Controllable Synthesis of Metal-Organic Framework/Polyethersulfone Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
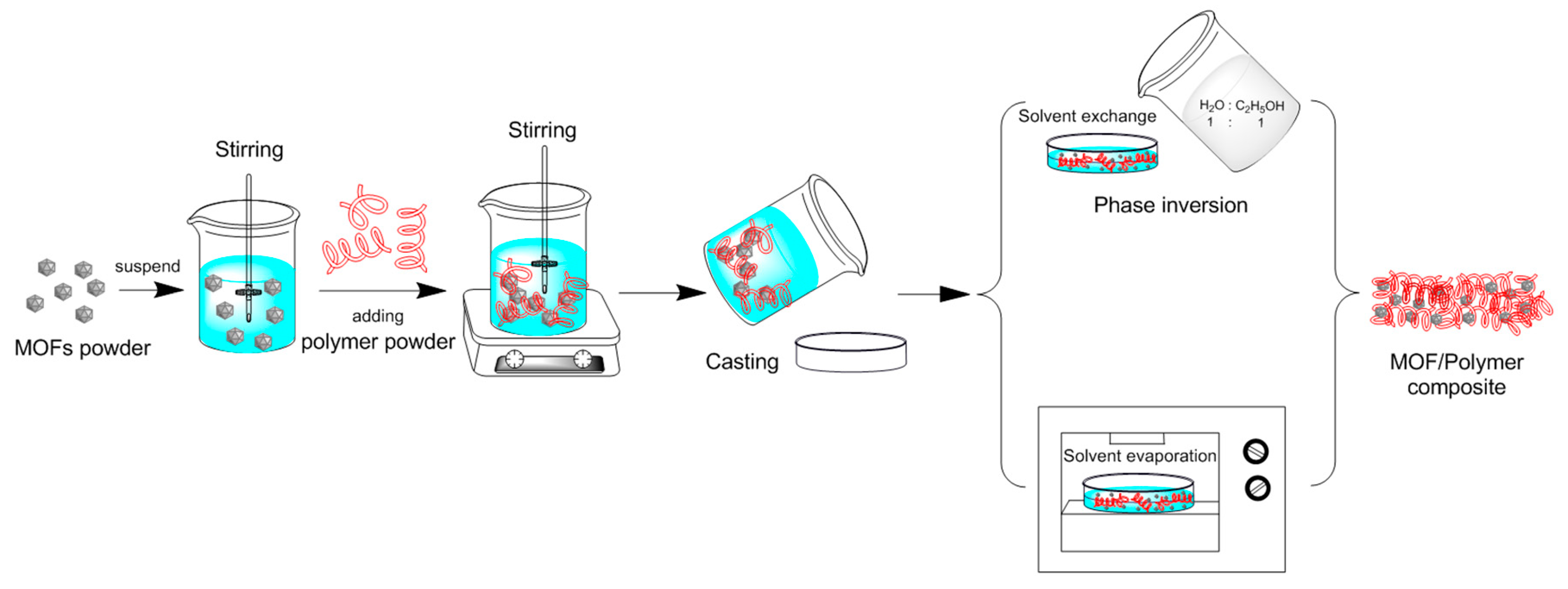
2.2. Synthesis of MOF and MOF/Polymer Composites
2.3. Measurements
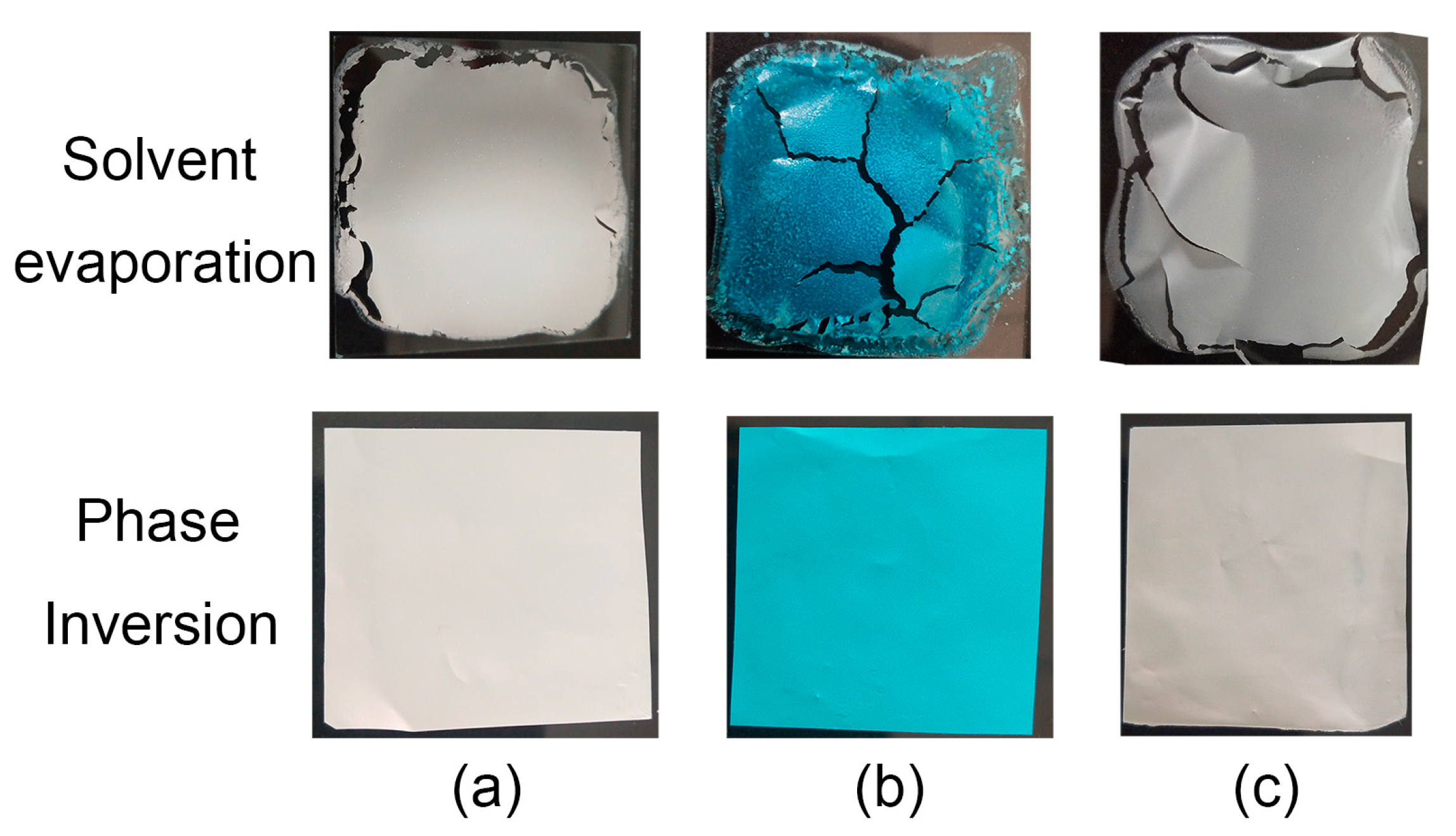
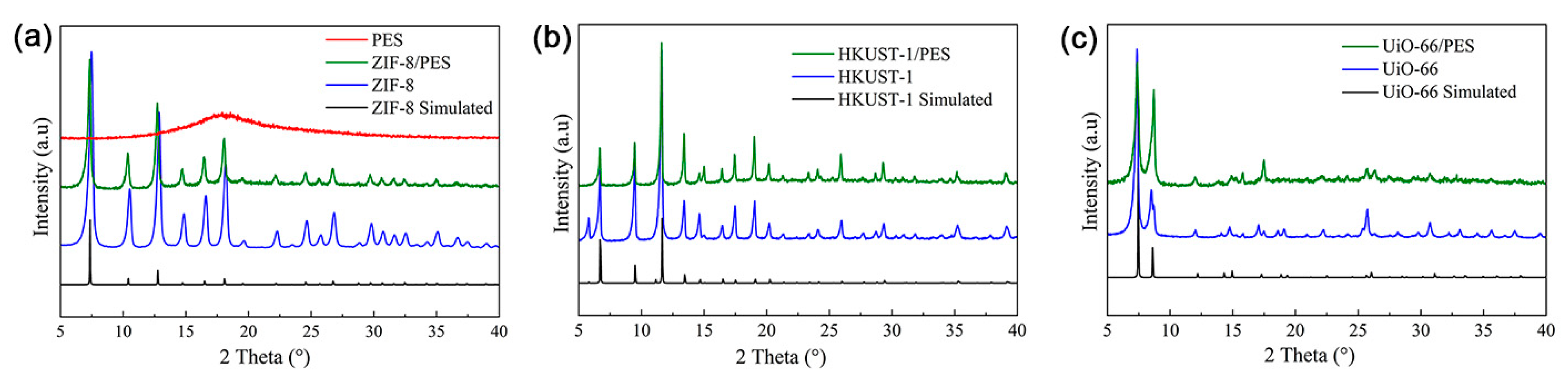
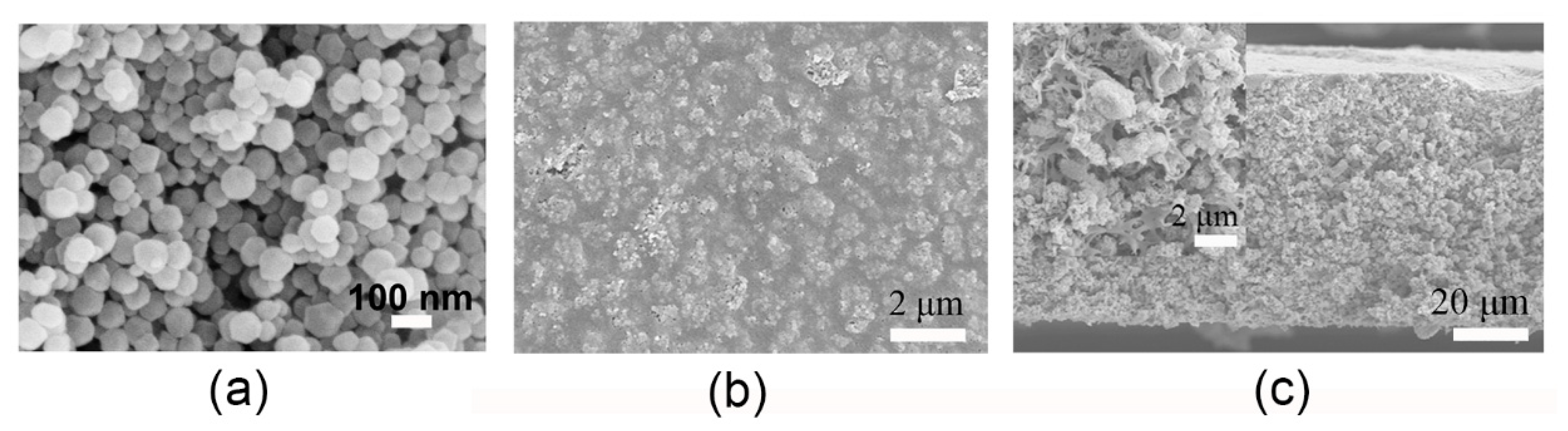
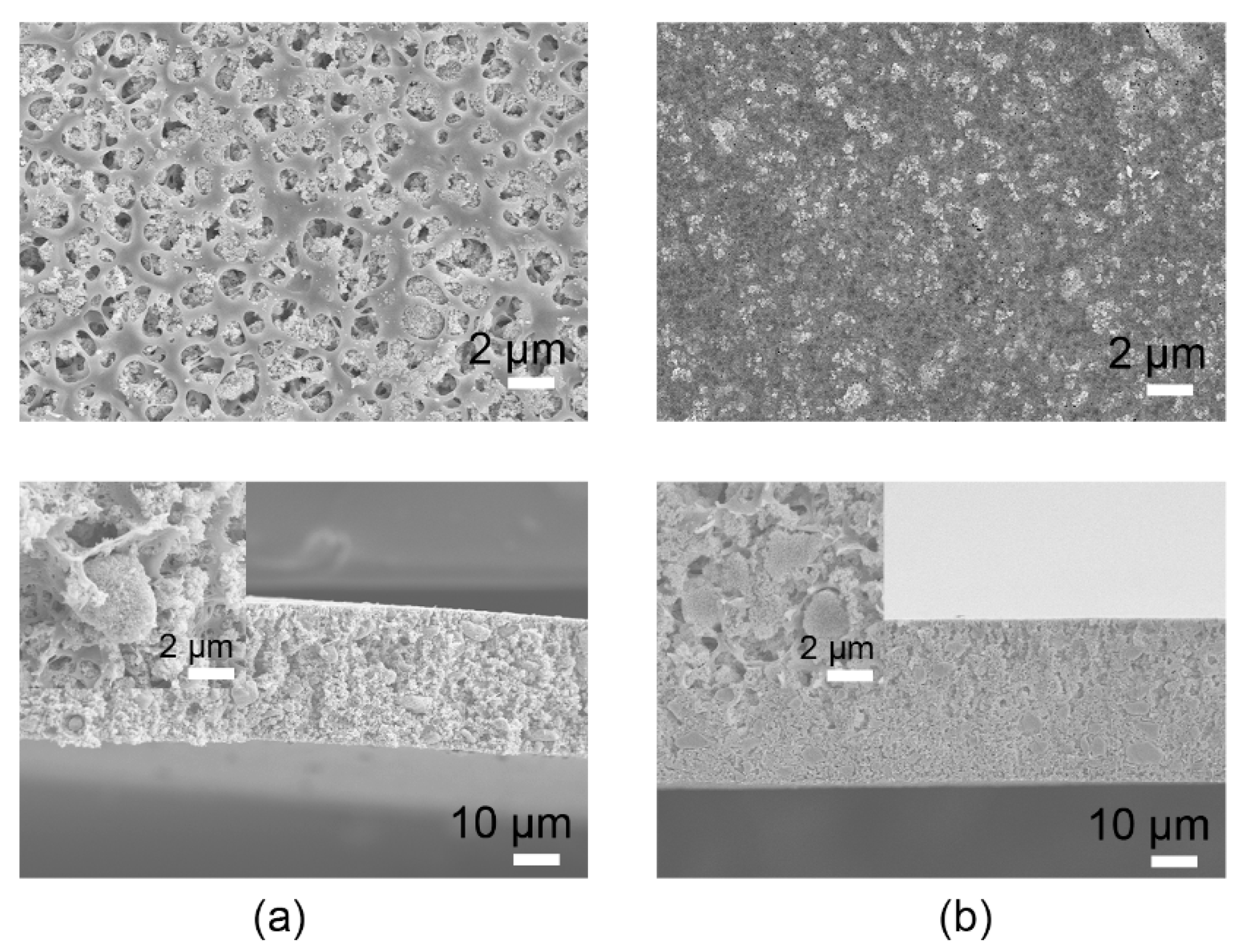
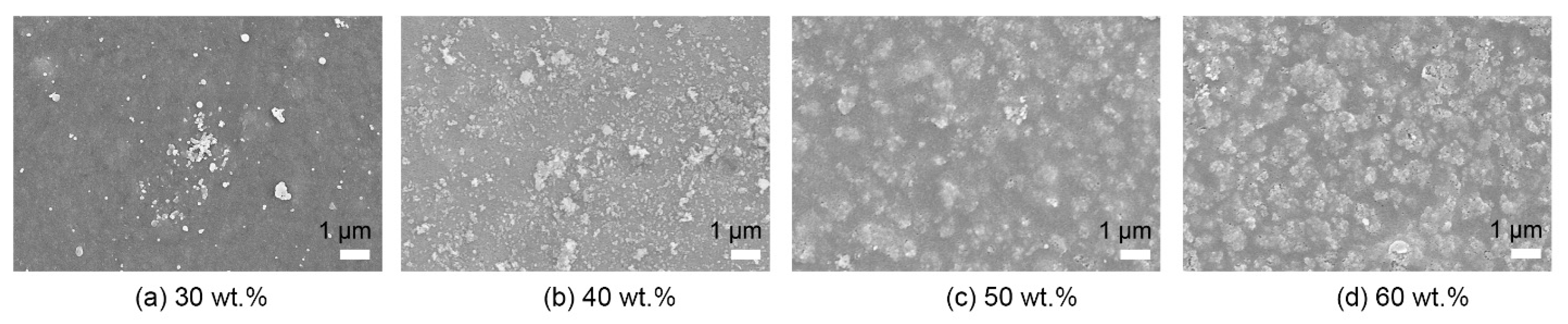
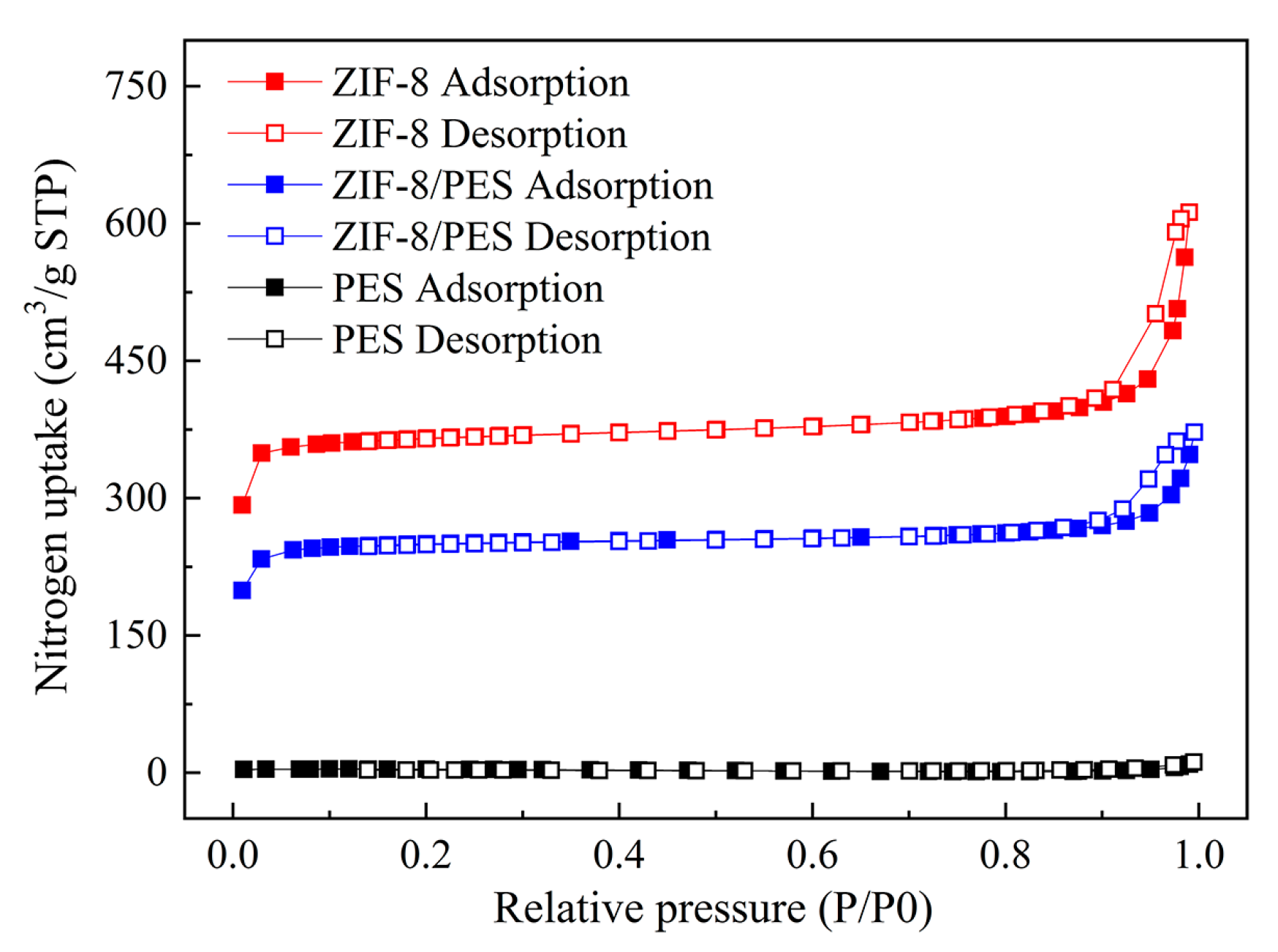
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Kitao, T.; Zhang, Y.; Kitagawa, S.; Wang, B.; Uemura, T. Hybridization of MOFs and polymers. Chem. Soc. Rev. 2017, 46, 3108–3133. [Google Scholar] [CrossRef]
- Ren, H.; Jin, J.; Hu, J.; Liu, H. Affinity between metal–organic frameworks and polyimides in asymmetric mixed matrix membranes for gas separations. Ind. Eng. Chem. Res. 2012, 51, 10156–10164. [Google Scholar] [CrossRef]
- Li, L.; Yao, J.; Xiao, P.; Shang, J.; Feng, Y.; Webley, P.A.; Wang, H.J.C.; Science, P. One-step fabrication of ZIF-8/polymer composite spheres by a phase inversion method for gas adsorption. Colloid. Polym. Sci. 2013, 291, 2711–2717. [Google Scholar] [CrossRef]
- Ma, J.; Guo, X.; Ying, Y.; Liu, D.; Zhong, C. Composite ultrafiltration membrane tailored by MOF@GO with highly improved water purification performance. Chem. Eng. J. 2017, 313, 890–898. [Google Scholar] [CrossRef]
- Hou, J.; Luan, Y.; Huang, X.; Gao, H.; Yang, M.; Lu, Y. Facile synthesis of Cu3(BTC)2/cellulose acetate mixed matrix membranes and their catalytic applications in continuous flow process. New J. Chem. 2017, 41, 9123–9129. [Google Scholar] [CrossRef]
- Xu, Y.M.; Chung, T.-S. High-performance UiO-66/polyimide mixed matrix membranes for ethanol, isopropanol and n-butanol dehydration via pervaporation. J. Membr. Sci. 2017, 531, 16–26. [Google Scholar] [CrossRef]
- Marti, A.M.; Venna, S.R.; Roth, E.A.; Culp, J.T.; Hopkinson, D.P. Simple fabrication method for mixed matrix membranes with in situ mof growth for gas separation. ACS Appl. Mater. Interfaces 2018, 10, 24784–24790. [Google Scholar] [CrossRef]
- Xiang, F.; Marti, A.M.; Hopkinson, D.P. Layer-by-layer assembled polymer/mof membrane for H2/CO2 separation. J. Membr. Sci. 2018, 556, 146–153. [Google Scholar] [CrossRef]
- Denny, M.S., Jr.; Cohen, S.M. In situ modification of metal–organic frameworks in mixed-matrix membranes. Angew. Chem. Int. Ed. 2015, 54, 9029–9032. [Google Scholar] [CrossRef]
- Gao, Y.; Qiao, Z.; Zhao, S.; Wang, Z.; Wang, J. In situ synthesis of polymer grafted ZIFs and application in mixed matrix membrane for CO2 separation. J. Mater. Chem. A 2018, 6, 3151–3161. [Google Scholar] [CrossRef]
- Jin, P.; Tan, W.; Huo, J.; Liu, T.; Liang, Y.; Wang, S.; Bradshaw, D. Hierarchically porous MOF/polymer composites via interfacial nanoassembly and emulsion polymerization. J. Mater. Chem. A 2018, 6, 20473–20479. [Google Scholar] [CrossRef]
- Benzaqui, M.; Semino, R.; Menguy, N.; Carn, F.; Kundu, T.; Guigner, J.-M.; McKeown, N.B.; Msayib, K.J.; Carta, M.; Malpass-Evans, R.; et al. Toward an understanding of the microstructure and interfacial properties of PIMs/ZIF-8 mixed matrix membranes. ACS Appl. Mater. Interfaces 2016, 8, 27311–27321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes – a review of methods. Prog. Mater Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Mohammadnezhad, F.; Feyzi, M.; Zinadini, S. A novel Ce-MOF/PES mixed matrix membrane; synthesis, characterization and antifouling evaluation. J. Ind. Eng. Chem. 2019, 71, 99–111. [Google Scholar] [CrossRef]
- Semino, R.; Moreton, J.C.; Ramsahye, N.A.; Cohen, S.M.; Maurin, G. Understanding the origins of metal–organic framework/polymer compatibility. Chem. Sci. 2018, 9, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Zhou, W.; Du, A.; Zhu, Z. Porous polyethersulfone-supported zeolitic imidazolate framework membranes for hydrogen separation. J. Phys. Chem. C 2012, 116, 13264–13270. [Google Scholar] [CrossRef]
- Kang, Z.; Peng, Y.; Qian, Y.; Yuan, D.; Addicoat, M.A.; Heine, T.; Hu, Z.; Tee, L.; Guo, Z.; Zhao, D. Mixed matrix membranes (mmms) comprising exfoliated 2D covalent organic frameworks (COFs) for efficient CO2 separation. Chem. Mater. 2016, 28, 1277–1285. [Google Scholar] [CrossRef]
- Valizadeh, B.; Nguyen, T.N.; Smit, B.; Stylianou, K.C. Porous metal–organic framework@polymer beads for iodine capture and recovery using a gas-sparged column. Adv. Funct. Mater. 2018, 28, 1801596. [Google Scholar] [CrossRef]
- Abbasi, Z.; Shamsaei, E.; Fang, X.-Y.; Ladewig, B.; Wang, H. Simple fabrication of zeolitic imidazolate framework ZIF-8/polymer composite beads by phase inversion method for efficient oil sorption. J. Colloid Interface Sci. 2017, 493, 150–161. [Google Scholar] [CrossRef]
- Yeo, Z.Y.; Tan, P.Y.; Chai, S.-P.; Zhu, P.W.; Mohamed, A.R. Continuous polycrystalline ZIF-8 membrane supported on CO2-selective mixed matrix supports for CO2/CH4 separation. RSC Adv. 2014, 4, 52461–52466. [Google Scholar] [CrossRef]
- Li, Y.; Wee, L.H.; Volodin, A.; Martens, J.A.; Vankelecom, I.F.J. Polymer supported ZIF-8 membranes prepared via an interfacial synthesis method. Chem. Commun. 2015, 51, 918–920. [Google Scholar] [CrossRef] [PubMed]
- Gholami, F.; Zinadini, S.; Zinatizadeh, A.A.; Abbasi, A.R. TMU-5 metal-organic frameworks (MOFs) as a novel nanofiller for flux increment and fouling mitigation in PES ultrafiltration membrane. Sep. Purif. Technol. 2018, 194, 272–280. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Wang, F.; Guo, H.; Chai, Y.; Li, Y.; Liu, C. The controlled regulation of morphology and size of HKUST-1 by “coordination modulation method”. Microporous Mesoporous Mater. 2013, 173, 181–188. [Google Scholar] [CrossRef]
- Lu, G.; Cui, C.; Zhang, W.; Liu, Y.; Huo, F. Synthesis and self-assembly of monodispersed metal-organic framework microcrystals. Chem.-Asian J. 2013, 8, 69–72. [Google Scholar] [CrossRef]
- Zhu, Y.; Ciston, J.; Zheng, B.; Miao, X.; Czarnik, C.; Pan, Y.; Sougrat, R.; Lai, Z.; Hsiung, C.-E.; Yao, K.; et al. Unravelling surface and interfacial structures of a metal–organic framework by transmission electron microscopy. Nat. Mater. 2017, 16, 532–536. [Google Scholar] [CrossRef]
- Amirjalayer, S.; Tafipolsky, M.; Schmid, R. Surface termination of the metal-organic framework HKUST-1: A theoretical investigation. J. Phys. Chem. Lett. 2014, 5, 3206–3210. [Google Scholar] [CrossRef]
- Adams, F.V.; Nxumalo, E.N.; Krause, R.W.M.; Hoek, E.M.V.; Mamba, B.B. The influence of solvent properties on the performance of polysulfone/β-cyclodextrin polyurethane mixed-matrix membranes. J. Appl. Polym. Sci. 2013, 130, 2005–2014. [Google Scholar] [CrossRef]
- Şener, T.; Okumuş, E.; Gürkan, T.; Yilmaz, L. The effect of different solvents on the performance of zeolite-filled composite pervaporation membranes. Desalination 2010, 261, 181–185. [Google Scholar] [CrossRef]
- Guan, R.; Dai, H.; Li, C.; Liu, J.; Xu, J. Effect of casting solvent on the morphology and performance of sulfonated polyethersulfone membranes. J. Membr. Sci. 2006, 277, 148–156. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Zheng, B.; Wang, J. Controllable Synthesis of Metal-Organic Framework/Polyethersulfone Composites. Crystals 2020, 10, 39. https://doi.org/10.3390/cryst10010039
Guo X, Zheng B, Wang J. Controllable Synthesis of Metal-Organic Framework/Polyethersulfone Composites. Crystals. 2020; 10(1):39. https://doi.org/10.3390/cryst10010039
Chicago/Turabian StyleGuo, Xiaomin, Bin Zheng, and Jinlei Wang. 2020. "Controllable Synthesis of Metal-Organic Framework/Polyethersulfone Composites" Crystals 10, no. 1: 39. https://doi.org/10.3390/cryst10010039
APA StyleGuo, X., Zheng, B., & Wang, J. (2020). Controllable Synthesis of Metal-Organic Framework/Polyethersulfone Composites. Crystals, 10(1), 39. https://doi.org/10.3390/cryst10010039





