| Deep Eutectic Solvent | Resin | Reference | ||
|---|---|---|---|---|
| Hydrogen-Bond Donor (HBD) | Hydrogen-Bond Acceptor (HBA) | Molar Ratio (HBD:HBA) | ||
| Urea | Choline chloride | 2:1 | Epoxy resin (silane-functionalized epoxy resin) | [46] |
| Imidazole | Choline chloride | 1:1 | Epoxy resin (bisphenol A-based low molecular weight epoxy resin) | [47] |
| ZnCl2/SnCl2 | Choline chloride | 2:1 | Epoxy resin (bisphenol A-based low molecular weight epoxy resin) | [48] |
| SnCl2 | Choline chloride | 2:1 | Epoxy resin (bisphenol A-based low molecular weight epoxy resin) | [48] |
| Aromatic amines (MPDA, DAT) | Choline chloride | 2:1 | Epoxy resin (bisphenol A-based low molecular weight epoxy resin) | [49] |
| Glycerol | Choline chloride | 2:1 | Epoxy resin (bisphenol F epoxy resin) | [50] |
| Ethylene glycol | Choline chloride | 2:1 | Epoxy resin (bisphenol F epoxy resin) | [50] |
| Oxalic acid | Choline chloride | 1:1 | Epoxy resin (bisphenol F epoxy resin) | [50] |
| Tris(hydroxymethyl)propane | Choline chloride | 1:1 | Epoxy resin (bisphenol A-based low molecular weight epoxy resin) | [51] |
| Urea | Choline chloride | 2:1 | Epoxy resin (waterborne epoxy emulsion) | [52] |
| Urea | ZnCl2 | 10:3 | Phenolic resin | [53] |
| ZnCl2 | Acetamide | 1:3 | Phenolic resin | [54] |
| ZnCl2 | Choline chloride | 2:1 | Phenolic resin | [55] |
| Urea | ZnCl2 | 1:1 | Phenolic resin | [56] |
| Urea | ZnCl2 | 10:3 | Phenolic resin | [57] |
| Itaconic acid | Choline chloride | 1:1 | Acrylic resins | [58] |
| Methacrylic acid | Choline chloride | 2:1 | Acrylic resins | [59] |
| Acrylic acid | Choline chloride | 1.6/2:1 | Acrylic resins | [60] |
| Acrylic acid | Choline chloride | 1.6/2:1 | Acrylic resins | [60] |
| Acrylic acid | Lidocaine hydrochloride | 3:1 | Acrylic resins | [61] |
| Acrylic acid | Choline chloride | 1.6/2:1 | Acrylic resins | [62] |
| Acrylic acid | Choline chloride | 1.6/2:1 | Acrylic resins | [63] |
| Acrylic acid | Choline chloride | 2:1 | Acrylic resins | [64] |
| Acrylic acid | Benzalkonium chloride | 2:1 | Acrylic resins | [23] |
| Urea | Choline chloride | 2:1 | Polyester resin (polyethylene terephthalate) | [65] |
| Ethylene glycol | Choline chloride | 2:1 | Polyester resin (polyethylene terephthalate) | [66] |
| Glycerol | Choline chloride | 2:1 | Polyester resin (polyethylene terephthalate) | [67] |
| Ethylene glycol | Potassium carbonate | 6:1 | Polyester resin (polyethylene terephthalate) | [68] |
| 1,3-dimethylurea | Zinc acetate | 4:1 | Polyester resin (polyethylene terephthalate) | [69] |
| Urea | Zinc chloride | 4:1 | Polyester resin (polyethylene terephthalate) | [70] |
| Choline chloride | Zinc acetate | 1:1 | Polyester resin (polyethylene terephthalate) | [71] |
| Urea | Choline chloride | 2:1 | Polyester resin (polyethylene terephthalate) | [72] |
| ZnCl2 | Choline chloride | 2:1 | Polyester resin (polyethylene terephthalate) | [72] |
| Methanesulfonic acid | Guanidine 1,5,7-triazabicyclo [4.4.0] dec-5-ene | 1.5:0.1 | Polyester resin (Polycaprolactone) | [73] |
| 1,4-butanediol | 3-(4-(4- (bis(2chloroethyl)amino)phenyl)butanoyloxy)-N,N,N-trimethylpropane-1-aminium chloride | 6/5:1 | Polyester resin (Polycaprolactone) | [74] |
| 1,8-octanediol | Tetraethylammonium bromide | 3:1 | Polyester resin (octanediol-co-citrate polyesters) | [75] |
| 1,8-octanediol | Hexadecyltrimethylammonium bromide | 3:1 | Polyester resin (octanediol-co-citrate polyesters) | [75] |
| 1,8-octanediol | Methyltriphenylphosphonium bromide | 3:0.75 | Polyester resin (octanediol-co-citrate polyesters) | [75] |
| Acetamide | Caprolactam | 1:1 | Polyester resin (methyl methacrylate) | [76] |
| Acetamide | Ammonium thiocyanate | 3:1 | Polyester resin (methyl methacrylate) | [76] |
| Ethylene | Tetrabutylammonium bromide | 2:1 | Polyester resin (methyl methacrylate) | [76] |
| Acrylic acid | Benzalkonium chloride | 2:1 | Polyester resin (ethoxylated bisphenol-a-glycidyl methacrylate and urethane dimethacrylate) | [77] |
| Caffeic acid | Choline chloride | 0.4:1:1 | Imprinted resin | [78] |
| Ethylene glycol | Choline chloride | 0.4:1:1 | Imprinted resin | [78] |
| Glycerol | Choline chloride | 2:1 | Imprinted resin | [79] |
| Ethylene glycol | Choline chloride | 2:1 | Imprinted resin | [80] |
| Ethylene glycol | Choline chloride | 2:1 | Imprinted resin | [81] |
| Ethylene glycol | Choline chloride | 1:1 | Imprinted resin | [14] |
| Glycerol | Choline chloride | 1:1 | Imprinted resin | [14] |
| Propylene glycol | Choline chloride | 1:1 | Imprinted resin | [14] |
| Glycerol | Allyl triethylammonium chloride | 1:1 | Imprinted resin | [82] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, J.; Wang, J.; Feng, D.; Huang, H.; Wang, M. Processing of Functional Composite Resins Using Deep Eutectic Solvent. Crystals 2020, 10, 864. https://doi.org/10.3390/cryst10100864
Xue J, Wang J, Feng D, Huang H, Wang M. Processing of Functional Composite Resins Using Deep Eutectic Solvent. Crystals. 2020; 10(10):864. https://doi.org/10.3390/cryst10100864
Chicago/Turabian StyleXue, Jing, Jing Wang, Daoshuo Feng, Haofei Huang, and Ming Wang. 2020. "Processing of Functional Composite Resins Using Deep Eutectic Solvent" Crystals 10, no. 10: 864. https://doi.org/10.3390/cryst10100864





