Inhomogeneity and Segregation Effect in the Surface Layer of Fe-Doped SrTiO3 Single Crystals
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
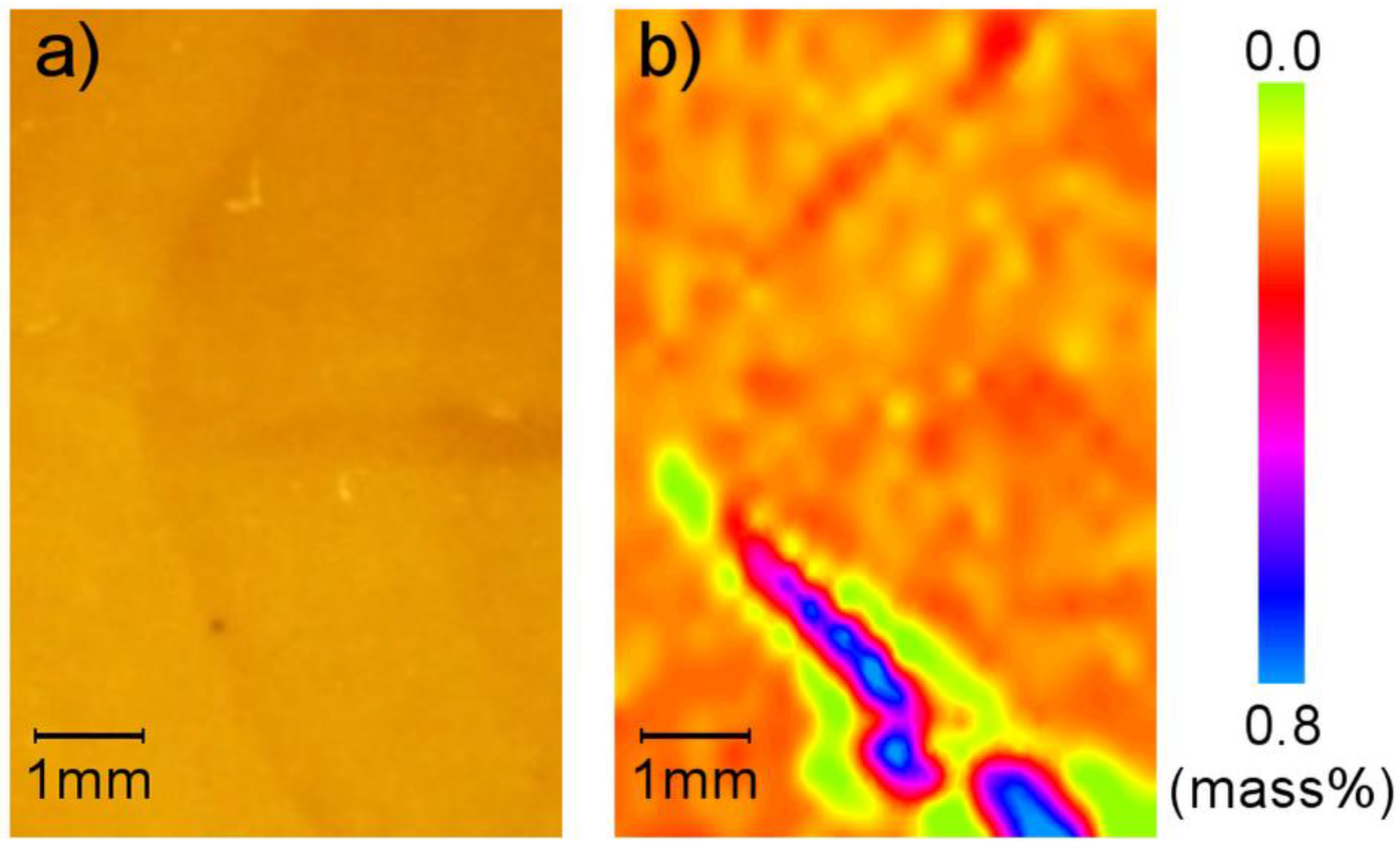
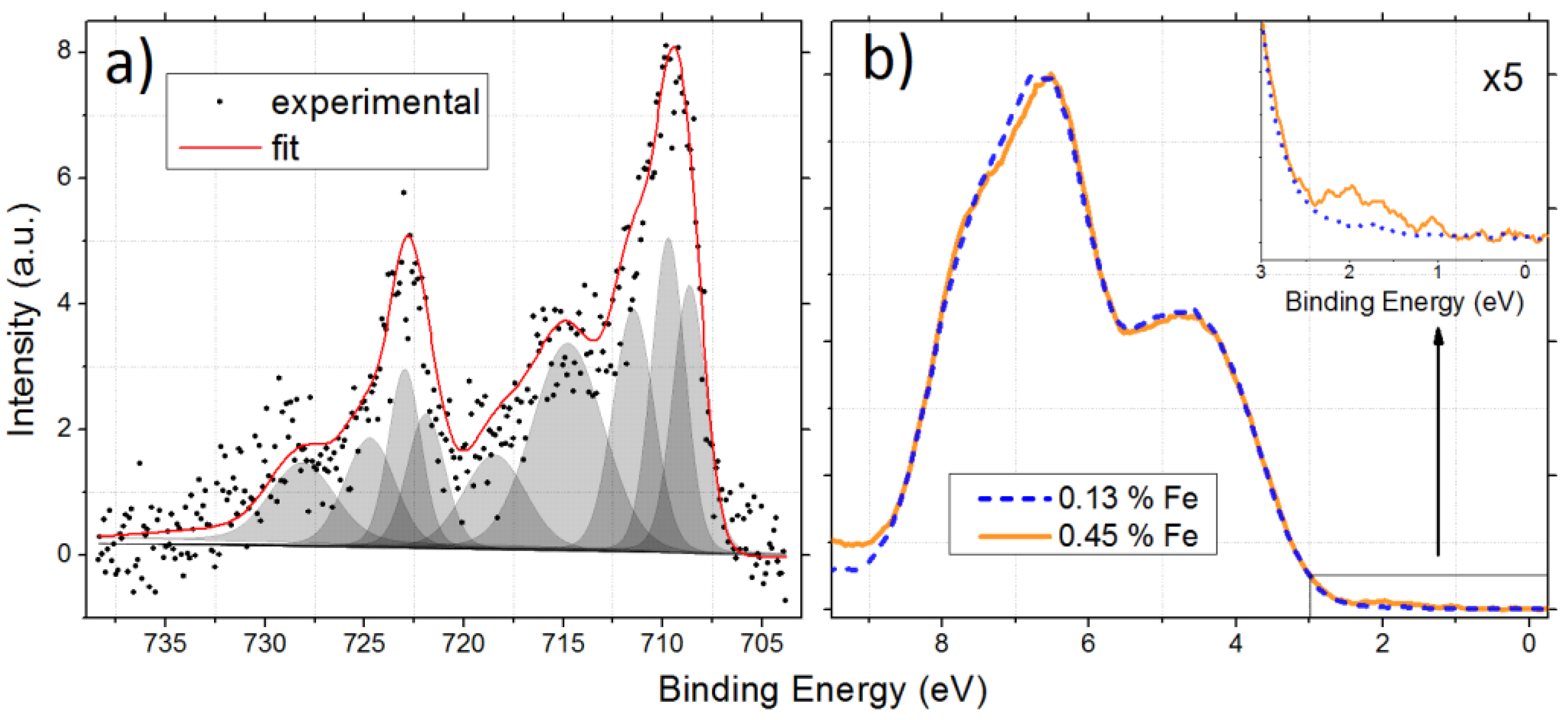
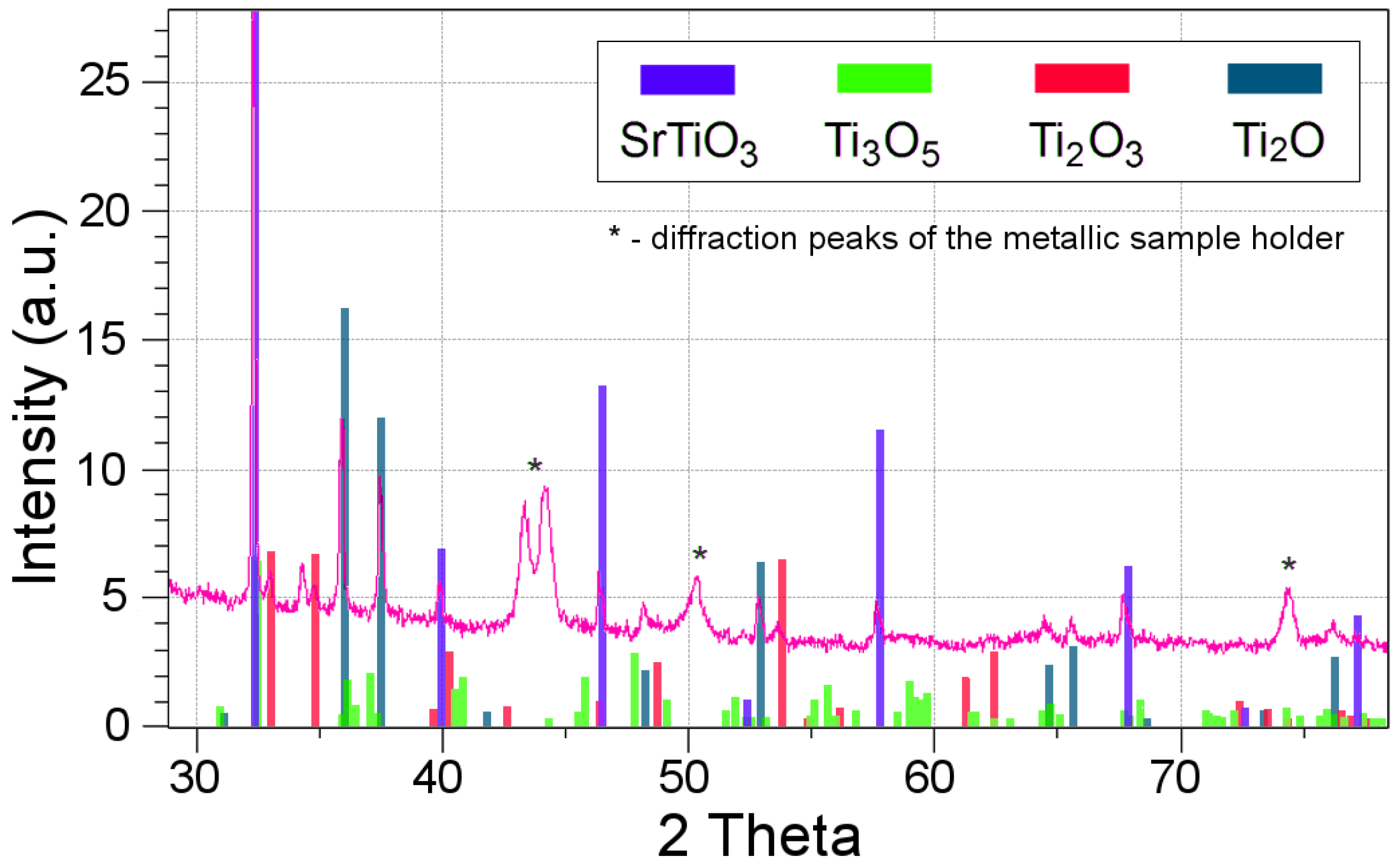
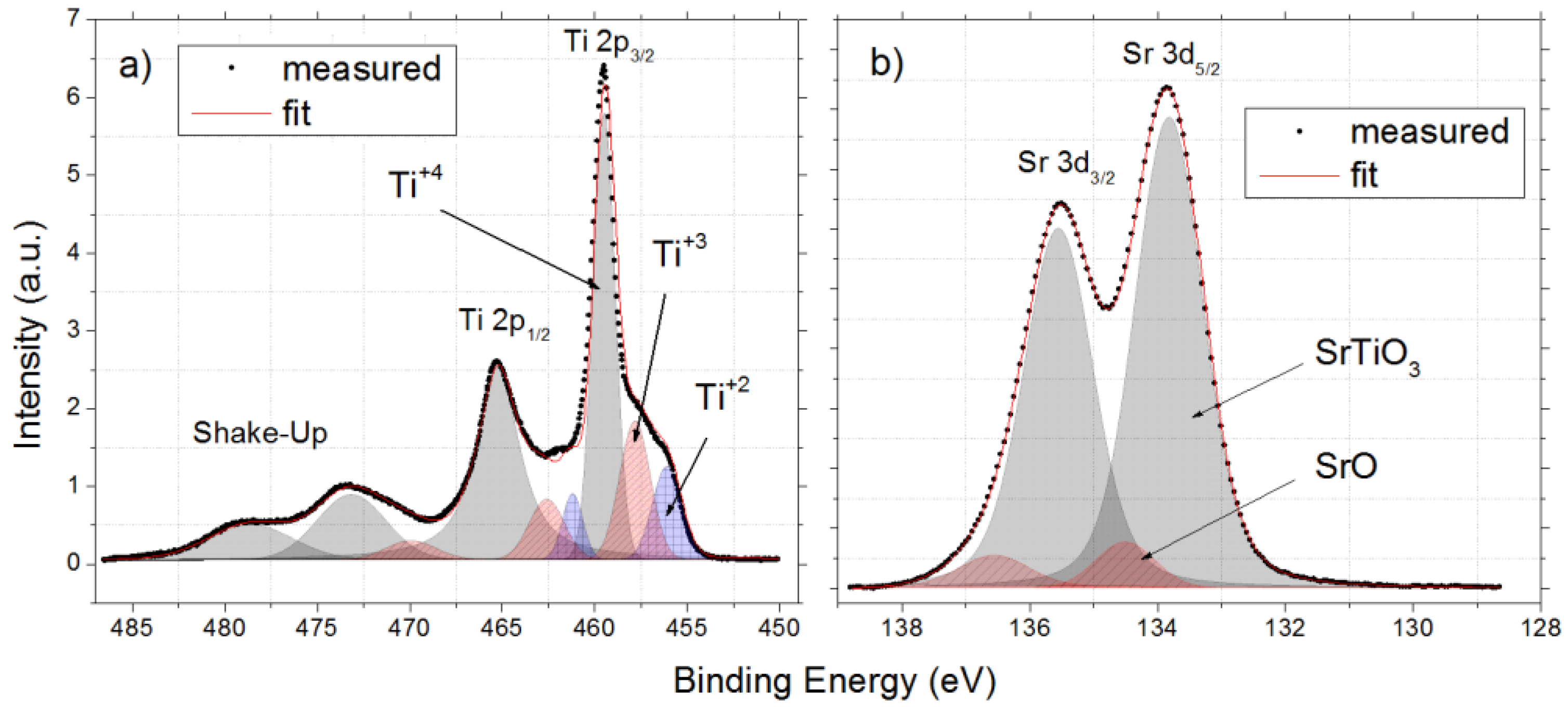
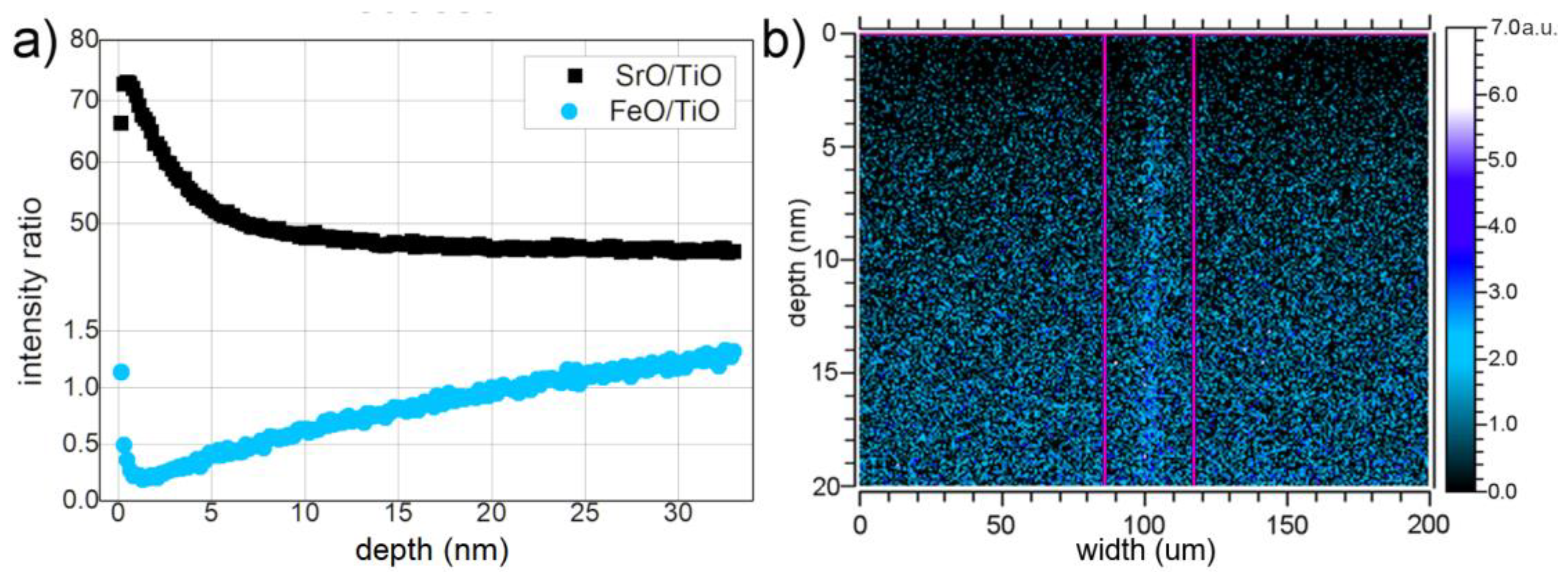
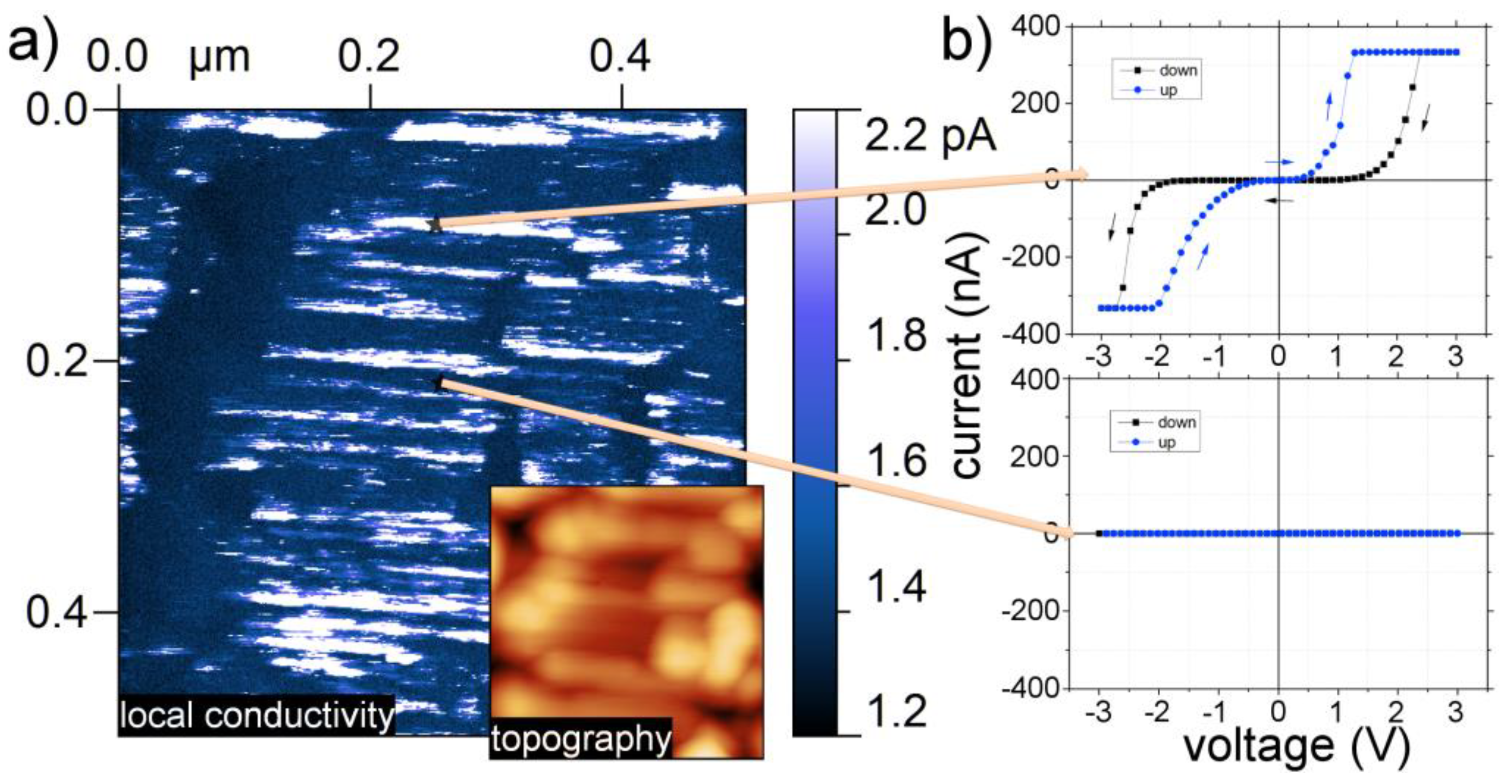
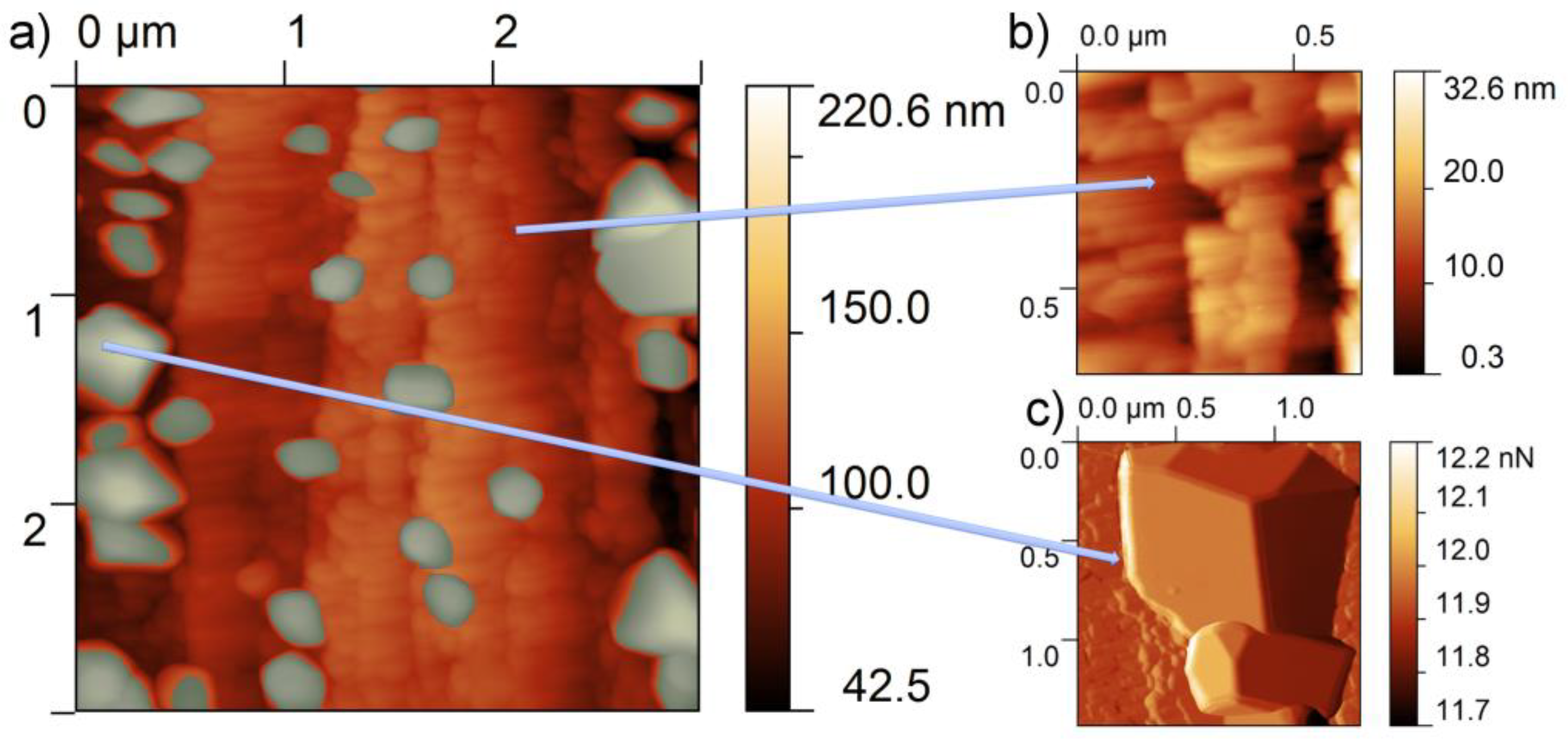
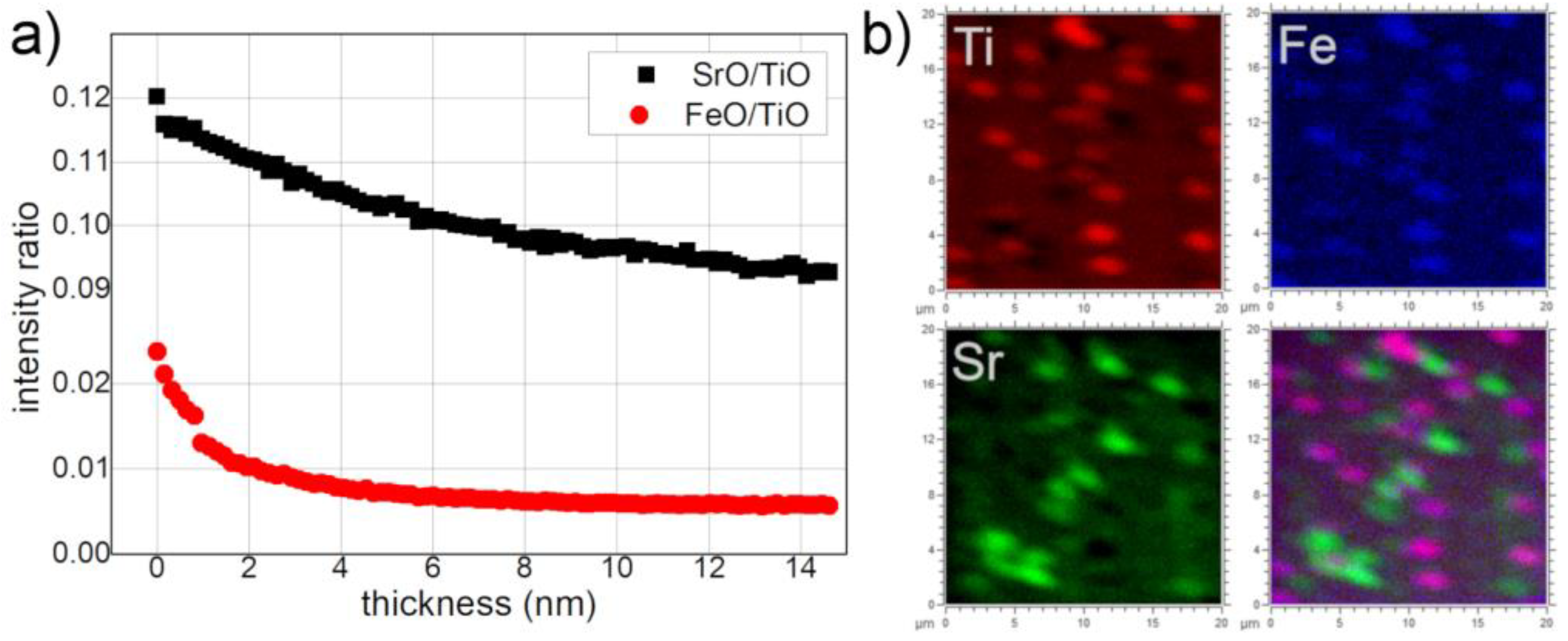
3.1. As Received Samples
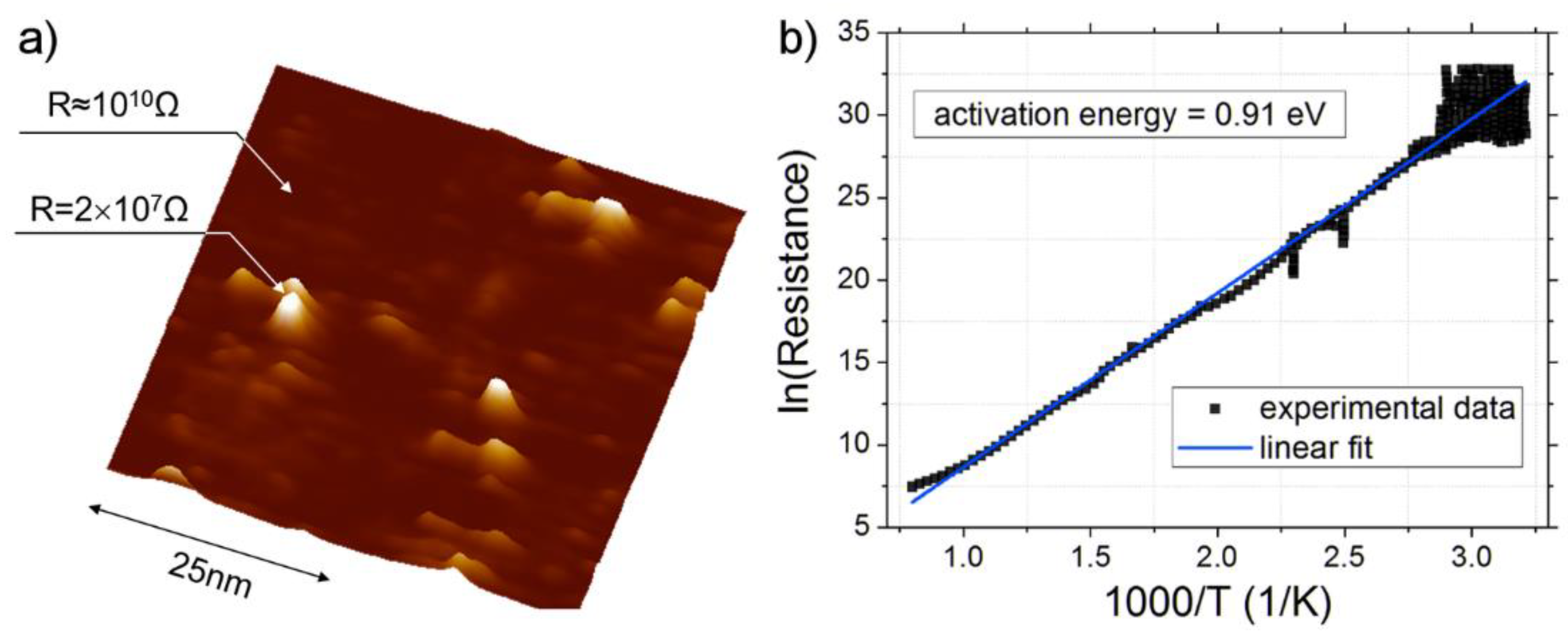
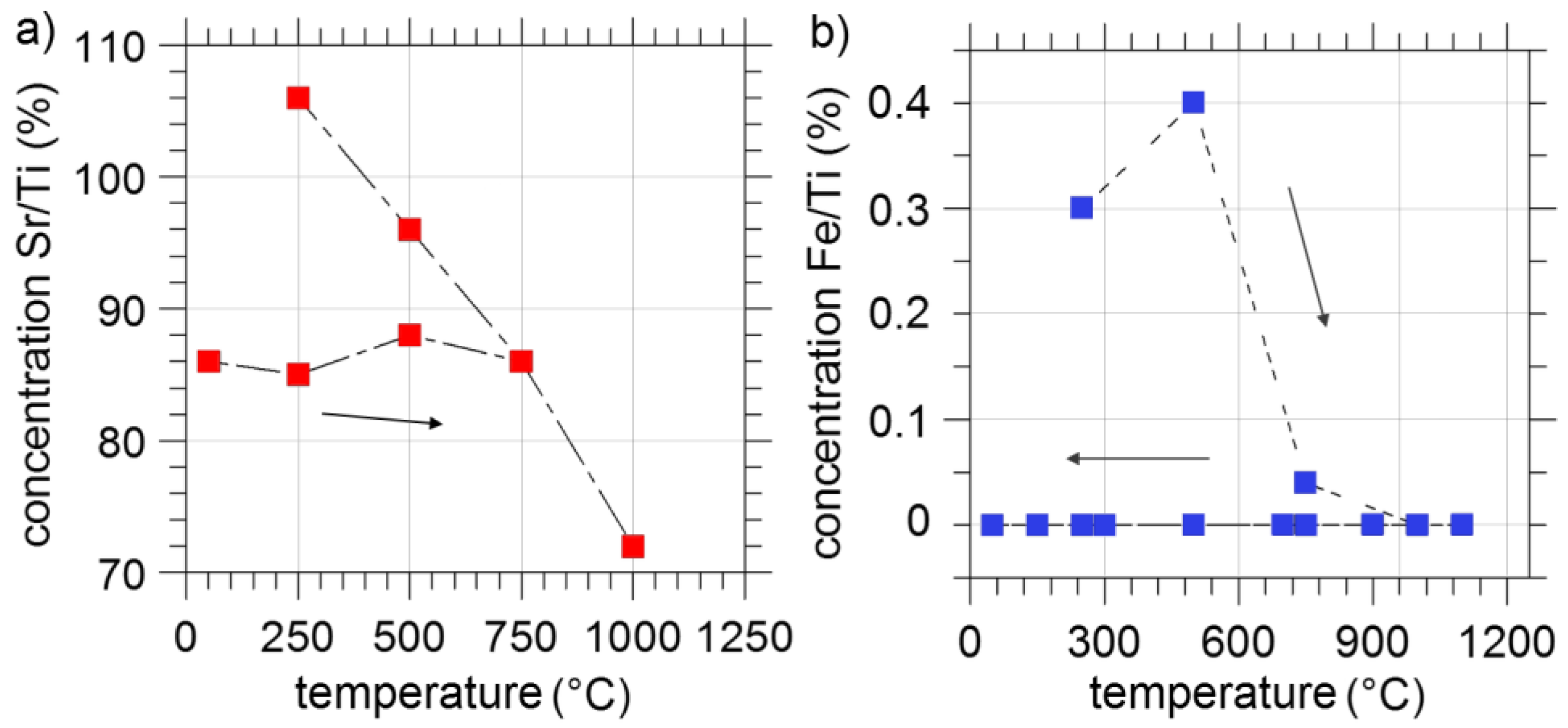
3.2. Annealing under Reducing Conditions
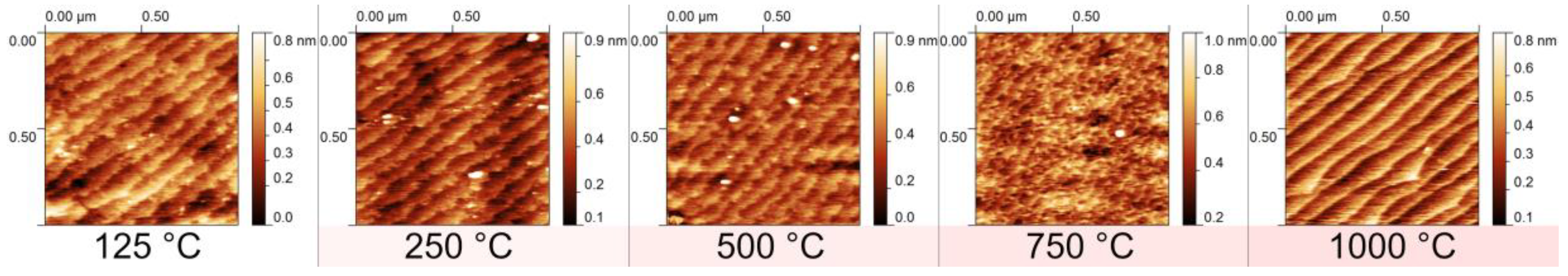
3.3. Annealing under Oxidizing Conditions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Russo, P.; Xiao, M.; Zhou, N.Y. Electrochemical Oxidation Induced Multi-Level Memory in Carbon-Based Resistive Switching Devices. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Minnekhanov, A.A.; Emelyanov, A.V.; Lapkin, D.A.; Nikiruy, K.E.; Shvetsov, B.S.; Nesmelov, A.A.; Erokhin, V.V.; Vyacheslav, A.D.; Rylkov, V.V. Parylene Based Memristive Devices with Multilevel Resistive Switching for Neuromorphic Applications. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ielmini, D. Resistive switching memories based on metal oxides: Mechanisms, reliability and scaling. Semicond. Sci. Technol. 2016, 31, 063002. [Google Scholar] [CrossRef]
- Ielmini, D.; Wong, H.S.P. In-memory computing with resistive switching devices. Nat. Electron. 2018, 1, 333–343. [Google Scholar] [CrossRef]
- Zidan, M.A.; Strachan, J.P.; Lu, W.D. The future of electronics based on memristive systems. Nat. Electron. 2018, 1, 22–29. [Google Scholar] [CrossRef]
- Mikhaylov, A.N.; Morozov, O.A.; Ovchinnikov, P.E.; Antonov, I.N.; Belov, A.I.; Korolev, D.S.; Kazantsev, V.B. One-Board Design and Simulation of Double-Layer Perceptron Based on Metal-Oxide Memristive Nanostructures. IEEE Trans. Emerg. Top. Comput. Intell. 2018, 2, 371–379. [Google Scholar] [CrossRef]
- Michalas, L.; Stathopoulos, S.; Khiat, A.; Prodromakis, T. An electrical characterisation methodology for identifying the switching mechanism in TiO2 memristive stacks. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef]
- Kwon, D.H.; Lee, S.; Kang, C.S.; Choi, Y.S.; Kang, S.J.; Cho, H.L.; Kim, M. Unraveling the Origin and Mechanism of Nanofilament Formation in Polycrystalline SrTiO3 Resistive-Switching Memories. Adv. Mater. 2019, 1901322, 1–12. [Google Scholar]
- Szot, K.; Speier, W.; Bihlmayer, G.; Waser, R. Switching the electrical resistance of individual dislocations in single-crystalline SrTiO3. Nat. Mater. 2006, 5, 312–320. [Google Scholar] [CrossRef]
- Szot, K.; Rogala, M.; Speier, W.; Klusek, Z.; Besmehn, A.; Waser, R. TiO2—A prototypical memristive material. Nanotechnology 2011, 22, 254001. [Google Scholar] [CrossRef] [PubMed]
- Waser, R.; Dittmann, R.; Staikov, G.; Szot, K. Redox-Based Resistive Switching Memories—Nanoionic Mechanisms, Prospects, and Challenges. Adv. Mater. 2009, 21, 2632–2663. [Google Scholar] [CrossRef]
- Szot, K.; Bihlmayer, G.; Speier, W. Chapter Four—Nature of the Resistive Switching Phenomena in TiO2 and SrTiO3: Origin of the Reversible Insulator–Metal Transition. Solid State Phys. 2004, 65, 353–559. [Google Scholar]
- Sawa, A. Resistive switching in rapid advances in information technology rely on high-speed and large-capacity nonvolatile memories. Mater. Today 2008, 11, 28–36. [Google Scholar] [CrossRef]
- Rodenbücher, C.; Speier, W.; Bihlmayer, G.; Breuer, U.; Waser, R.; Szot, K. Cluster-like resistive switching of SrTiO3: Nb surface layers. New J. Phys. 2013, 15, 103017. [Google Scholar] [CrossRef]
- Hirose, S.; Nakayama, A.; Niimi, H.; Kageyama, K.; Takagi, H. Resistance switching and retention behaviors in polycrystalline La-doped SrTiO3 ceramics chip devices. J. Appl. Phys. 2008, 104, 053712. [Google Scholar] [CrossRef]
- Janousch, M.; Meijer, G.I.; Staub, U.; Delley, B.; Karg, S.F.; Andreasson, B.P. Role of Oxygen Vacancies in Cr-Doped SrTiO3 for Resistance-Change Memory. Adv. Mater. 2007, 19, 2232–2235. [Google Scholar] [CrossRef]
- Wang, J.J.; Huang, H.B.; Bayer, T.J.M.; Moballegh, A.; Cao, Y.; Klein, A.; Chen, L.Q. Defect chemistry and resistance degradation in Fe-doped SrTiO3 single crystal. Acta. Mater. 2016, 108, 229–240. [Google Scholar] [CrossRef]
- Szot, K.; Speier, W. Surfaces of reduced and oxidized SrTiO3 from atomic force microscopy. Phys. Rev. B 1999, 60, 5909–5926. [Google Scholar] [CrossRef]
- Szot, K.; Speier, W.; Carius, R.; Zastrow, U.; Beyer, W. Localized Metallic Conductivity and Self-Healing during Thermal Reduction of SrTiO3. Phys. Rev. Lett. 2002, 88, 2–5. [Google Scholar] [CrossRef]
- Blanc, J.; Staebler, D.L. Electrocoloration in SrTiO3: Vacancy Drift and Oxidation-Reduction of Transition Metals. Phys. Rev. B 1971, 4, 3548–3557. [Google Scholar] [CrossRef]
- Menke, T.; Meuffels, P.; Dittmann, R.; Szot, K.; Waser, R. Separation of bulk and interface contributions to electroforming and resistive switching behavior of epitaxial Fe-doped SrTiO3. J. Appl. Phys. 2009, 105, 066104. [Google Scholar] [CrossRef]
- Chen, X.G.; Ma, X.B.; Yang, Y.B.; Chen, L.P.; Xiong, G.C.; Lian, G.J.; Yang, J.B. Comprehensive study of the resistance switching in SrTiO3 and Nb-doped SrTiO3. Appl. Phys. Lett. 2011, 98, 122102. [Google Scholar] [CrossRef]
- Leonarska, A.; Szot, K.; Ratuszna, A. Temperature evolution of the crystal structure in SrTiO3 doped by W6+, Ni3+, Fe3+ and La3+. Phase Transit. 2011, 84, 1015–1027. [Google Scholar] [CrossRef]
- Faughnan, B. Photochromism in Transition-Metal-Doped SrTiO3. Phys. Rev. B 1971, 4, 3623–3636. [Google Scholar] [CrossRef]
- Lenser, C.; Kalinko, A.; Kuzmin, A.; Berzins, D.; Purans, J.; Szot, K.; Dittmann, R. Spectroscopic study of the electric field induced valence change of Fe-defect centers in SrTiO3. Phys. Chem. Chem. Phys. 2011, 13, 20779–20786. [Google Scholar] [CrossRef]
- Wojtyniak, M.; Szot, K.; Wrzalik, R.; Rodenbücher, C.; Roth, G.; Waser, R. Electro-degradation and resistive switching of Fe-doped SrTiO3 single crystal. J. Appl. Phys. 2013, 113, 083713. [Google Scholar] [CrossRef]
- Berney, R.; Cowan, D.; Morin, F. ESR of Fe3+-Rhombic, Fe2+-Vo and Fe1+-Vo complexes in SrTiO3. Solid State Commun. 1978, 26, 579–582. [Google Scholar] [CrossRef]
- Kool, T.; Glasbeek, M. Electron paramagnetic resonance of photochromic Fe2+-O-in SrTiO3. J. Phys. Condens. Matter 1993, 5, 361–370. [Google Scholar] [CrossRef]
- Szade, J.; Szot, K.; Kulpa, M.; Kubacki, J.; Lenser, C.; Dittmann, R.; Waser, R. Electronic structure of epitaxial Fe-doped SrTiO3 thin films. Phase Transit. 2011, 84, 489–500. [Google Scholar] [CrossRef]
- Kohl, A.; Kajewski, D.; Kubacki, J.; Lenser, C.; Dittmann, R.; Meuffels, P.; Szade, J. Detection of Fe2+ valence states in Fe doped SrTiO3 epitaxial thin films grown by pulsed laser deposition. Phys. Chem. Chem. Phys. 2013, 15, 8311–8317. [Google Scholar] [CrossRef]
- Drahus, M.D.; Jakes, P.; Erdem, E.; Eichel, R.-A. Defect structure of the mixed ionic–electronic conducting Sr[Ti,Fe]Ox solid-solution system—Change in iron oxidation states and defect complexation. Solid State Ion. 2011, 184, 47–51. [Google Scholar] [CrossRef]
- Adler, P.; Lebon, A.; Damljanović, V.; Ulrich, C.; Bernhard, C.; Boris, A.V.; Keimer, B. Magnetoresistance Effects in SrFeO3−δ: Dependence on Phase Composition and Relation to Magnetic and Charge Order. Phys. Rev. B 2006, 73, 1–50. [Google Scholar] [CrossRef]
- Bocquet, A.; Fujimori, A.; Mizokawa, T.; Saitoh, T.; Namatame, H.; Suga, S.; Takano, M. Electronic Structure of SrFe4+O3 and Related Fe Perovskite Oxides. Phys. Rev. B 1992, 45, 1561. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Takahashi, K.; Maeda, T.; Tsuchiya, R.; Shinohara, M.; Ishiyama, O.; Yonezawa, T.; Yoshimoto, M.; Koinuma, H. Atomic Control of the SrTiO3 Crystal Surface. Science 1994, 266, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Rodenbücher, C.; Wojtyniak, M.; Szot, K. Conductive AFM for Nanoscale Analysis of High-k Dielectric Metal Oxides; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Strachan, J.P.; Pickett, M.D.; Yang, J.J.; Aloni, S.; David Kilcoyne, A.L.; Medeiros-Ribeiro, G.; Stanley Williams, R. Direct Identification of the Conducting Channels in a Functioning Memristive Device. Adv. Mater. 2010, 22, 3573–3577. [Google Scholar] [CrossRef] [PubMed]
- Rodenbücher, C.; Menzel, S.; Wrana, D.; Gensch, T.; Korte, C.; Krok, F.; Szot, K. Current channeling along extended defects during electroreduction of SrTiO3. Sci. Rep. 2019, 9, 1–9. [Google Scholar]
- Szot, K.; Speier, W.; Herion, J.; Freiburg, C. Restructuring of the surface region in SrTiO3. Appl. Phys. A Mater. Sci. Process. 1996, 64, 55–59. [Google Scholar] [CrossRef]
- Tenne, D.; Soukiassian a Zhu, M.; Clark a Xi, X.; Choosuwan, H.; Bhalla, A. Raman study of BaxSr1-xTiO3 films: Evidence for the existence of polar nanoregions. Phys. Rev. B 2003, 67, 012302. [Google Scholar] [CrossRef]
- Fuks, D.; Dorfman, S.; Piskunov, S.; Kotomin, E. Ab initio thermodynamics of BacSr(1−c)TiO3 solid solutions. Phys. Rev. B 2005, 71, 014111. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtyniak, M.; Balin, K.; Szade, J.; Szot, K. Inhomogeneity and Segregation Effect in the Surface Layer of Fe-Doped SrTiO3 Single Crystals. Crystals 2020, 10, 33. https://doi.org/10.3390/cryst10010033
Wojtyniak M, Balin K, Szade J, Szot K. Inhomogeneity and Segregation Effect in the Surface Layer of Fe-Doped SrTiO3 Single Crystals. Crystals. 2020; 10(1):33. https://doi.org/10.3390/cryst10010033
Chicago/Turabian StyleWojtyniak, Marcin, Katarzyna Balin, Jacek Szade, and Krzysztof Szot. 2020. "Inhomogeneity and Segregation Effect in the Surface Layer of Fe-Doped SrTiO3 Single Crystals" Crystals 10, no. 1: 33. https://doi.org/10.3390/cryst10010033
APA StyleWojtyniak, M., Balin, K., Szade, J., & Szot, K. (2020). Inhomogeneity and Segregation Effect in the Surface Layer of Fe-Doped SrTiO3 Single Crystals. Crystals, 10(1), 33. https://doi.org/10.3390/cryst10010033




