1. Introduction
Micro-mesoporous composite molecular sieves have been widely used in the petrochemical field, due to the simultaneous presence of a microporous and mesoporous structure. Among them, micro-mesoporous composite molecular sieves containing a core-shell structure have received significant attention recently; in these sieves, the outer surface of the active core (e.g., ZSM-5) can be modified with a pure silicon molecular sieve (e.g., Silicalite 1 or SBA-15) to adjust the surface acidity to prevent adverse events, while retaining the acidity and porous size of their inner surface. Up to now, there have been mainly two methods to prepare these core-shell structured composite molecular sieves: the isomorphic growth method and the epitaxial growth method. In particular, the epitaxial growth enables the growth of another structure or composition on the outer surface of a core, forming a composite core-shell structure with some particular properties, such as catalytic activity [
1,
2].
Significant attention has been paid to studying the forming mechanism of core-shell structured composite molecular sieves, due to their properties heavily depending on their structure [
3,
4,
5,
6,
7,
8,
9]. Especially, the effect of synthesis conditions including temperature and time on multiple-layer pore structure have been extensively investigated. Many studies have been done to investigate the effect of these experimental parameters on the structure of the composite molecular sieves based on the epitaxial method. Most of them focused on experimental parameters’ effect on the crystallization process. For example, Wang et al. [
3] systematically investigated the influence of crystallization temperature and time on the formation of shell and core, obtaining high density and coverage shell, this core-shell structured molecular sieve displays rhombic crystal morphology with a complete MFI/CHA composite core-shell structure. Our team also utilized the cation-exchange effect to grow the template agent on the surface of the Y microporous molecular sieve [
10], and investigated the crystallization mechanism of Y/SBA-15 composite molecular sieve. We found that the replacement of aluminum with silicon in the four-membered ring can reduce the stability of the unit cell since the size of the silicon atom is smaller than aluminum’s. This further leads to irregular contraction of the unit cell. Thus, reduced stability plays a guiding role.
Recently, the nucleation stage of single molecular sieves has been extensively studied [
11,
12,
13]. Yang et al [
14] found that the introduction of iron (Fe) could promote the crystal grain formation of Eu-1 molecular sieve, and further investigated the relationship between nucleation rate and crystal growth rate of Fe. Xing et al [
15] reviewed recent advances in pre-crystallization phase formation during the crystallization of SAPO-34 molecular sieve and the nucleation during the induction period, concluding that an unstable layered pre-crystallization phase structure was first formed during the crystallization process, and further developed into the orderly crystal lattice arrangement. It is deduced that crystallization kinetics control the nucleation and growth rate of crystals, of which temperature is the key factor. However, although nucleation stage plays a key role in the porous structure of composite molecular sieves, there were few reports studying the nucleation stage of composite molecular sieves. For example, Hammed et al [
16] reported a SuZSM-5 composite molecular sieve, and investigated the effect of crystallization conditions (e.g., pH, temperature, time) on its loading capacity to methylene blue, which increased the maximum loading capacity from 20.68 mg/g to 86.70 mg/g. Therefore, it is necessary to unmask the nucleation mechanism of composite molecular sieves, due to its significant role in the controllable preparation of composite molecular sieves. Taken together, the prepared molecular sieves displayed microporous and mesoporous structures, which are significantly different from hybrid materials. Thus, these verify that they are the micro-mesoporous composite molecular sieves. In addition, temperature plays an important role in porous structures of the molecular sieves. Low temperature can result in bad porous structure and connectivity.
In this work, we applied the epitaxial growth to prepare composite molecular sieves, and investigated the effect of temperature on porous structure. A range of techniques including XRD, SEM, and TEM were used to characterize the morphology and porous structure of composite molecular sieves, studying the effect of nucleation temperature on the porous structure. Furthermore, using n-pentane as a template agent, the performance of these composite molecular sieves on hydroisomerization of n-pentane were investigated by a continuous-flow high-pressure microreactor-chromatography apparatus.
2. Results and Discussion
2.1. Characterization of YSV Micro-Mesoporous Composite Molecular Sieve
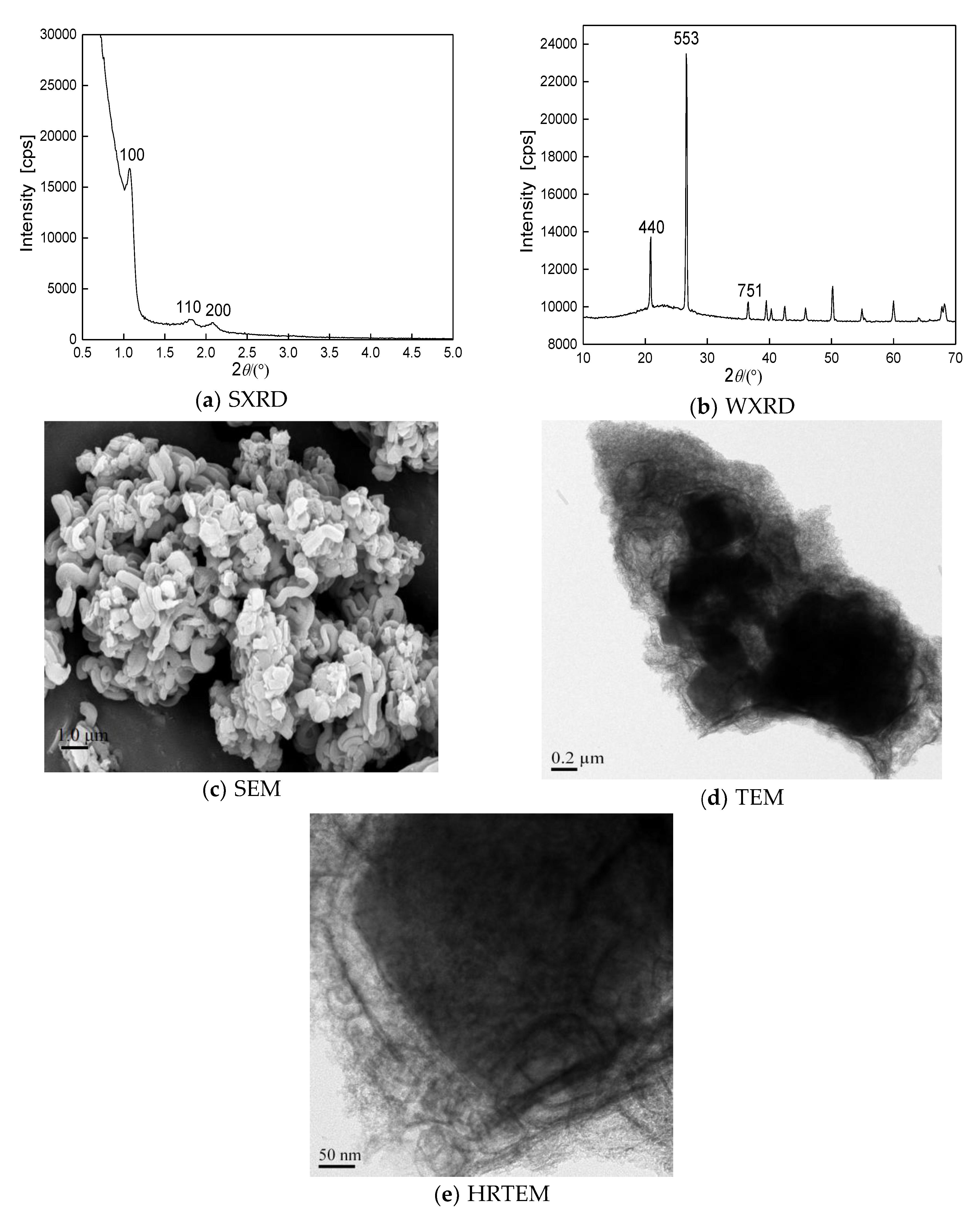
We first investigated the morphology and structure of the prepared YSV composite molecular sieve; during the reaction the temperature at the nucleation stage was 10 °C. As shown in
Figure 1, the simultaneous presence of microporous and mesoporous characteristic structures was observed (
Figure 1a,b), confirming the generation of micro-mesoporous composite molecular sieves under 10 °C of the nucleation temperature. As seen from
Figure 1c–e, the samples displayed foam or cotton-like morphology. The cores showed Y-type microporous porous structure, while the shells showed cotton-like SBA-15 mesoporous porous structure around the cores. Moreover, the boundary between microporous and mesoporous was clearly observed.
2.2. Characterization of YSG Micro-Mesoporous Composite Molecular Sieve
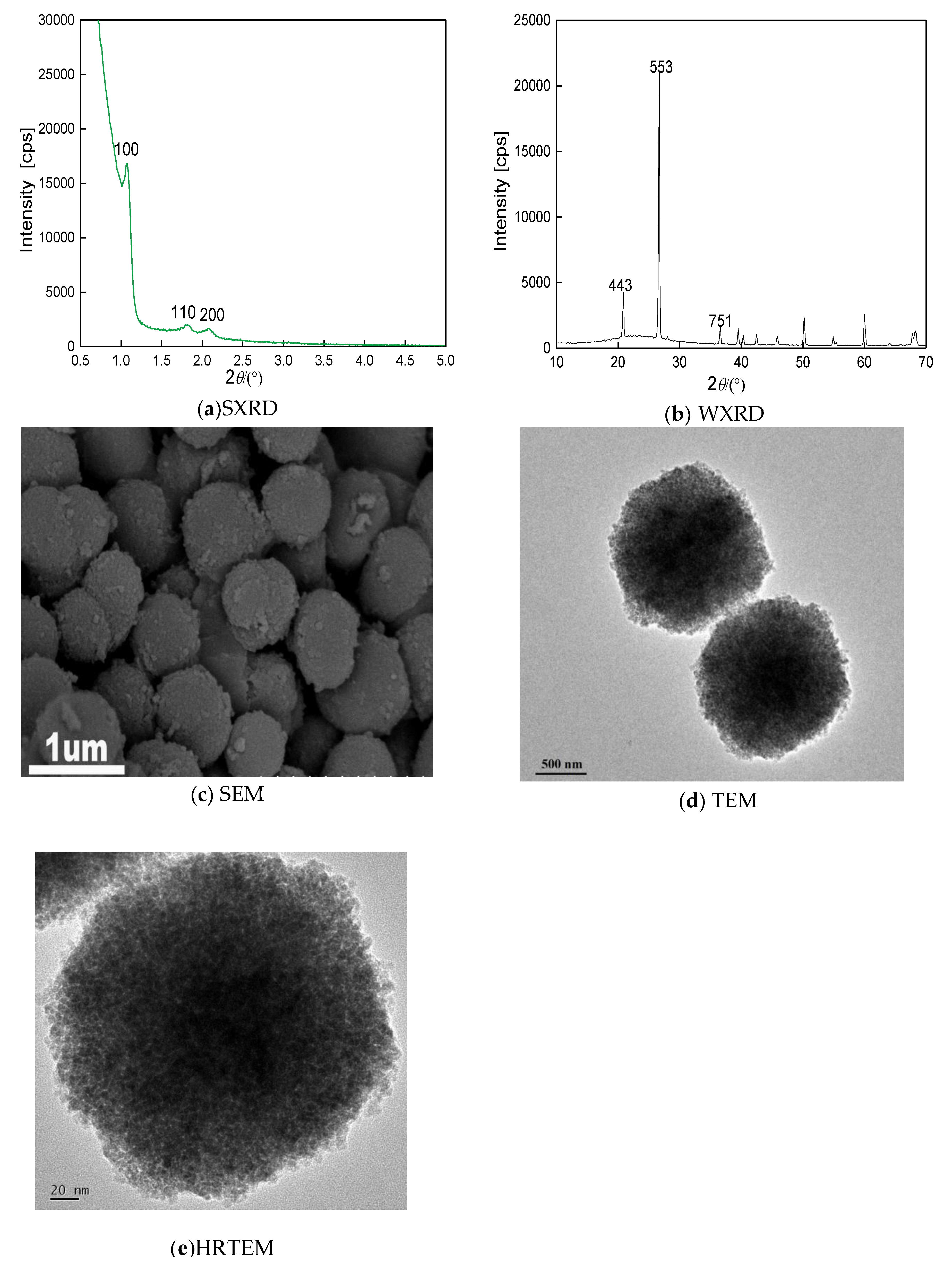
XRD results of YSG composite molecular sieves are shown in
Figure 2a,b, both small angle and wide angle were observed, which are characteristic peaks of mesoporous and microporous, respectively. The SEM result showed they were spherical particles with a slightly rough surface, a quite uniform size and high surface density, which was consistent with the TEM result (
Figure 2d,e). Taken together, YSG micro-mesoporous composite molecular sieve showed Y-type cores and SBA-15 mesoporous shell around the core. However, it showed a Y-type microporous structure, while the shells showed cotton-like SBA-15 mesoporous structure around the cores. This may be contributed to by low nucleation temperature, which can lead to the incomplete growth of the mesoporous.
2.3. Characterization of YST Micro-Mesoporous Composite Molecular Sieves
As shown in
Figure 3, the characteristic peak of SBA-15 was observed (
Figure 3a), while the amorphous scattering peaks of SBA-15 and the crystalline scattering peak of Y-type molecular sieve were clearly observed (
Figure 3b), indicating their co-existence.
Figure 3c showed that no octagonal crystal grain of single Y-type microporous zeolite was observed, while the particle morphology was also different from that of SBA-15 mesoporous molecular sieve. Their overall shape looked like pipes and was regular, being composed of column-like molecular sieves. These indicated that SBA-15 mesoporous molecular sieves grew along the outer surface of Y-type microporous zeolite. As shown in
Figure 3d,e, the mesoporous was in a regular and ordered shape, indicating that the appropriate temperature during the nucleation stage was key to the growth of the mesoporous.
To further confirm the simultaneous presence of microporous and mesoporous structures, the nitrogen adsorption desorption test was performed for the YST. As shown in
Figure 4, the adsorption desorption isotherms for Y is a typical line, which is the characteristic for microporous molecular sieves, and that for SBA-15 it was a typical line, which is the characteristic for microporous molecular sieves, while that for YST was IV line at a low relative pressure stage, which is characteristic for micro-mesoporous molecular sieves [
17].
2.4. Kinetics Analysis of the Synthesis
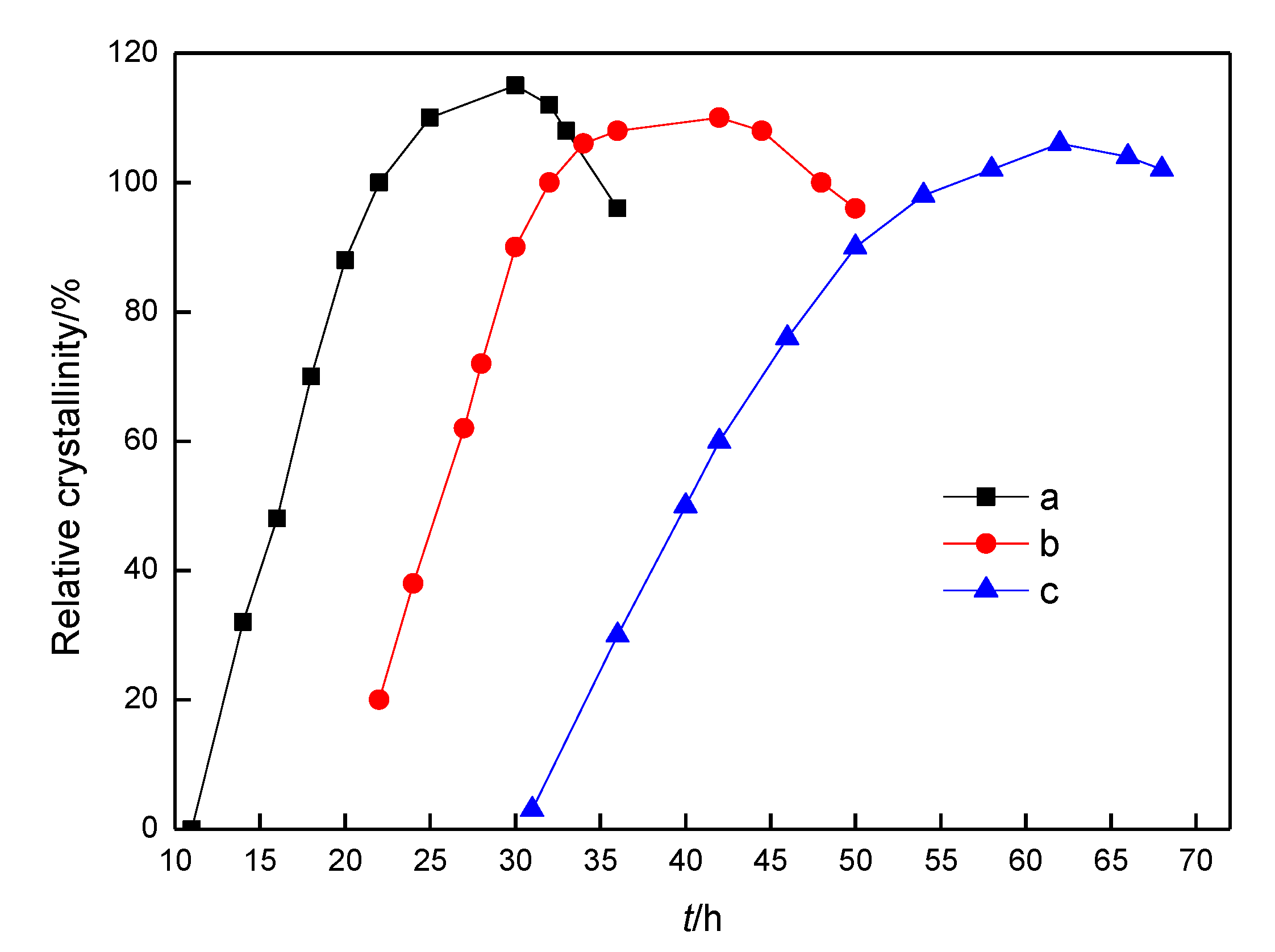
The crystallization kinetics curves of YST, YSG, and YSV micro-mesoporous composite molecular sieves were first produced at different nucleation temperature but under the same crystallization temperature (373 K). As shown in
Figure 5, the relative crystallinity of the YSV composite molecular sieve rose slowly under the same crystallization temperature, while its nucleation induction period was long, and the nucleation rate and growth rate of crystal were also correspondingly slow. With the extension of crystallization time, the relative crystallinity of YSG and YSV molecular sieves increased rapidly in a short time. After a period of crystallization, the crystallization rate slowed down, and declined after reaching their respective peaks.
A computer simulation was also carried out to stimulate the rapid rising section of the crystallization curves according to a previous report, which the curves were extended to zero crystallinity [
17,
18,
19]. Obtained data, including the nucleation induction period, apparent nucleation rate, and apparent crystal growth rate, are listed in
Table 1. The results showed that with the increase in the nucleation temperature, the nucleation induction period was significantly shortened [
20,
21,
22,
23], while the nucleation rate was significantly accelerated, and the crystal growth rate was also increased. As compared with crystal growth, nucleation is a slow process. Therefore, when the crystallization temperature and time keep the same, the degree of mesoporous phase crystallization is impaired if the nucleation temperature is low. Therefore, the nucleation temperature plays an important role in the crystallization degree of mesoporous structures of composite molecular sieves.
2.5. Investigation Their Performance on Isomerization of N-Pentane
The prepared YST, YSG, and YSV composite molecular sieves were then investigated as catalysts on isomerization of
n-pentane, which were compared with the traditional Y-type single molecular sieve without composite porous structure. The reactions were carried out under the conditions in the experimental section, and their performance was summarized in
Table 2. The results showed that the isomerization performance of YST, YSG, and YSV composite molecular sieves was significantly higher than that of the traditional Y-type single molecular sieve. The isomerization performance of the composite molecular sieves was more improved than the single molecular sieve, regardless of the regularity of the composite sieve [
24]. Moreover, the YSG composite molecular sieve with higher mesoporous regularity than the YSV composite molecular sieve exhibited higher isomerization selectivity than YSV composite molecular sieve, while the YST composite molecular sieve with the best mesoporous channel regularity displayed the best isomerization selectivity among these four molecular sieves, which reached 95.81%. The improved isomerization performance of YST, YSG, YSV composite molecular sieves is mainly due to the introduction of mesoporous, improving the mass transfer efficiency, thus shortening the reaction time of
n-pentane on the molecular sieves. Thus, it inhibits further decomposition of
n-pentane on molecular sieves, improving the isomerization selectivity of the reaction. With respect to YST, YSG, and YSV composite molecular sieves, the regularity of mesoporous pores tends to be good to bad due to different nucleation temperatures. Therefore, the mass transfer effect of YSV and YSG composite molecular sieves with more irregular mesoporous is far less than that of the YST composite molecular sieve, thus their isomerization performance is lower than that of YST composite molecular sieve.
3. Experimental
Sodium aluminate, sodium hydroxide, sodium silicate, cerium nitrate, heptanes, toluene and tetraethyl orthosilicate (TEOS) were all analytical pure reagents and purchased from Sinopharm Group Co. LTD. (Shanghai, China) Triblock copolymer poly (ethylene glycol)-block-poly (propylene glycol)-block-poly (ethylene glycol) (Pluronic P123) was purchased from Sigma Aldrich (St. Louis, MA, USA). Y type microporous materials is provided by Fushun Petrochemical Research Institute.
In a three-necked flask, 4.0 g triblock copolymer copolymer P123 was completely dissolved into 30.0mL water, 9.50 g TEOS was then added and stirred thoroughly. Then 4.0 g of prepared HY was added in the mixtures five times. Next, 120 mL hydrochloric acid at 30% volume fraction was added, respectively, and adding an appropriate amount of water to adjust the solution pH value to 2.5, and the resulting mixture was stirred at 10, 25, 40 °C temperature for 24 h, respectively. The resulting mixture was transferred into a hydrothermal reactor kettle, and placed in an oven under 100 °C, which was used for crystallization for 10−70 h. After the reaction, the reaction solution was taken out and filtered, the products were repeatedly washed with distilled water and ethanol, and then dried under 110 °C. Finally, Y/SBA-15 microporous-mesoporous composite molecular sieves (YSV, YSG, YST) were obtained through calcination at 550 °C for 6 h.
XRD patterns of the catalysts were obtained with an XRD-700 X-ray fractometer in the 2 theta ranges of 0.5–70° by using Cu Ka radiation combined with a Ni filter (Shimadzu Co. Ltd., Tokyo, Japan). Surface morphology of the samples was studied by scanning electron microscopy (SEM) at a magnification of 250,000–1,000,000 microscope (JEOL Co. Ltd., Tokyo, Japan). Transmission electron microscopy (TEM) micrographs were collected on a Philips CM200 microscope (Philips Co. Ltd., Amsterdam, Holland). NH3-TPD patterns were recorded on a RC-IR-TP-3030 (Science and Technology Development Co. Ltd., Shanghai, China) to detect surface acidity of the molecular sieves.
The probe reaction was performed on a continuous-flow high-pressure microreactor-chromatography apparatus, which was assembled by our lab. The parameters were set at: 220 °C of reaction temperature, 2 h of reaction time, 8:1 of hydrogen/n-pentane ratio, 3 h−1 of space velocity. The prepared YSV, YSG, and YST served as catalysts to conduct hydroisomerization reaction under these conditions.
4. Conclusions
In this work, three core-shell structured microporous-mesoporous composite molecular sieves were synthesized based on the epitaxial growth. They were thoroughly characterized by a range of techniques including SEM, TEM, and XRD, and the results demonstrated that the order of mesoporous regularity of them from good to bad was YST, YSG, and YSV. The effect of the nucleation temperature on the synthesis of composite molecular sieves was also investigated under the same crystallization conditions. The results indicated that with the increase of the nucleation temperature, the nucleation rate was significantly accelerated. Nucleation was a slow process compared with crystal growth. Therefore, lower nucleation temperature impaired the degree of mesoporous phase crystallization, indicating that the nucleation temperature is key to porous structures of composite molecular sieves. Furthermore, employing isomerization of n-pentane as a model reaction, their performance was examined. Rresults showed that the higher nucleation temperature lead to the higher isomerization selectivity of n-pentane, indicating that the regularity of the mesoporous structure determines the isomerization selectivity of alkane.