Bimetallic Iron–Cobalt Catalysts and Their Applications in Energy-Related Electrochemical Reactions
Abstract
:1. Introduction
2. Synthesis Methods for Fe/Co Bimetallic Catalysts
2.1. Bimetallic Alloy-Based Hybrid Catalysts
2.2. Carbon Materials Supported Bimetallic Hybrid Catalysts
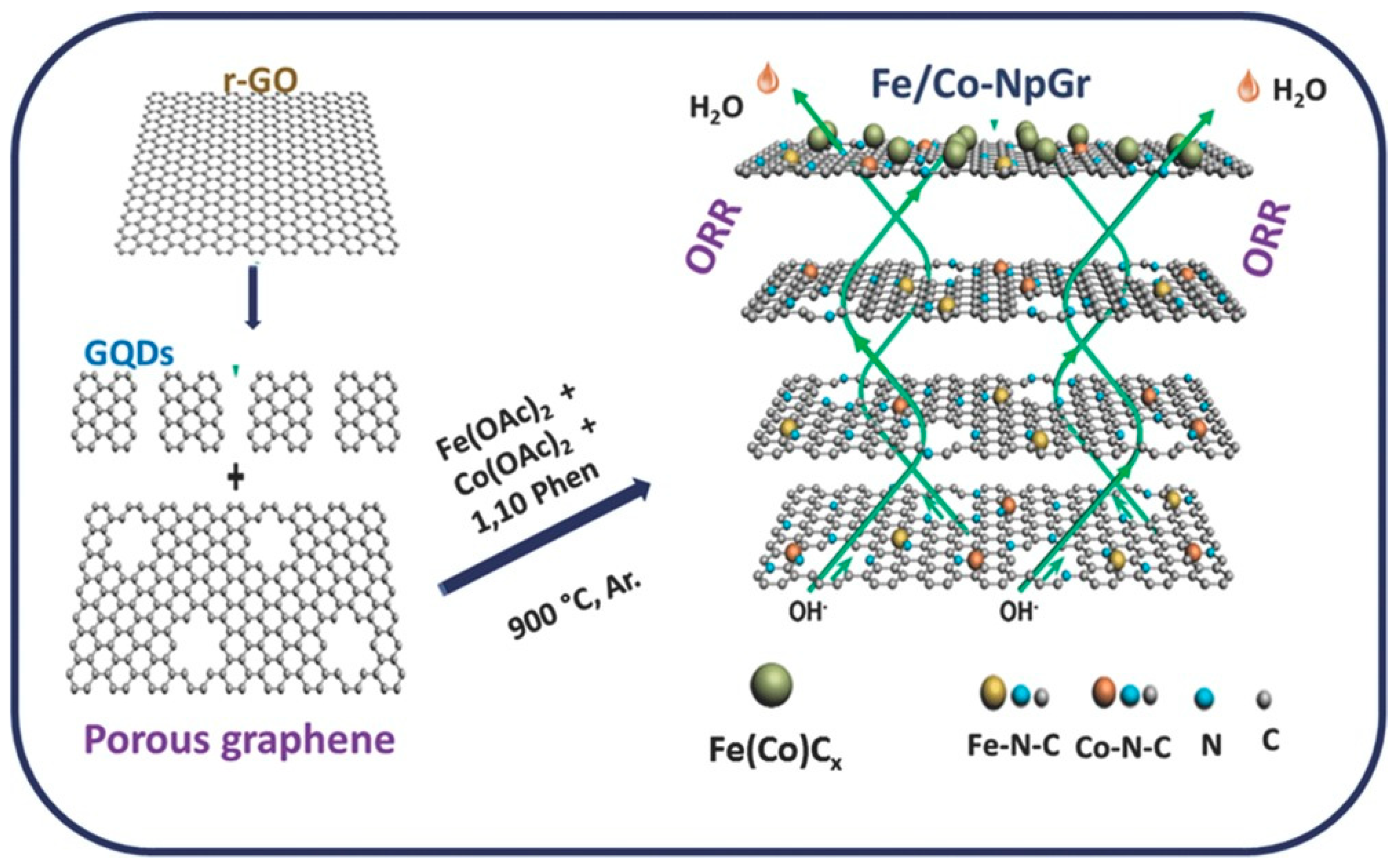
2.2.1. Graphene-Based Bimetallic Hybrid Catalysts
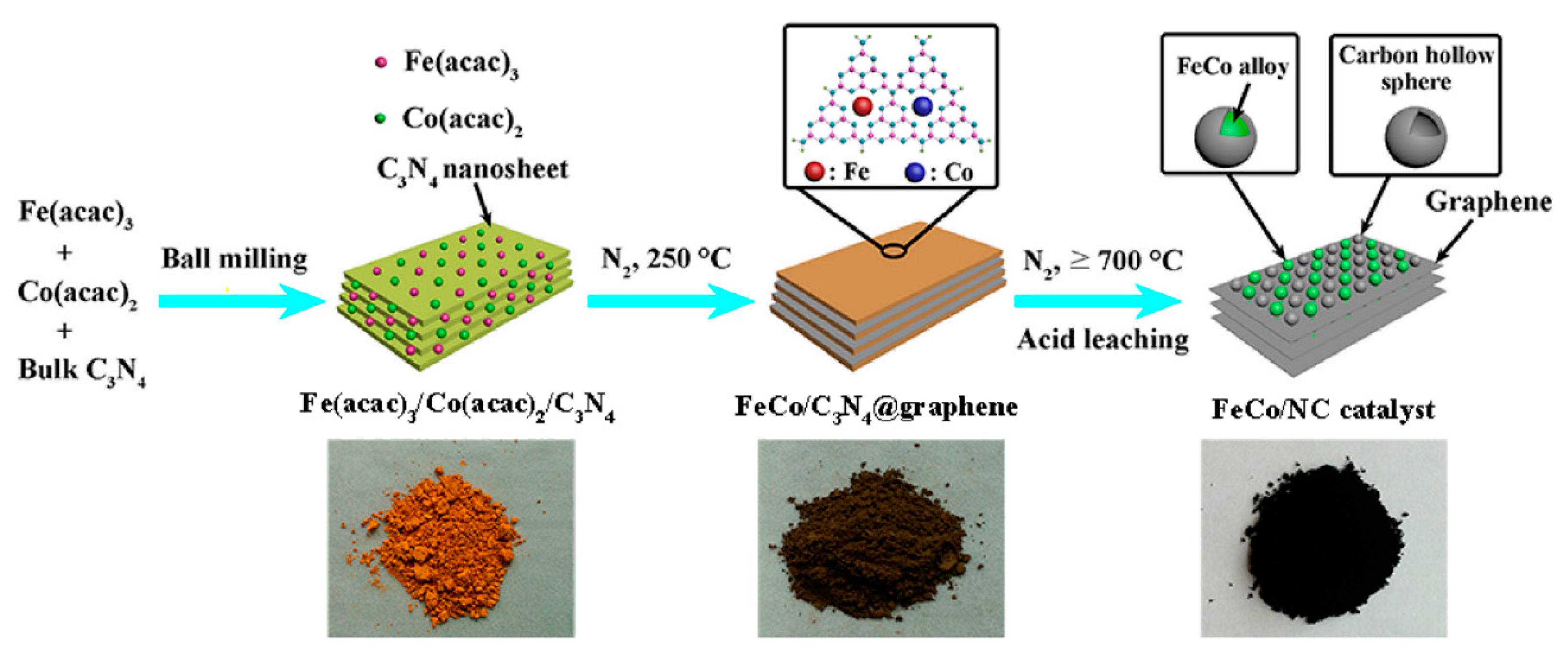
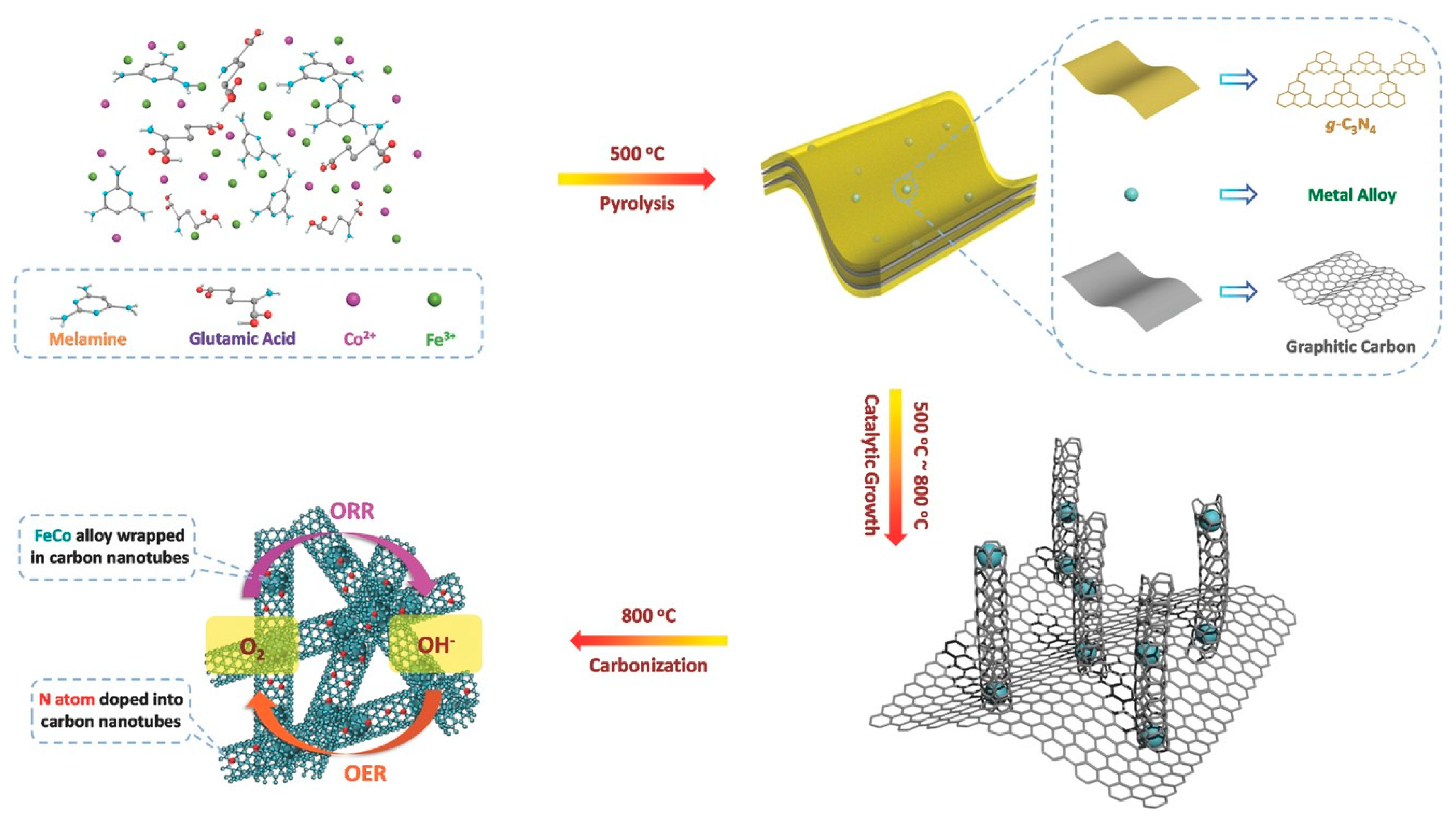
2.2.2. Other Carbon Materials Supported Bimetallic Hybrid Catalysts
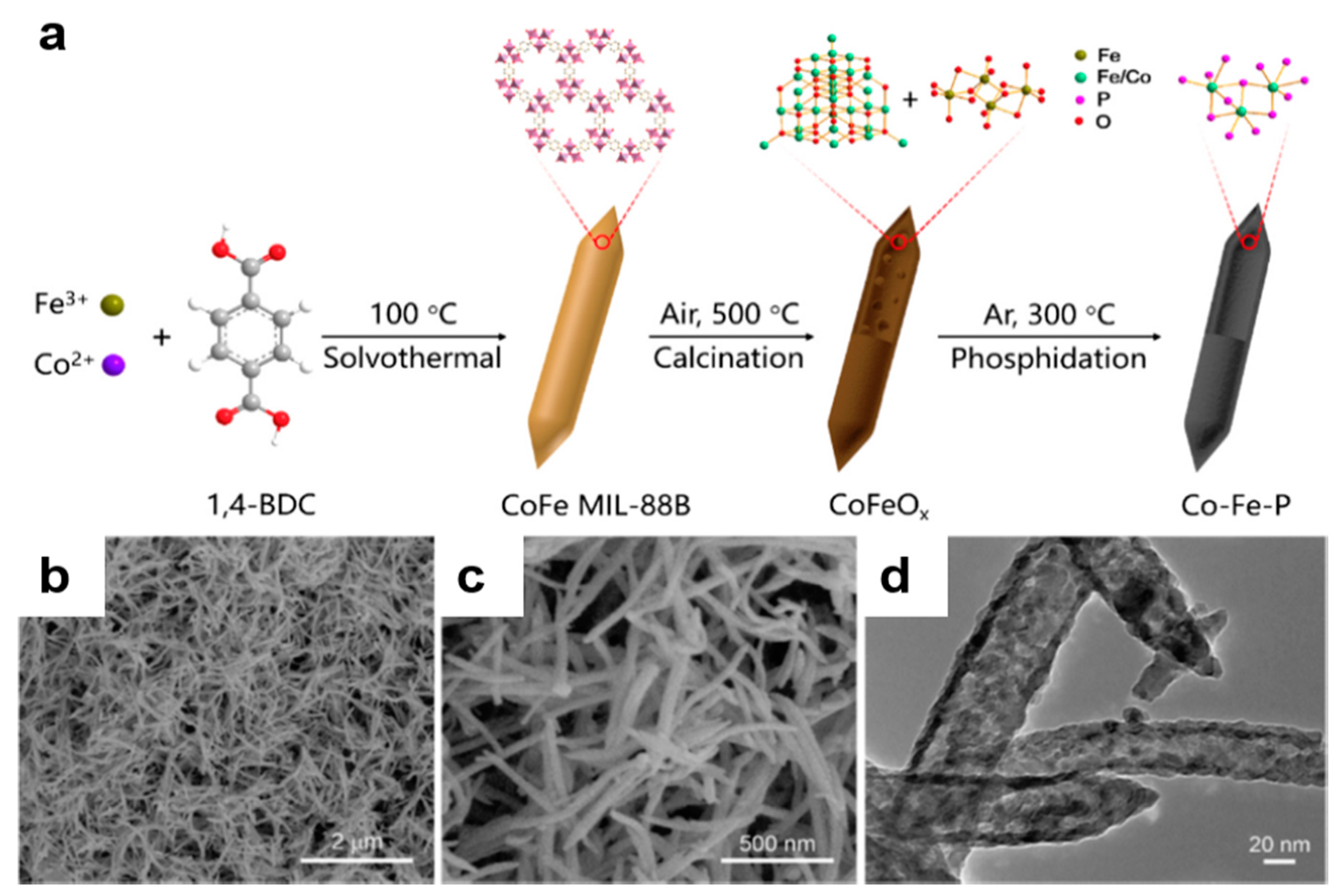
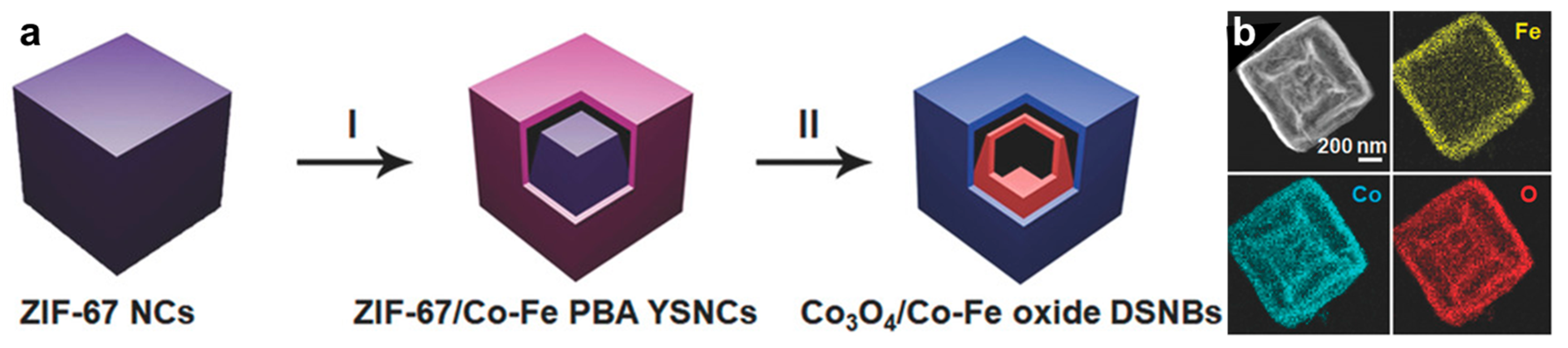
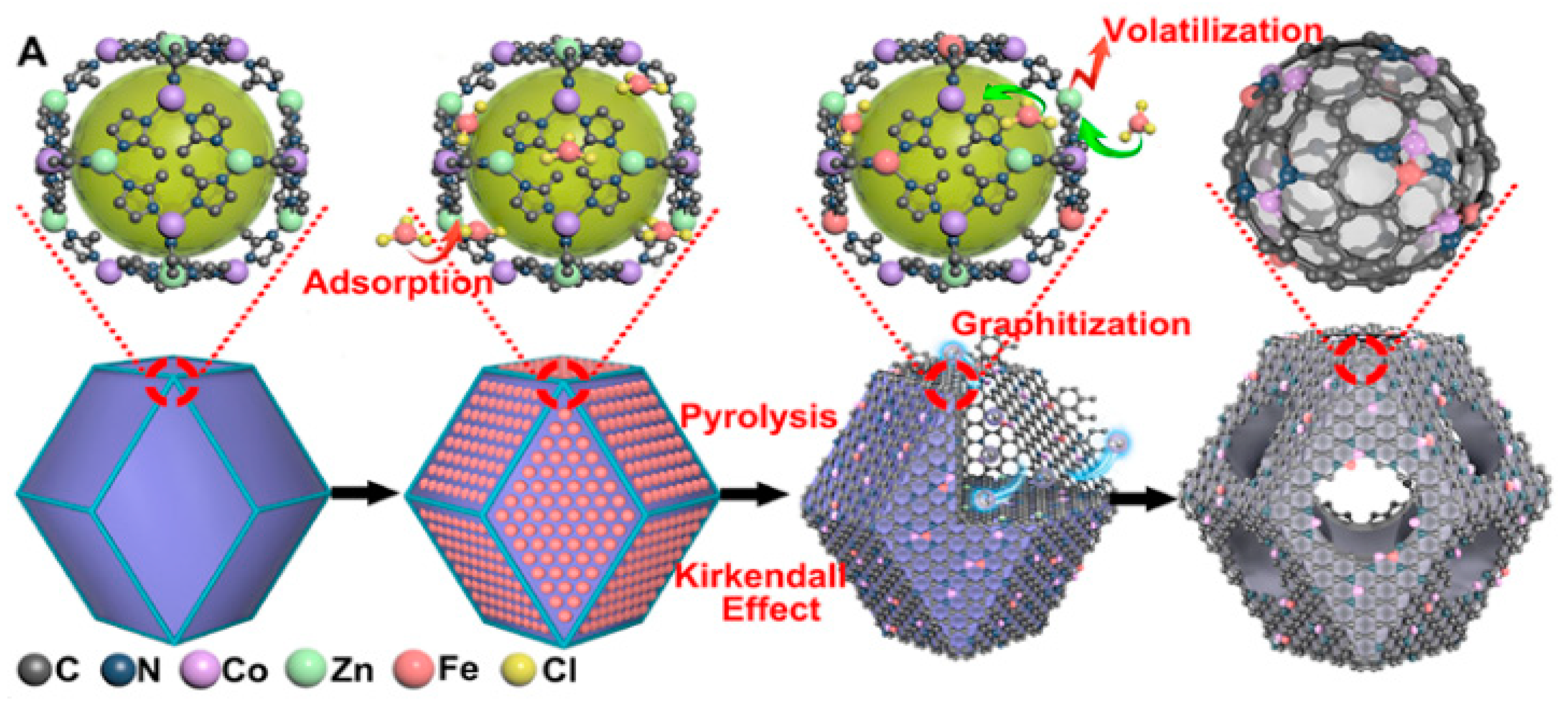
2.3. MOF-Based Bimetallic Hybrid Catalysts
3. Applications of Fe/Co Bimetallic Electrocatalysts in Electrochemical Reactions
3.1. HER
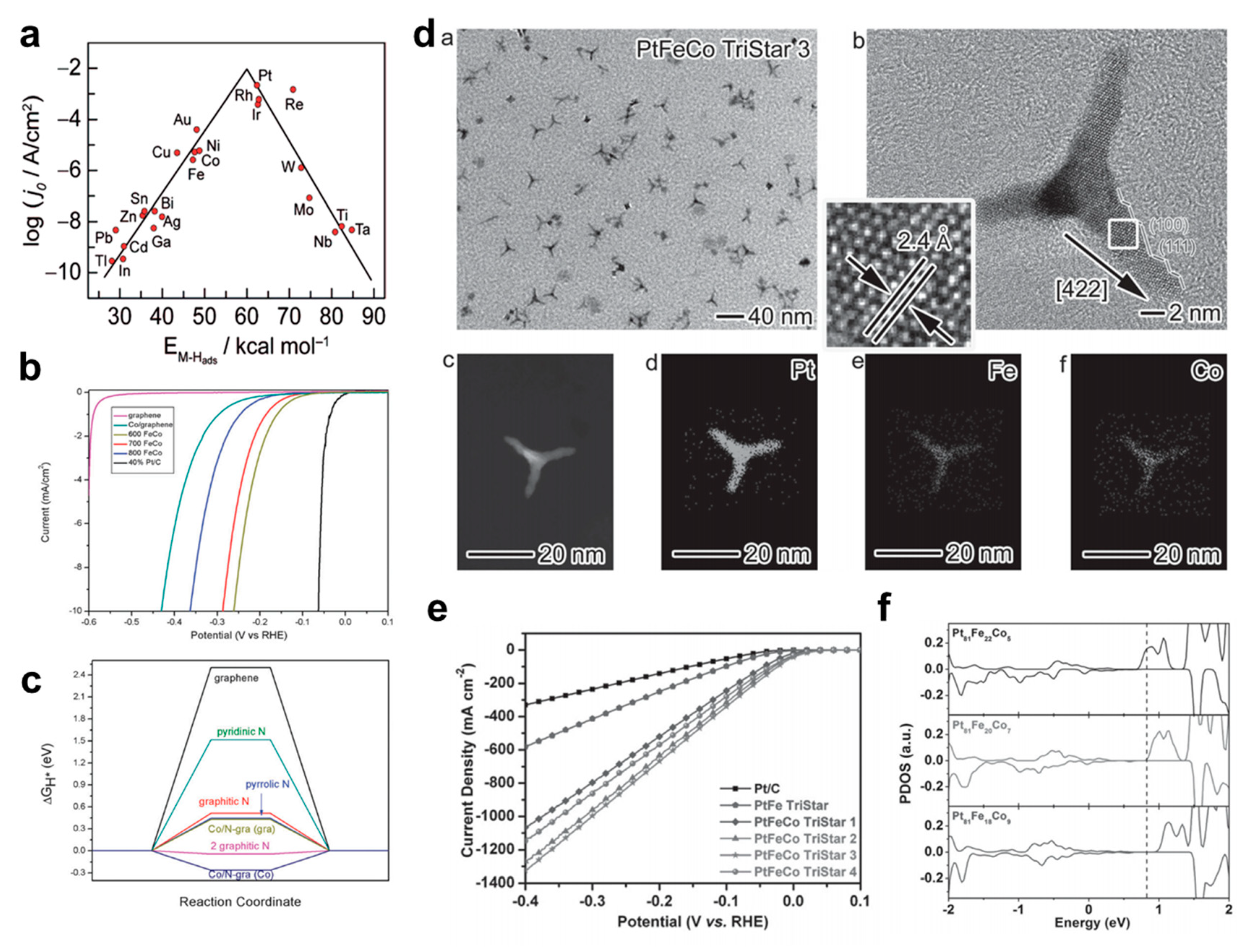
3.1.1. Bimetallic Alloy-Based Electrocatalysts
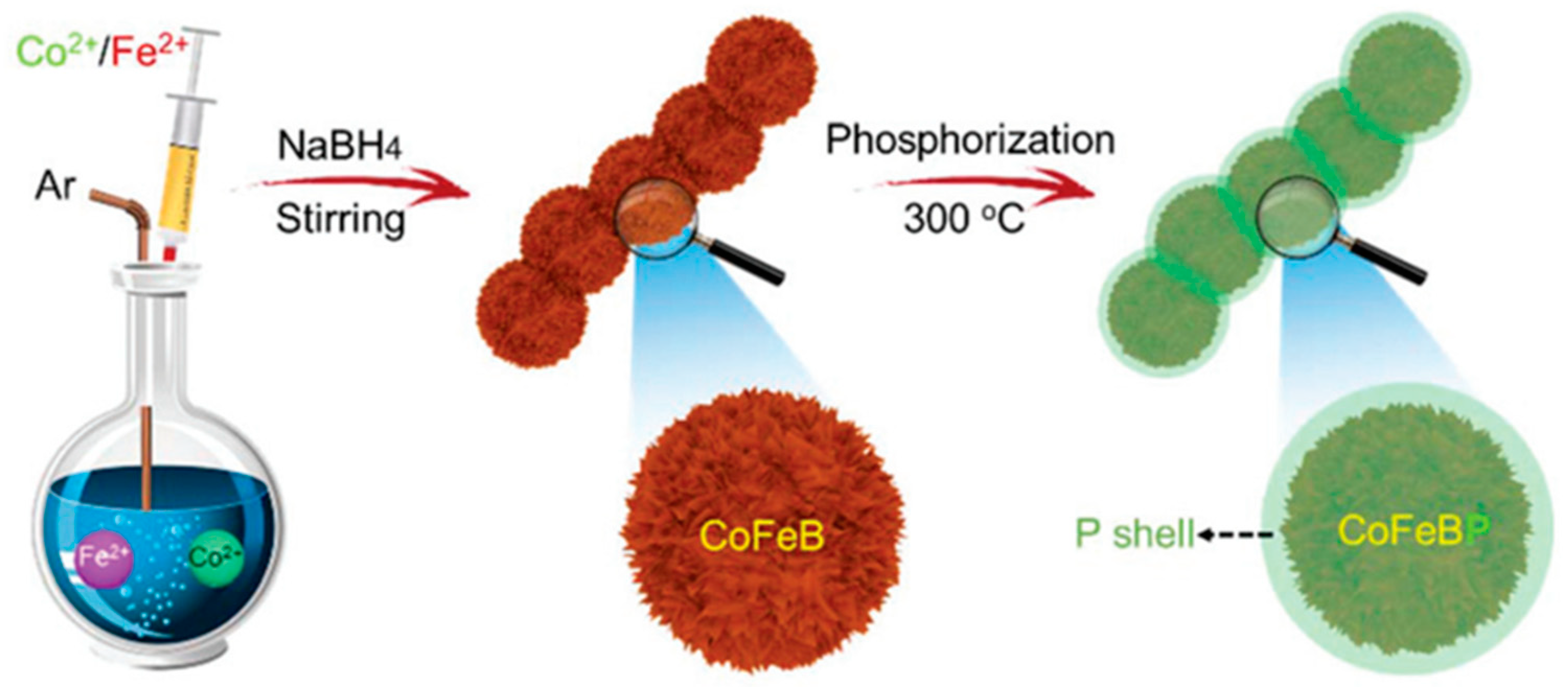
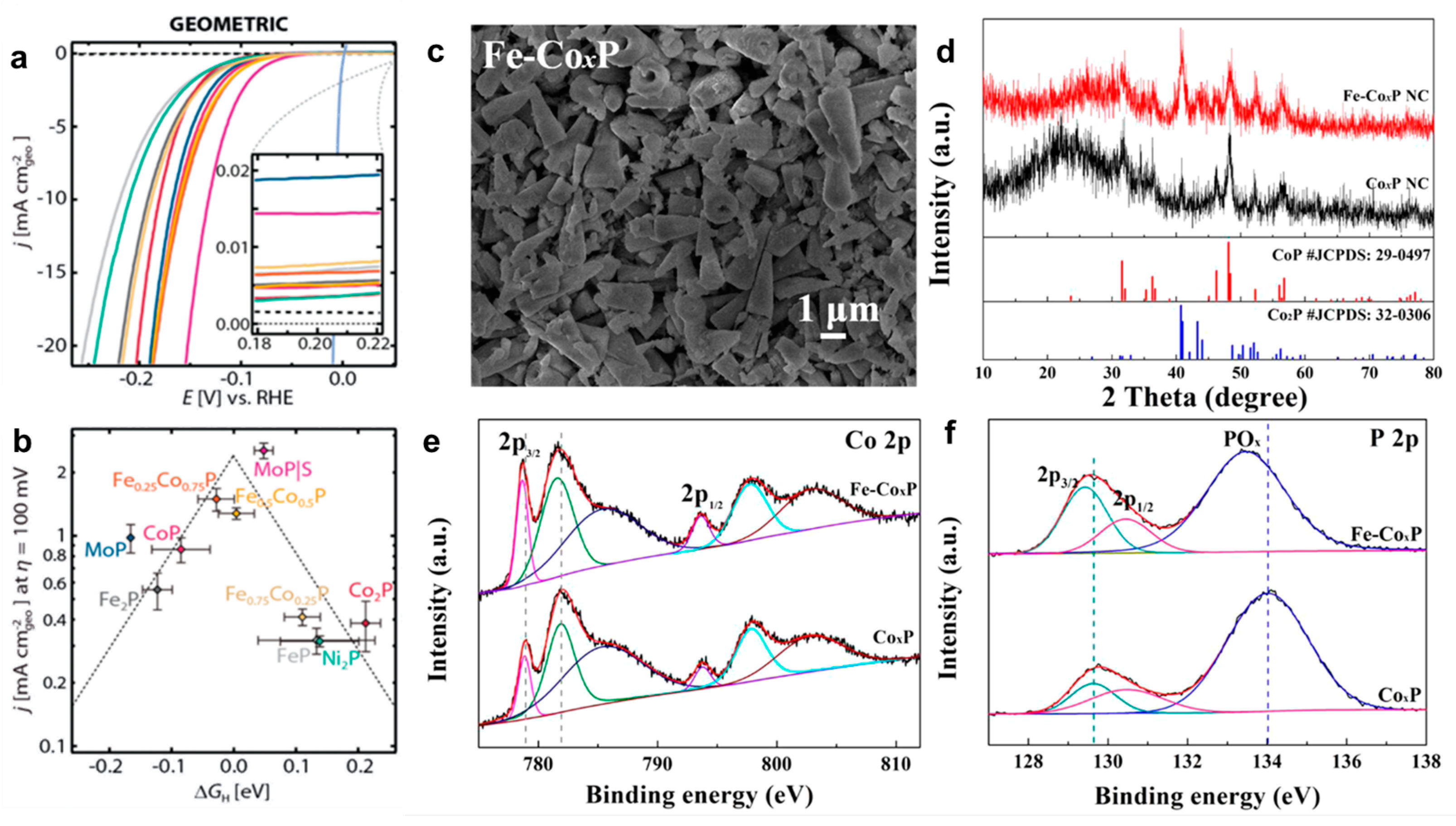
3.1.2. Bimetal Phosphide Electrocatalysts
3.2. OER
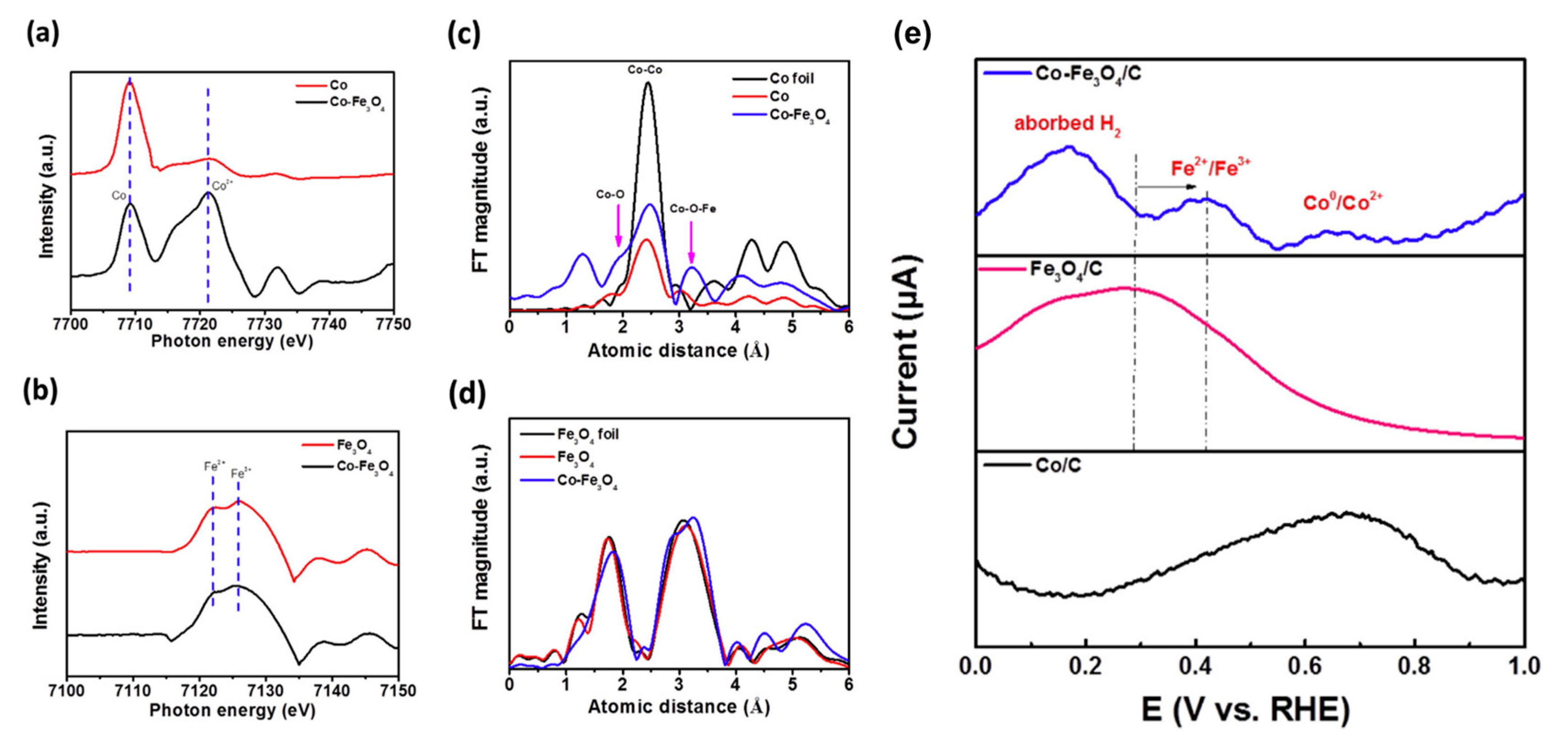
3.2.1. Bimetallic Alloy-Based Electrocatalysts
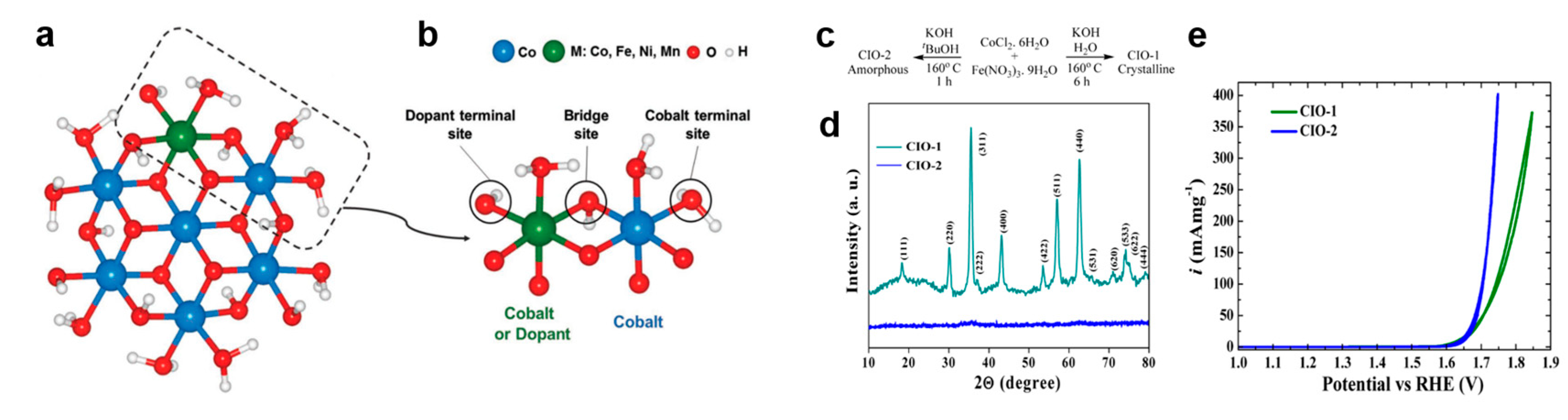
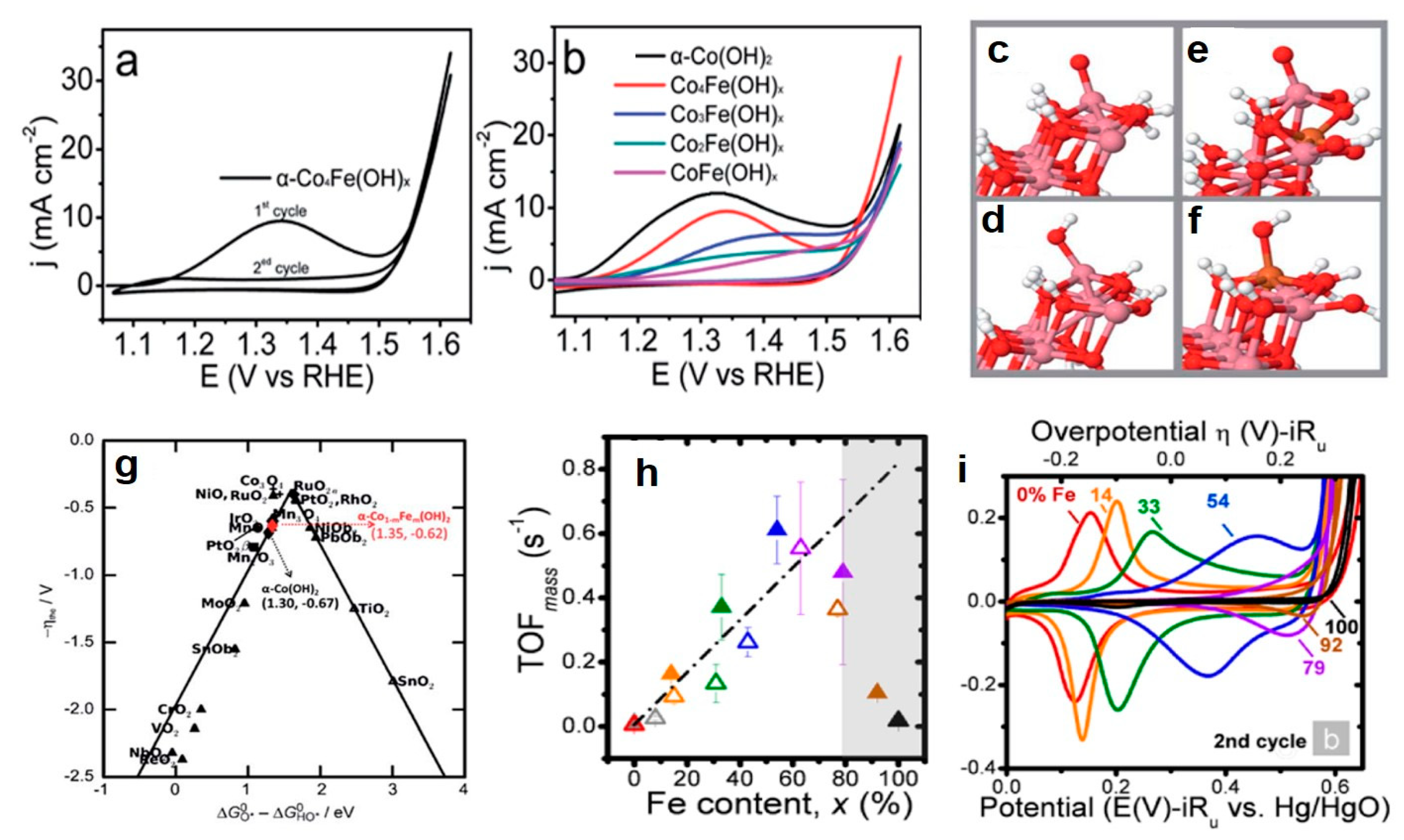
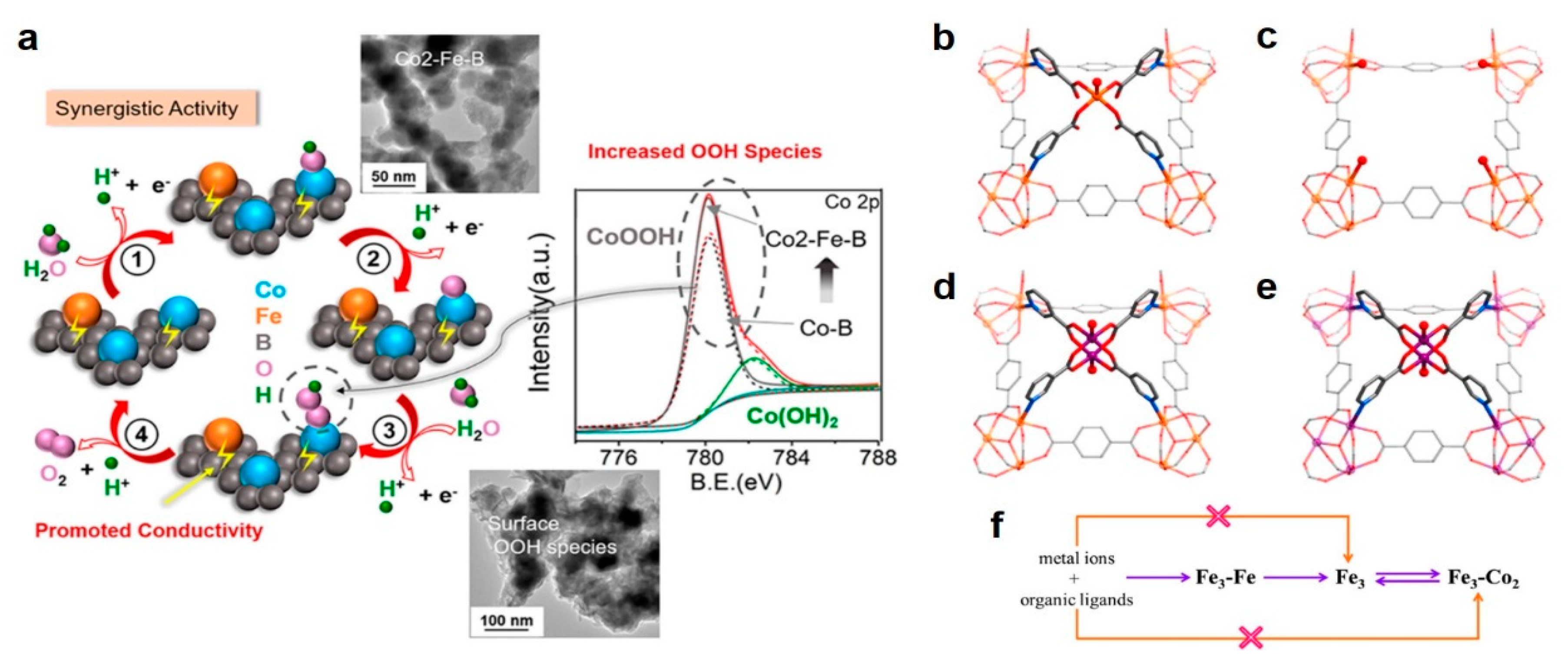
3.2.2. Bimetal Oxides and Hydroxides Electrocatalysts
3.2.3. Dual-Metal M–N–C Site-Based Electrocatalysts
3.3. ORR
3.3.1. Bimetallic Alloy-Based Electrocatalysts
3.3.2. Bimetal Derivatives Electrocatalysts
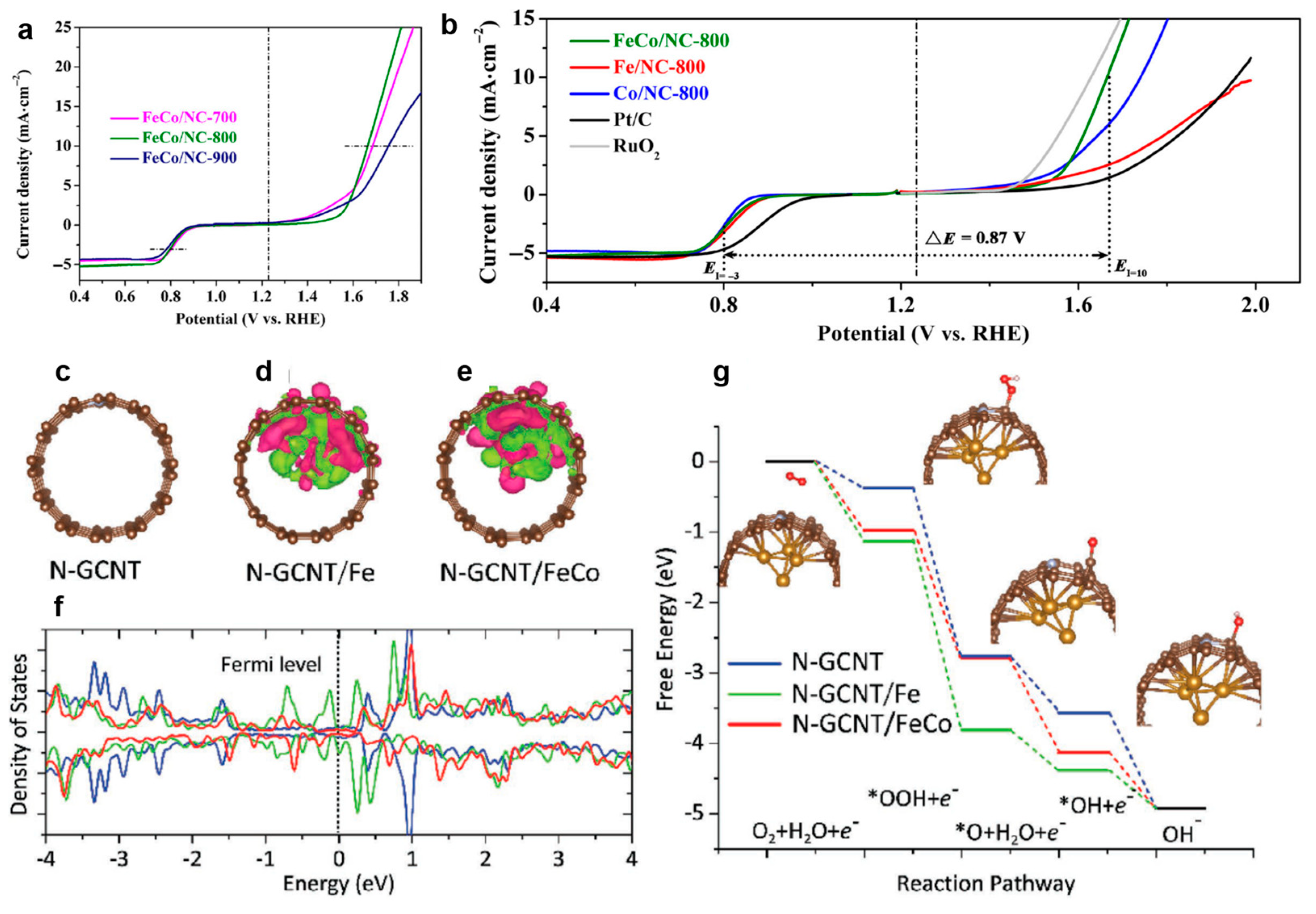
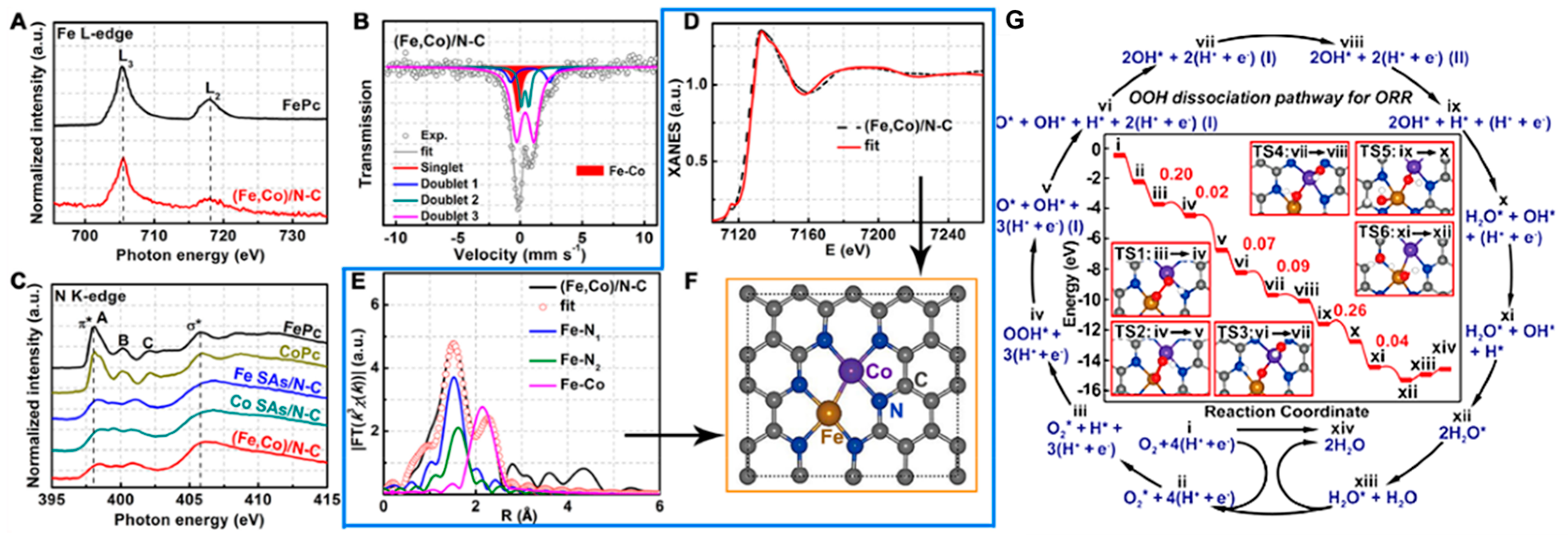
3.3.3. Dual Metal M–N–C Sites-Based Electrocatalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tao, L.; Wang, Y.; Zou, Y.; Zhang, N.; Zhang, Y.; Wu, Y.; Wang, Y.; Chen, R.; Wang, S. Charge Transfer Modulated Activity of Carbon-Based Electrocatalysts. Adv. Energy Mater. 2019, 1901227. [Google Scholar] [CrossRef]
- Dong, D.; Wu, Z.; Wang, J.; Fu, G.; Tang, Y. Recent progress in Co9S8-based materials for hydrogen and oxygen electrocatalysis. J. Mater. Chem. A 2019, 7, 16068–16088. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Zhao, B.; Wang, X. A review on noble-metal-free bifunctional heterogeneous catalysts for overall electrochemical water splitting. J. Mater. Chem. A 2016, 4, 17587–17603. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, J.; Kumar, B.; Mugweru, A.M.; Trinh, P.; Ramanujachary, K.V.; Lofland, S.E.; Govind; Ganguli, A.K. Binary Fe-Co Alloy Nanoparticles Showing Significant Enhancement in Electrocatalytic Activity Compared with Bulk Alloys. J. Phys. Chem. C 2010, 114, 18779–18784. [Google Scholar] [CrossRef]
- Peng, P.; Lin, X.-M.; Liu, Y.; Filatov, A.S.; Li, D.; Stamenkovic, V.R.; Yang, D.; Prakapenka, V.B.; Lei, A.; Shevchenko, E.V. Binary Transition-Metal Oxide Hollow Nanoparticles for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2018, 10, 24715–24724. [Google Scholar] [CrossRef]
- Babar, P.; Lokhande, A.; Shin, H.H.; Pawar, B.; Gang, M.G.; Pawar, S.; Kim, J.H. Cobalt Iron Hydroxide as a Precious Metal-Free Bifunctional Electrocatalyst for Efficient Overall Water Splitting. Small 2018, 14, 1702568. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, G.; Liu, Y.; Xia, J.; Ji, Z.; Shen, X.; Wu, S. Fe3O4-Decorated Co9S8 Nanoparticles in Situ Grown on Reduced Graphene Oxide: A New and Efficient Electrocatalyst for Oxygen Evolution Reaction. Adv. Funct. Mater. 2016, 26, 4712–4721. [Google Scholar] [CrossRef]
- Kibsgaard, J.; Tsai, C.; Chan, K.; Benck, J.D.; Norskov, J.K.; Abild-Pedersen, F.; Jaramillo, T.F. Designing an improved transition metal phosphide catalyst for hydrogen evolution using experimental and theoretical trends. Energy Environ. Sci. 2015, 8, 3022–3029. [Google Scholar] [CrossRef]
- Jo, C.; Lee, J.I.; Jang, Y. Electronic and Magnetic Properties of Ultrathin Fe−Co Alloy Nanowires. Chem. Mater. 2005, 17, 2667–2671. [Google Scholar] [CrossRef]
- Bergmann, A.; Martinez-Moreno, E.; Teschner, D.; Chernev, P.; Gliech, M.; de Araujo, J.F.; Reier, T.; Dau, H.; Strasser, P. Reversible amorphization and the catalytically active state of crystalline Co3O4 during oxygen evolution. Nat. Commun. 2015, 6, 8625. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Mao, S.; Zhan, G.; Xu, F.; Bao, X.; Wang, Y. Fe incorporated α-Co(OH)2 nanosheets with remarkably improved activity towards the oxygen evolution reaction. J. Mater. Chem. A 2017, 5, 1078–1084. [Google Scholar] [CrossRef]
- Yang, F.; Sliozberg, K.; Sinev, I.; Antoni, H.; Baehr, A.; Ollegott, K.; Xia, W.; Masa, J.; Gruenert, W.; Roldan Cuenya, B.; et al. Synergistic Effect of Cobalt and Iron in Layered Double Hydroxide Catalysts for the Oxygen Evolution Reaction. Chemsuschem 2017, 10, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.S.; Kast, M.G.; Trotochaud, L.; Smith, A.M.; Boettcher, S.W. Cobalt-Iron (Oxy)hydroxide Oxygen Evolution Electrocatalysts: The Role of Structure and Composition on Activity, Stability, and Mechanism. J. Am. Chem. Soc. 2015, 137, 3638–3648. [Google Scholar] [CrossRef] [PubMed]
- Griboval-Constant, A.; Butel, A.; Ordomsy, V.V.; Chernavskii, P.A.; Khodakova, A.Y. Cobalt and iron species in alumina supported bimetallic catalysts for Fischer-Tropsch reaction. Appl. Catal. A 2014, 481, 116–126. [Google Scholar] [CrossRef]
- Antonio Diaz, J.; Akhavan, H.; Romero, A.; Maria Garcia-Minguillan, A.; Romero, R.; Giroir-Fendler, A.; Luis Valverde, J. Cobalt and iron supported on carbon nanofibers as catalysts for Fischer-Tropsch synthesis. Fuel Process. Technol. 2014, 128, 417–424. [Google Scholar] [CrossRef]
- Masa, J.; Weide, P.; Peeters, D.; Sinev, I.; Xia, W.; Sun, Z.; Somsen, C.; Muhler, M.; Schuhmann, W. Amorphous Cobalt Boride (Co2B) as a Highly Efficient Nonprecious Catalyst for Electrochemical Water Splitting: Oxygen and Hydrogen Evolution. Adv. Energy Mater. 2016, 6, 1502313. [Google Scholar] [CrossRef]
- Chen, H.; Ouyang, S.; Zhao, M.; Li, Y.; Ye, J. Synergistic Activity of Co and Fe in Amorphous Cox-Fe-B Catalyst for Efficient Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 40333–40343. [Google Scholar] [CrossRef]
- Wu, Z.; Nie, D.; Song, M.; Jiao, T.; Fu, G.; Liu, X. Facile synthesis of Co-Fe-B-P nanochains as an efficient bifunctional electrocatalyst for overall water-splitting. Nanoscale 2019, 11, 7506–7512. [Google Scholar] [CrossRef]
- Yang, L.; Guo, Z.; Huang, J.; Xi, Y.; Gao, R.; Su, G.; Wang, W.; Cao, L.; Dong, B. Vertical Growth of 2D Amorphous FePO4 Nanosheet on Ni Foam: Outer and Inner Structural Design for Superior Water Splitting. Adv. Mater. 2017, 29, 1704574. [Google Scholar] [CrossRef]
- Yang, Y.; Zhuang, L.; Lin, R.; Li, M.; Xu, X.; Rufford, T.E.; Zhu, Z. A facile method to synthesize boron-doped Ni/Fe alloy nano-chains as electrocatalyst for water oxidation. J. Power Sources 2017, 349, 68–74. [Google Scholar] [CrossRef]
- Li, Y.; Fan, X.; Qi, J.; Ji, J.; Wang, S.; Zhang, G.; Zhang, F. Palladium nanoparticle-graphene hybrids as active catalysts for the Suzuki reaction. Nano Res. 2010, 3, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ma, J.; Fan, X.; Peng, W.; Zhang, G.; Zhang, F.; Li, Y. Graphene supported Au-Pd-Fe3O4 alloy trimetallic nanoparticles with peroxidase-like activities as mimic enzyme. Catal. Commun. 2017, 89, 148–151. [Google Scholar] [CrossRef]
- Mattioli, G.; Giannozzi, P.; Bonapasta, A.A.; Guidonili, L. Reaction Pathways for Oxygen Evolution Promoted by Cobalt Catalyst. J. Am. Chem. Soc. 2013, 135, 15353–15363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Yu, C.; Zhou, S.; Zhao, C.; Huang, H.; Yang, J.; Liu, Z.; Zhao, J.; Qiu, J. Ultrasensitive Iron-Triggered Nanosized Fe–CoOOH Integrated with Graphene for Highly Efficient Oxygen Evolution. Adv. Energy Mater. 2017, 7, 1602148. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Jaroniec, M.; Qiao, S.Z. Origin of the Electrocatalytic Oxygen Reduction Activity of Graphene-Based Catalysts: A Roadnnap to Achieve the Best Performance. J. Am. Chem. Soc. 2014, 136, 4394–4403. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Lei, Y.; Wang, Q.; Wang, B.; Han, C.; Wang, Y. Facile synthesis of FeCo@NC core–shell nanospheres supported on graphene as an efficient bifunctional oxygen electrocatalyst. Nano Res. 2017, 10, 2332–2343. [Google Scholar] [CrossRef]
- Seo, W.S.; Lee, J.H.; Sun, X.; Suzuki, Y.; Mann, D.; Liu, Z.; Terashima, M.; Yang, P.C.; McConnell, M.V.; Nishimura, D.G.; et al. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat. Mater. 2006, 5, 971–976. [Google Scholar] [CrossRef]
- Zhu, S.J.; Zhang, J.H.; Qiao, C.Y.; Tang, S.J.; Li, Y.F.; Yuan, W.J.; Li, B.; Tian, L.; Liu, F.; Hu, R.; et al. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Navalon, S.; Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Carbocatalysis by Graphene-Based Materials. Chem. Rev. 2014, 114, 6179–6212. [Google Scholar] [CrossRef]
- Palaniselvam, T.; Kashyap, V.; Bhange, S.N.; Baek, J.-B.; Kurungot, S. Nanoporous Graphene Enriched with Fe/Co-N Active Sites as a Promising Oxygen Reduction Electrocatalyst for Anion Exchange Membrane Fuel Cells. Adv. Funct. Mater. 2016, 26, 2150–2162. [Google Scholar] [CrossRef]
- Palaniselvam, T.; Valappil, M.O.; Illathvalappil, R.; Kurungot, S. Nanoporous graphene by quantum dots removal from graphene and its conversion to a potential oxygen reduction electrocatalyst via nitrogen doping. Energy Environ. Sci. 2014, 7, 1059–1067. [Google Scholar] [CrossRef]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-Atom Catalysts: A New Frontier in Heterogeneous Catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.T.; Wang, A.Q.; Yang, X.F.; Allard, L.F.; Jiang, Z.; Cui, Y.T.; Liu, J.Y.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt-1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Bakandritsos, A.; Kadam, R.G.; Kumar, P.; Zoppellaro, G.; Medved, M.; Tucek, J.; Montini, T.; Tomanec, O.; Andryskova, P.; Drahos, B.; et al. Mixed-Valence Single-Atom Catalyst Derived from Functionalized Graphene. Adv. Mater. 2019, 31, e1900323. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Babu, D.D.; Huang, Y.; Lv, J.; Wang, Y.; Wu, M. Atomic dispersion of Fe/Co/N on graphene by ball-milling for efficient oxygen evolution reaction. Int. J. Hydrog. Energy 2018, 43, 10351–11035. [Google Scholar] [CrossRef]
- Li, M.; Liu, T.; Bo, X.; Zhou, M.; Guo, L. A novel flower-like architecture of FeCo@NC-functionalized ultra-thin carbon nanosheets as a highly efficient 3D bifunctional electrocatalyst for full water splitting. J. Mater. Chem. A 2017, 5, 5413–5425. [Google Scholar] [CrossRef]
- Samanta, A.; Raj, C.R. Catalyst Support in Oxygen Electrocatalysis: A Case Study with CoFe Alloy Electrocatalyst. J. Phys. Chem. C 2018, 122, 15843–15852. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, S.; Qiu, Y.; Zhao, L. Bimetallic Fe-Co promoting one-step growth of hierarchical nitrogen-doped carbon nanotubes/nanofibers for highly efficient oxygen reduction reaction. Mater. Sci. Eng. B 2017, 223, 159–166. [Google Scholar] [CrossRef]
- Li, C.; Wu, M.; Liu, R. High-performance bifunctional oxygen electrocatalysts for zinc-air batteries over mesoporous Fe/Co-N-C nanofibers with embedding FeCo alloy nanoparticles. Appl. Catal. B 2019, 244, 150–158. [Google Scholar] [CrossRef]
- Yan, Y.; Miao, J.; Yang, Z.; Xiao, F.-X.; Yang, H.B.; Liu, B.; Yang, Y. Carbon nanotube catalysts: Recent advances in synthesis, characterization and applications. Chem. Soc. Rev. 2015, 44, 3295–3346. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-Y.; Cheng, H.; Li, W.; Liu, Z.-Q.; Li, N.; Hou, Z.; Bai, F.-Q.; Zhang, H.-X.; Ma, T.-Y. Atomic Modulation of FeCo-Nitrogen-Carbon Bifunctional Oxygen Electrodes for Rechargeable and Flexible All-Solid-State Zinc-Air Battery. Adv. Energy Mater. 2017, 7, 1602420. [Google Scholar] [CrossRef]
- Yang, Y.; Lun, Z.; Xia, G.; Zheng, F.; He, M.; Chen, Q. Non-precious alloy encapsulated in nitrogen-doped graphene layers derived from MOFs as an active and durable hydrogen evolution reaction catalyst. Energy Environ. Sci. 2015, 8, 3563–3571. [Google Scholar] [CrossRef]
- Lian, Y.; Sun, H.; Wang, X.; Qi, P.; Mu, Q.; Chen, Y.; Ye, J.; Zhao, X.; Deng, Z.; Peng, Y. Carved nanoframes of cobalt-iron bimetal phosphide as a bifunctional electrocatalyst for efficient overall water splitting. Chem. Sci. 2019, 10, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, H.; Luo, R.; Zhang, M.; Yan, X.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Li, J. Prussian blue analogues-derived bimetallic iron-cobalt selenides for efficient overall water splitting. J. Colloid Interface Sci. 2019, 548, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, L.; Zhao, Z.-J.; Li, A.; Chang, X.; Gong, J. Synergism of Geometric Construction and Electronic Regulation: 3D Se-(NiCo)Sx/(OH)x Nanosheets for Highly Efficient Overall Water Splitting. Adv. Mater. 2018, 30, 1705538. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, J.; Xie, J.-Q.; Ye, H.; Fu, X.-Z.; Sun, R.; Wong, C.-P. Co-Fe-P nanotubes electrocatalysts derived from metal-organic frameworks for efficient hydrogen evolution reaction under wide pH range. Nano Energy 2019, 56, 225–233. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Jin, H.; Chen, Y.; Wang, Y. Non-Noble Metal-based Carbon Composites in Hydrogen Evolution Reaction: Fundamentals to Applications. Adv. Mater. 2017, 29, 1605838. [Google Scholar] [CrossRef]
- Wang, X.; Yu, L.; Guan, B.Y.; Song, S.; Lou, X.W. Metal–Organic Framework Hybrid-Assisted Formation of Co3O4/Co-Fe Oxide Double-Shelled Nanoboxes for Enhanced Oxygen Evolution. Adv. Mater. 2018, 30, 1801211. [Google Scholar] [CrossRef] [PubMed]
- Trotochaud, L.; Young, S.L.; Ranney, J.K.; Boettcher, S.W. Nickel–Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation. J. Am. Chem. Soc. 2014, 136, 6744–6753. [Google Scholar] [CrossRef]
- Chen, W.X.; Pei, J.J.; He, C.T.; Wan, J.W.; Ren, H.L.; Zhu, Y.Q.; Wang, Y.; Dong, J.C.; Tian, S.B.; Cheong, W.C.; et al. Rational Design of Single Molybdenum Atoms Anchored on N-Doped Carbon for Effective Hydrogen Evolution Reaction. Angew. Chem. Int. Edit. 2017, 56, 16086–16090. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, Z.; Liu, W.; Chang, C.; Tang, H.; Li, Z.; Chen, W.; Jia, C.; Yao, T.; Wei, S.; et al. Design of N-Coordinated Dual-Metal Sites: A Stable and Active Pt-Free Catalyst for Acidic Oxygen Reduction Reaction. J. Am. Chem. Soc. 2017, 139, 17281–17284. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Dogutan, D.K.; Reece, S.Y.; Surendranath, Y.; Teets, T.S.; Nocera, D.G. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010, 110, 6474–6502. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Wang, C.; Wang, X.; Lin, Y.; Jiang, J.; Xiong, Y. Trimetallic TriStar Nanostructures: Tuning Electronic and Surface Structures for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2016, 28, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.I.; Sellschopp, K.; Tegel, M.; Rauscher, T.; Kieback, B.; Röntzsch, L. The activity of nanocrystalline Fe-based alloys as electrode materials for the hydrogen evolution reaction. J. Power Sources 2016, 304, 196–206. [Google Scholar] [CrossRef]
- Liu, W.; Du, K.; Liu, L.; Zhang, J.; Zhu, Z.; Shao, Y.; Li, M. One-step electroreductively deposited iron-cobalt composite films as efficient bifunctional electrocatalysts for overall water splitting. Nano Energy 2017, 38, 576–584. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Xu, H.; Wu, Z.; Wang, H.; Liang, Y. Iron-Doped Cobalt Monophosphide Nanosheet/ Carbon Nanotube Hybrids as Active and Stable Electrocatalysts for Water Splitting. Adv. Funct. Mater. 2017, 27, 1606635. [Google Scholar] [CrossRef]
- Guo, X.; Yu, X.; Feng, Z.; Liang, J.; Li, Q.; Lv, Z.; Liu, B.; Hao, C.; Li, G. Intercalation Synthesis of Prussian Blue Analogue Nanocone and Their Conversion into Fe-Doped CoxP Nanocone for Enhanced Hydrogen Evolution. ACS Sustain. Chem. Eng. 2018, 6, 8150–8158. [Google Scholar] [CrossRef]
- Li, F.; Bu, Y.; Lv, Z.; Mahmood, J.; Han, G.-F.; Ahmad, I.; Kim, G.; Zhong, Q.; Baek, J.-B. Porous Cobalt Phosphide Polyhedrons with Iron Doping as an Efficient Bifunctional Electrocatalyst. Small 2017, 13, 1701167. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.M.; Hu, Y.W.; Tang, S.F.; Iljin, A.; Wang, J.W.; Zhang, Z.M.; Lu, T.B. Fe-CoP Electrocatalyst Derived from a Bimetallic Prussian Blue Analogue for Large-Current-Density Oxygen Evolution and Overall Water Splitting. Adv. Sci. 2018, 5, 1800949. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Oh, S.; Cho, E.; Kwon, H. 3D Porous Cobalt–Iron–Phosphorus Bifunctional Electrocatalyst for the Oxygen and Hydrogen Evolution Reactions. ACS Sustain. Chem. Eng. 2018, 6, 6305–6311. [Google Scholar] [CrossRef]
- Kuo, T.-R.; Chen, W.-T.; Liao, H.-J.; Yang, Y.-H.; Yen, H.-C.; Liao, T.-W.; Wen, C.-Y.; Lee, Y.-C.; Chen, C.-C.; Wang, D.-Y. Improving Hydrogen Evolution Activity of Earth-Abundant Cobalt-Doped Iron Pyrite Catalysts by Surface Modification with Phosphide. Small 2017, 13, 1603356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, F.; Yang, H.; Li, J.; Ma, P.; Zhu, Y.; Ma, J. Two-Step Synthesis of Cobalt Iron Alloy Nanoparticles Embedded in Nitrogen-Doped Carbon Nanosheets/Carbon Nanotubes for the Oxygen Evolution Reaction. ChemSusChem 2018, 11, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Yu, P.; Tian, C.; Sun, F.; Ma, J.; Li, W.; Fu, H. A Stable Bifunctional Catalyst for Rechargeable Zinc-Air Batteries: Iron-Cobalt Nanoparticles Embedded in a Nitrogen-Doped 3D Carbon Matrix. Angew. Chem. Int. Ed. Engl. 2018, 57, 16166–16170. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Bo, X.; Guo, L. CoM(M = Fe,Cu,Ni)-embedded nitrogen-enriched porous carbon framework for efficient oxygen and hydrogen evolution reactions. J. Power Sources 2018, 389, 249–259. [Google Scholar] [CrossRef]
- Zhu, X.; Jin, T.; Tian, C.; Lu, C.; Liu, X.; Zeng, M.; Zhuang, X.; Yang, S.; He, L.; Liu, H.; et al. In Situ Coupling Strategy for the Preparation of FeCo Alloys and Co4N Hybrid for Highly Efficient Oxygen Evolution. Adv. Mater. 2017, 29, 1704091. [Google Scholar] [CrossRef]
- Li, T.; Lu, Y.; Zhao, S.; Gao, Z.-D.; Song, Y.-Y. Co3O4-doped Co/CoFe nanoparticles encapsulated in carbon shells as bifunctional electrocatalysts for rechargeable Zn–Air batteries. J. Mater. Chem. A 2018, 6, 3730–3737. [Google Scholar] [CrossRef]
- Kim, B.; Park, I.; Yoon, G.; Kim, J.S.; Kim, H.; Kang, K. Atomistic Investigation of Doping Effects on Electrocatalytic Properties of Cobalt Oxides for Water Oxidation. Adv. Sci. 2018, 5, 1801632. [Google Scholar] [CrossRef]
- Indra, A.; Menezes, P.W.; Sahraie, N.R.; Bergmann, A.; Das, C.; Tallarida, M.; Schmeisser, D.; Strasser, P.; Driess, M. Unification of Catalytic Water Oxidation and Oxygen Reduction Reactions: Amorphous Beat Crystalline Cobalt Iron Oxides. J. Am. Chem. Soc. 2014, 136, 17530–17536. [Google Scholar] [CrossRef]
- Chen, W.; Wang, H.; Li, Y.; Liu, Y.; Sun, J.; Lee, S.; Lee, J.-S.; Cui, Y. In Situ Electrochemical Oxidation Tuning of Transition Metal Disulfides to Oxides for Enhanced Water Oxidation. ACS Cent. Sci. 2015, 1, 244–251. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Jin, Z.; Chen, X.; Xiao, D. A robust water oxidation electrocatalyst from amorphous cobalt-iron bimetallic phytate nanostructures. J. Mater. Chem. A 2016, 4, 15888–15895. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, C.; Sun, Y.; Zhang, G.; Shen, X.; Zou, F.; Zhang, H.; Wu, Z.; Wegener, E.C.; Taubert, C.J.; et al. High-Performance Transition Metal Phosphide Alloy Catalyst for Oxygen Evolution Reaction. ACS Nano 2018, 12, 158–167. [Google Scholar] [CrossRef]
- Shen, J.-Q.; Liao, P.-Q.; Zhou, D.-D.; He, C.-T.; Wu, J.-X.; Zhang, W.-X.; Zhang, J.-P.; Chen, X.-M. Modular and Stepwise Synthesis of a Hybrid Metal–Organic Framework for Efficient Electrocatalytic Oxygen Evolution. J. Am. Chem. Soc. 2017, 139, 1778–1781. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D.; Li, Y. Single-Atom Catalysts: Synthetic Strategies and Electrochemical Applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Cheng, C.; Zhao, X.; Schmidt, J.; Thomas, A. Active Salt/Silica-Templated 2D Mesoporous FeCo-Nx -Carbon as Bifunctional Oxygen Electrodes for Zinc-Air Batteries. Angew. Chem. Int. Ed. Engl. 2018, 57, 1856–1862. [Google Scholar] [CrossRef]
- Sultan, S.; Tiwari, J.N.; Jang, J.-H.; Harzandi, A.M.; Salehnia, F.; Yoo, S.J.; Kim, K.S. Highly Efficient Oxygen Reduction Reaction Activity of Graphitic Tube Encapsulating Nitrided CoxFey Alloy. Adv. Energy Mater. 2018, 8, 1801002. [Google Scholar] [CrossRef]
- Lim, S.H.; Li, Z.; Poh, C.K.; Lai, L.; Lin, J. Highly active non-precious metal catalyst based on poly(vinylpyrrolidone)–wrapped carbon nanotubes complexed with iron–cobalt metal ions for oxygen reduction reaction. J. Power Sources 2012, 214, 15–20. [Google Scholar] [CrossRef]
- Guan, B.Y.; Lu, Y.; Wang, Y.; Wu, M.; Lou, X.W.D. Porous Iron-Cobalt Alloy/Nitrogen-Doped Carbon Cages Synthesized via Pyrolysis of Complex Metal-Organic Framework Hybrids for Oxygen Reduction. Adv. Funct. Mater. 2018, 28, 1706738. [Google Scholar] [CrossRef]
- Tan, M.; He, T.; Liu, J.; Wu, H.; Li, Q.; Zheng, J.; Wang, Y.; Sun, Z.; Wang, S.; Zhang, Y. Supramolecular bimetallogels: A nanofiber network for bimetal/nitrogen co-doped carbon electrocatalysts. J. Mater. Chem. A 2018, 6, 8227–8232. [Google Scholar] [CrossRef]
- Wang, J.; Xin, H.L.; Zhu, J.; Liu, S.; Wu, Z.; Wang, D. 3D hollow structured Co2FeO4/MWCNT as an efficient non-precious metal electrocatalyst for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 1601–1608. [Google Scholar] [CrossRef]
- Kim, J.; Gwon, O.; Kwon, O.; Mahmood, J.; Kim, C.; Yang, Y.; Lee, H.; Lee, J.H.; Jeong, H.Y.; Baek, J.B.; et al. Synergistic Coupling Derived Cobalt Oxide with Nitrogenated Holey Two-Dimensional Matrix as an Efficient Bifunctional Catalyst for Metal-Air Batteries. ACS Nano 2019, 13, 5502–5512. [Google Scholar] [CrossRef]
- Wang, C.-H.; Yang, C.-W.; Lin, Y.-C.; Chang, S.-T.; Chang, S.L.Y. Cobalt–iron (II, III) oxide hybrid catalysis with enhanced catalytic activities for oxygen reduction in anion exchange membrane fuel cell. J. Power Sources 2015, 277, 147–154. [Google Scholar] [CrossRef]
- Jin, Y.Q.; Lin, Z.; Zhong, R.; Huang, J.; Liang, G.; Li, J.; Jin, Y.; Meng, H. Cobalt iron carbonate hydroxide hydrate on 3D porous carbon as active and stable bifunctional oxygen electrode for Zn–air battery. J. Power Sources 2018, 402, 388–393. [Google Scholar] [CrossRef]
- Sun, K.; Li, J.; Huang, L.; Ji, S.; Kannan, P.; Li, D.; Liu, L.; Liao, S. Biomass-derived 3D hierarchical N-doped porous carbon anchoring cobalt-iron phosphide nanodots as bifunctional electrocatalysts for Li O2 batteries. J. Power Sources 2019, 412, 433–441. [Google Scholar] [CrossRef]
- Zhao, Y.; Watanabe, K.; Hashimoto, K. Efficient oxygen reduction by a Fe/Co/C/N nano-porous catalyst in neutral media. J. Mater. Chem. A 2013, 1, 1450–1456. [Google Scholar] [CrossRef]
- Lin, Q.; Bu, X.; Kong, A.; Mao, C.; Bu, F.; Feng, P. Heterometal-Embedded Organic Conjugate Frameworks from Alternating Monomeric Iron and Cobalt Metalloporphyrins and Their Application in Design of Porous Carbon Catalysts. Adv. Mater. 2015, 27, 3431–3436. [Google Scholar] [CrossRef]
- Zhang, Z.; Dou, M.; Liu, H.; Dai, L.; Wang, F. A Facile Route to Bimetal and Nitrogen-Codoped 3D Porous Graphitic Carbon Networks for Efficient Oxygen Reduction. Small 2016, 12, 4193–4199. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Li, Y.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Bimetallic Iron–Cobalt Catalysts and Their Applications in Energy-Related Electrochemical Reactions. Catalysts 2019, 9, 762. https://doi.org/10.3390/catal9090762
Li K, Li Y, Peng W, Zhang G, Zhang F, Fan X. Bimetallic Iron–Cobalt Catalysts and Their Applications in Energy-Related Electrochemical Reactions. Catalysts. 2019; 9(9):762. https://doi.org/10.3390/catal9090762
Chicago/Turabian StyleLi, Kai, Yang Li, Wenchao Peng, Guoliang Zhang, Fengbao Zhang, and Xiaobin Fan. 2019. "Bimetallic Iron–Cobalt Catalysts and Their Applications in Energy-Related Electrochemical Reactions" Catalysts 9, no. 9: 762. https://doi.org/10.3390/catal9090762
APA StyleLi, K., Li, Y., Peng, W., Zhang, G., Zhang, F., & Fan, X. (2019). Bimetallic Iron–Cobalt Catalysts and Their Applications in Energy-Related Electrochemical Reactions. Catalysts, 9(9), 762. https://doi.org/10.3390/catal9090762




