Enzyme-Catalyzed Glycosylation of Curcumin and Its Analogues by Glycosyltransferases from Bacillus subtilis ATCC 6633
Abstract
:1. Introduction
2. Result
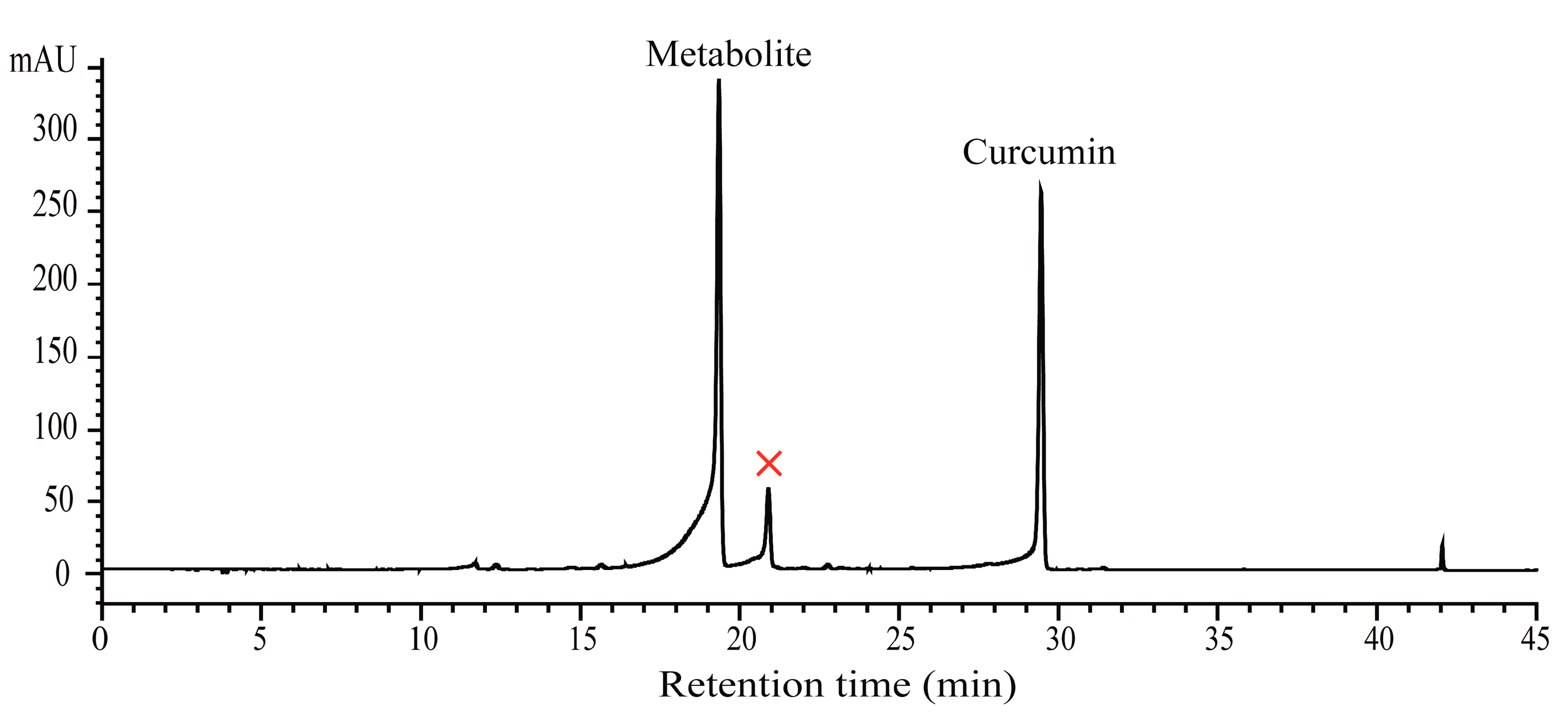
2.1. Biotransformation of Curcumin with B. Subtilis ATCC 6633
2.2. Purification of Recombinant BsGT1 and BsGT2
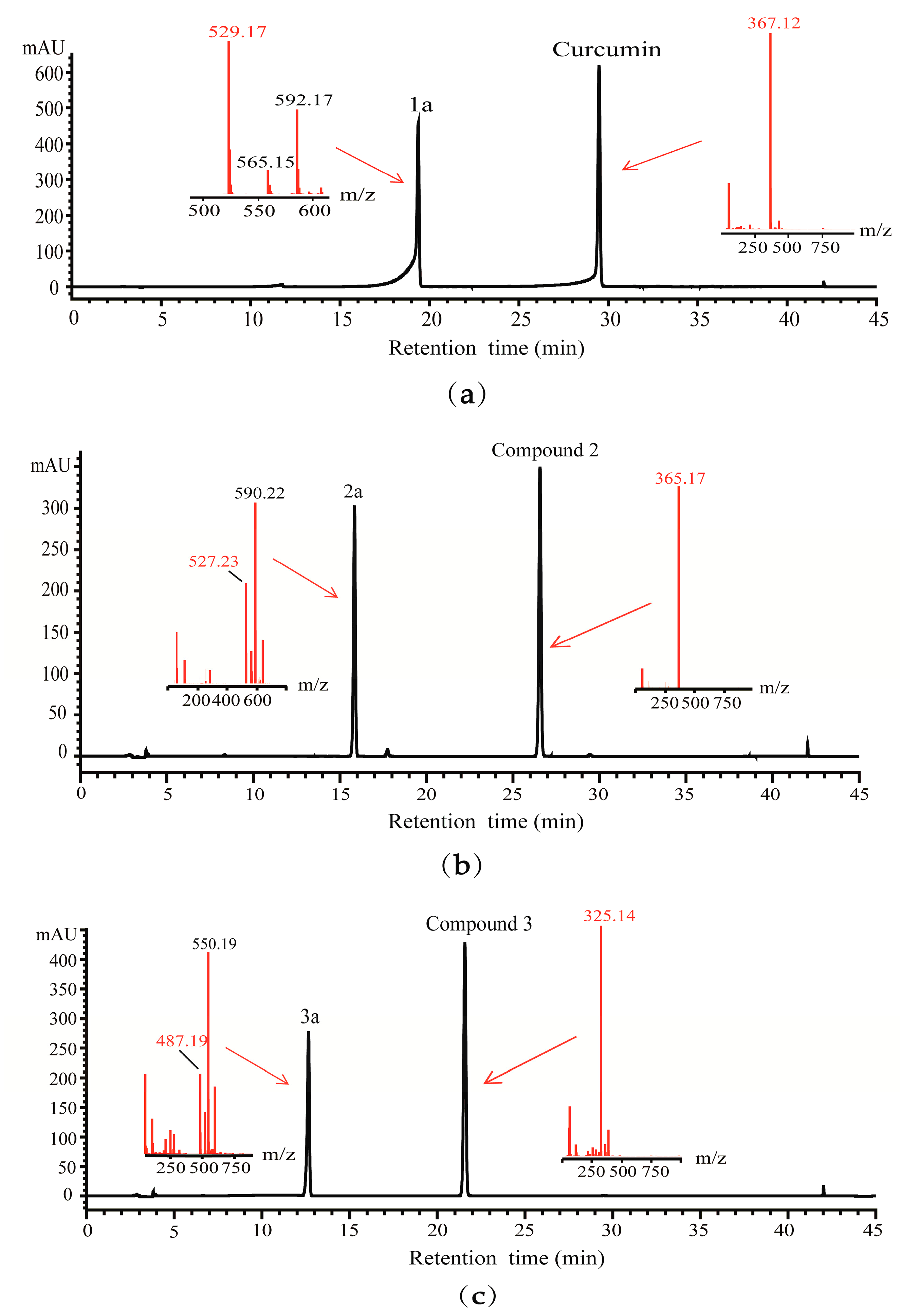
2.3. Detection of Glycosylated Products by HPLC-QTOF-MS
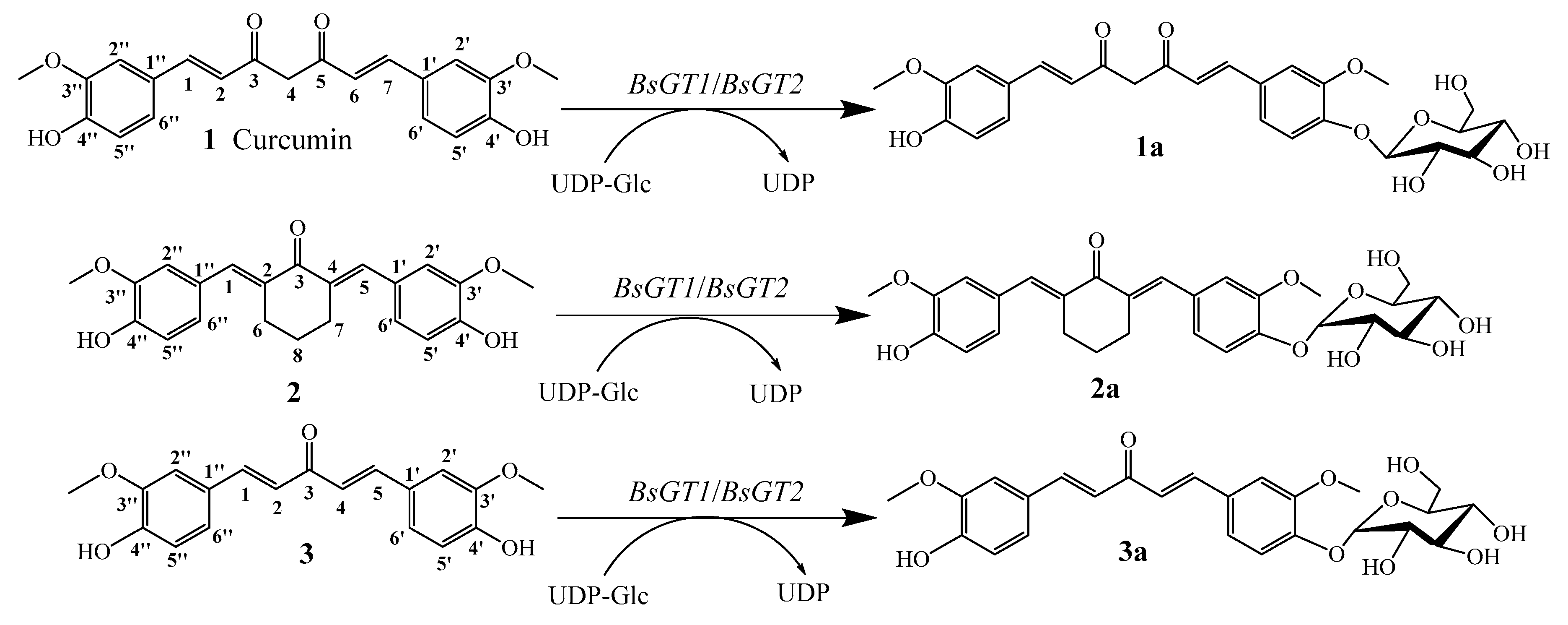
2.4. Structural Elucidation of Curcumin and Its Two Analogues Glucoside Derivatives
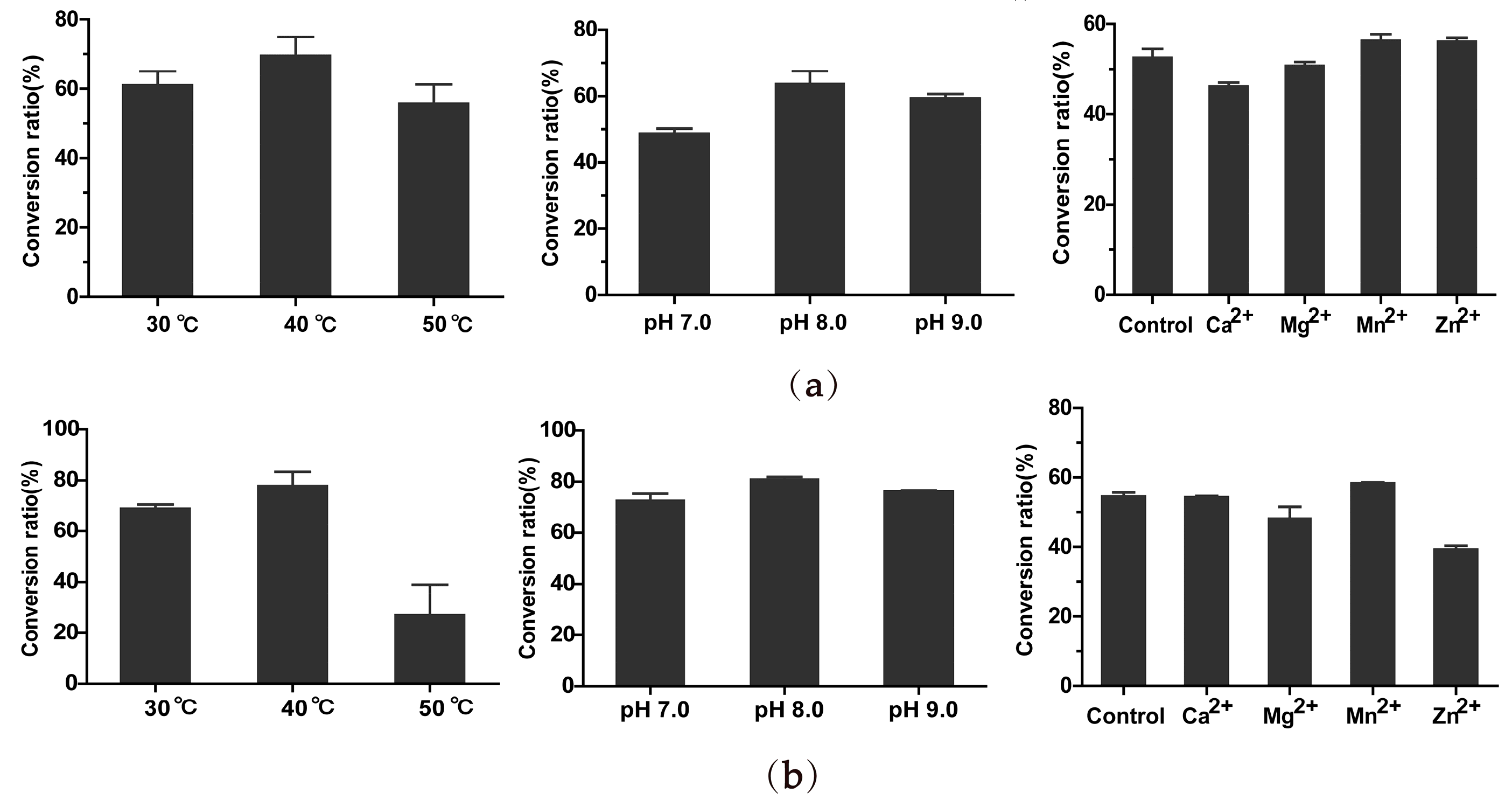
2.5. Optimal Catalyzing Conditions and Kinetic Parameters of BsGT1 and BsGT2 toward Curcumin
2.6. Determination of Solubility of Curcumin and Curcumin 4′-O-β-D-glucoside
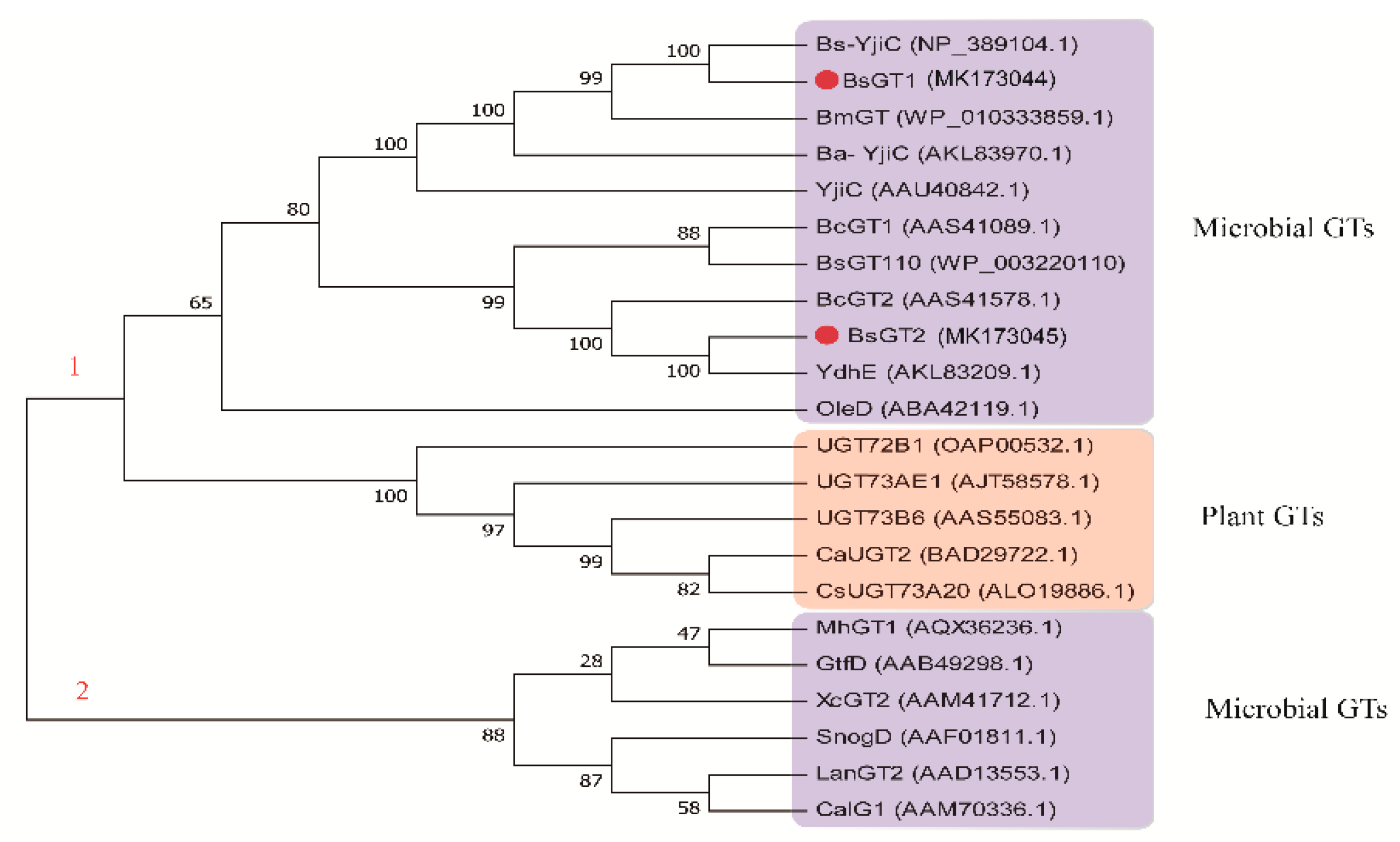
2.7. Phylogenetic Analysis and Sequence Alignment
3. Discussion
4. Materials and Methods
4.1. Microorganisms, Plasmids, and Chemicals
4.2. Identification of Biotransformation Activity of B. subtilis ATCC 6633 to Curcumin
4.3. Expression and Purification of Recombinant BsGT1 and BsGT2
4.4. Preparative-Scale Reactions and Structural Analysis of the Glucosidic Derivatives
4.5. Enzyme Activity Assay, Optimal Catalyzing Conditions, and Kinetic Parameters of BsGT1 and BsGT2 toward Curcumin
4.6. Solubility Test
4.7. Phylogenetic Analysis and Sequence Alignment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scartezzini, P.; Speroni, E. Review on some plants of Indian traditional medicine with antioxidant activity. J. Ethnopharmacol. 2000, 71, 23–43. [Google Scholar] [CrossRef]
- Schmandke, H. D-Limonene in citrus fruit with anticarcinogenic action. Ernährungs Umschau 2003, 50, 264–266. [Google Scholar]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Evangelopoulos, A.; Schizas, N.; Kazazis, C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015, 35, 645–651. [Google Scholar] [PubMed]
- Zhang, S.S.; Gong, Z.J.; Li, W.H.; Wang, X.; Ling, T.Y. Antifibrotic effect of curcumin in TGF-beta 1-induced myofibroblasts from human oral mucosa. Asian. Pac. J. Cancer. Prev. 2012, 13, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Hashish, E.A.; Elgaml, S.A. Hepatoprotective and Nephroprotective Effect of Curcumin Against Copper Toxicity in Rats. Indian. J. Clin. Biochem. 2016, 31, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell. Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.H.; Loo, C.Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its derivatives: Their application in neuropharmacology and neuroscience in the 21st century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef]
- Burgos-Moron, E.; Calderon-Montano, J.M.; Salvador, J.; Robles, A.; Lopez-Lazaro, M. The dark side of curcumin. Int. J. Cancer. 2010, 126, 1771–1775. [Google Scholar] [CrossRef]
- Baell, J.B. Feeling Nature’s PAINS: Natural Products, Natural Product Drugs, and Pan Assay Interference Compounds (PAINS). J. Nat. Prod. 2016, 79, 616–628. [Google Scholar] [CrossRef]
- Chin, D.; Huebbe, P.; Pallauf, K.; Rimbach, G. Neuroprotective properties of curcumin in Alzheimer’s disease--merits and limitations. Curr. Med. Chem. 2013, 20, 3955–3985. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Thorson, J.S. Development of a universal glycosyltransferase assay amenable to high-throughput formats. Anal. Biochem. 2011, 418, 85–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palcic, M.M. Glycosyltransferases as biocatalysts. Curr. Opin. Chem. Biol. 2011, 15, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Thibodeaux, C.J.; Melancon, C.E.; Liu, H.W. Unusual sugar biosynthesis and natural product glycodiversification. Nature 2007, 446, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Honke, K.; Fukuda, M.; Narimatsu, H.; Yamaguchi, Y.; Angata, T. Handbook of Glycosyltransferases and Related Genes; Springer: Berlin/Heidelberg, Germany, 2002; pp. 885–903. [Google Scholar]
- Dai, L.; Li, J.; Yang, J.; Zhu, Y.; Men, Y.; Zeng, Y.; Cai, Y.; Dong, C.; Dai, Z.; Zhang, X.; et al. Use of a Promiscuous Glycosyltransferase from Bacillus subtilis 168 for the Enzymatic Synthesis of Novel Protopanaxatriol-Type Ginsenosides. J. Agric. Food. Chem. 2018, 66, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.P.; Li, T.F.; Kim, E.H.; Yamaguchi, T.; Park, Y.I.; Kim, J.S.; Sohng, J.K. Enzymatic synthesis of novel phloretin glucosides. Appl. Environ. Microbiol. 2013, 79, 3516–3521. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, P.; Cui, Y.; Li, K.; Qiao, X.; Zhang, Y.T.; Li, S.M.; Cox, R.J.; Wu, B.; Ye, M.; et al. Regio and Stereo-specific O-Glycosylation of Phenolic Compounds Catalyzed by a Fungal Glycosyltransferase from Mucor hiemalis. Adv. Synth. Catal. 2017, 359, 995–1006. [Google Scholar] [CrossRef]
- Wang, W.W.; Xu, S.H.; Zhao, Y.Z.; Zhang, C.; Zhang, Y.Y.; Yu, B.Y.; Zhang, J. Microbial hydroxylation and glycosylation of pentacyclic triterpenes as inhibitors on tissue factor procoagulant activity. Bioorg. Med. Chem. Lett. 2017, 27, 1026–1030. [Google Scholar] [CrossRef]
- Lim, E.K. Plant glycosyltransferases: Their potential as novel biocatalysts. Chemistry 2005, 11, 5486–5494. [Google Scholar] [CrossRef]
- Ge, H.X.; Chen, L.; Zhang, J.; Kou, J.P.; Yu, B.Y. Inhibitory effect of curcumin analogs on tissue factor procoagulant activity and their preliminary structure–activity relationships. Med. Chem. Res. 2013, 22, 3242–3246. [Google Scholar] [CrossRef]
- Luo, S.L.; Dang, L.Z.; Zhang, K.Q.; Liang, L.M.; Li, G.H. Cloning and heterologous expression of UDP-glycosyltransferase genes from Bacillus subtilis and its application in the glycosylation of ginsenoside Rh1. Lett. Appl. Microbiol. 2015, 60, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.D.; Jin, Y.; Wang, C.; Kim, Y.J.; Perez, Z.E.J.; Baek, N.I.; Mathiyalagan, R.; Markus, J.; Yang, D.C. Rare ginsenoside Ia synthesized from F1 by cloning and overexpression of the UDP-glycosyltransferase gene from Bacillus subtilis: Synthesis, characterization, and in vitro melanogenesis inhibition activity in BL6B16 cells. J. Ginseng. Res. 2018, 42, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Z.; Jang, J.H.; Woo, M.; Ahn, J.S.; Kim, J.S.; Hong, Y.S. Enzymatic glycosylation of nonbenzoquinone geldanamycin analogs via Bacillus UDP-glycosyltransferase. Appl. Environ. Microbiol. 2012, 78, 7680–7686. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qin, W.; Liu, Q.; Zhang, J.; Li, H.; Xu, S.; Ren, P.; Tian, L.; Li, W. Genome-wide identification and characterization of macrolide glycosyltransferases from a marine-derived Bacillus strain and their phylogenetic distribution. Environ. Microbiol. 2016, 18, 4770–4781. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.; Li, H.M.; Lee, J.K.; Li, J.; Ma, T.; Zhang, X.; Dai, Y.; Hong, Y.S.; Wu, C.Z. Biosynthesis of Novel Glucosides Geldanamycin Analogs by Enzymatic Synthesis. J. Microbiol. Biotechnol. 2016, 26, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Solenberg, P.J.; Matsushima, P.; Stack, D.R.; Wilkie, S.C.; Thompson, R.C.; Baltz, R.H. Production of hybrid glycopeptide antibiotics in vitro and in Streptomyces toyocaensis. Chem. Biol. 1997, 4, 195. [Google Scholar] [CrossRef]
- Chiang, C.M.; Wang, T.Y.; Yang, S.Y.; Wu, J.Y.; Chang, T.S. Production of New Isoflavone Glucosides from Glycosylation of 8-Hydroxydaidzein by Glycosyltransferase from Bacillus subtilis ATCC 6633. Catalysts 2018, 8, 387. [Google Scholar] [CrossRef]
- Yoon, J.A.; Kim, B.G.; Lee, W.J.; Lim, Y.; Chong, Y.; Ahn, J.H. Production of a novel quercetin glycoside through metabolic engineering of Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 4256–4262. [Google Scholar] [CrossRef]
- Gurung, R.B.; Kim, E.H.; Oh, T.J.; Sohng, J.K. Enzymatic synthesis of apigenin glucosides by glucosyltransferase (YjiC) from Bacillus licheniformis DSM 13. Mol. Cells. 2013, 36, 355–361. [Google Scholar] [CrossRef]
- Jeon, Y.M. Enzymatic Glycosylation of Phenolic Compounds Using BsGT-3 Based on Molecular Docking Simulation. J. Korean Soc. Appl. Bi. 2009, 52, 98–101. [Google Scholar] [CrossRef]
- Gurung, R.B.; Gong, S.Y.; Dhakal, D.; Le, T.T.; Jung, N.R.; Jung, H.J.; Oh, T.J.; Sohng, J.K. Erratum to: Synthesis of Curcumin Glycosides with Enhanced Anticancer Properties Using One-Pot Multienzyme Glycosylation Technique. J. Microbiol. Biotechnol. 2018, 28, 347. [Google Scholar] [CrossRef] [PubMed]
- Kaminaga, Y.; Sahin, F.P.; Mizukami, H. Molecular cloning and characterization of a glucosyltransferase catalyzing glucosylation of curcumin in cultured Catharanthus roseus cells. FEBS Lett. 2004, 567, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Dewitte, G.; Walmagh, M.; Diricks, M.; Lepak, A.; Gutmann, A.; Nidetzky, B.; Desmet, T. Screening of recombinant glycosyltransferases reveals the broad acceptor specificity of stevia UGT-76G1. J. Biotechnol. 2016, 233, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Song, M.C.; Kim, E.; Ban, Y.H.; Yoo, Y.J.; Kim, E.J.; Park, S.R.; Pandey, R.P.; Sohng, J.K.; Yoon, Y.J. Achievements and impacts of glycosylation reactions involved in natural product biosynthesis in prokaryotes. Appl. Microbiol. Biotechnol. 2013, 97, 5691–5704. [Google Scholar] [CrossRef] [PubMed]


| GTs | Km (μM) | Kcat (S−1) | Vmax (μM·min−1) |
|---|---|---|---|
| BsGT1 | 200.1 ± 23.73 | 0.47 ± 0.03 | 45.31 ± 2.50 |
| BsGT2 | 118.3 ± 17.69 | 0.18 ± 0.01 | 24.87 ± 1.37 |
| Compound | Aqueous Solubility (mg/L) |
|---|---|
| Curcumin | N.D 1 |
| Curcumin 4′-O-β-D-glucoside | 18.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Zhang, J.; Shao, Y.; Xu, Y.; Ge, H.; Yu, B.; Wang, W. Enzyme-Catalyzed Glycosylation of Curcumin and Its Analogues by Glycosyltransferases from Bacillus subtilis ATCC 6633. Catalysts 2019, 9, 734. https://doi.org/10.3390/catal9090734
Cheng Y, Zhang J, Shao Y, Xu Y, Ge H, Yu B, Wang W. Enzyme-Catalyzed Glycosylation of Curcumin and Its Analogues by Glycosyltransferases from Bacillus subtilis ATCC 6633. Catalysts. 2019; 9(9):734. https://doi.org/10.3390/catal9090734
Chicago/Turabian StyleCheng, Yatian, Jian Zhang, Yan Shao, Yixiang Xu, Haixia Ge, Boyang Yu, and Weiwei Wang. 2019. "Enzyme-Catalyzed Glycosylation of Curcumin and Its Analogues by Glycosyltransferases from Bacillus subtilis ATCC 6633" Catalysts 9, no. 9: 734. https://doi.org/10.3390/catal9090734
APA StyleCheng, Y., Zhang, J., Shao, Y., Xu, Y., Ge, H., Yu, B., & Wang, W. (2019). Enzyme-Catalyzed Glycosylation of Curcumin and Its Analogues by Glycosyltransferases from Bacillus subtilis ATCC 6633. Catalysts, 9(9), 734. https://doi.org/10.3390/catal9090734





