Magnetic Nanoparticles for Photocatalytic Ozonation of Organic Pollutants
Abstract
1. Introduction
2. Results
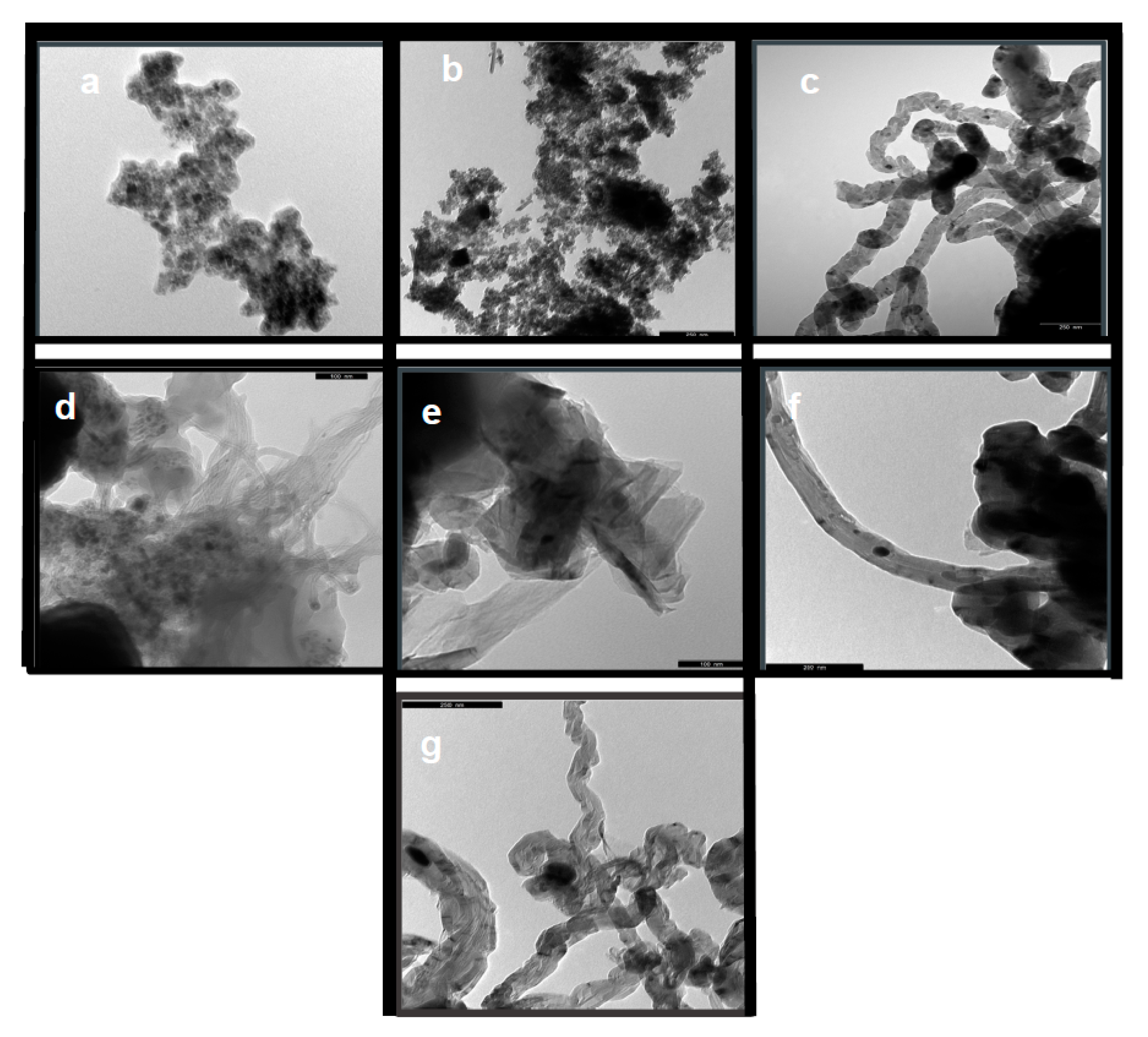
2.1. Catalyst Characterisation
2.2. OMA Removal by MNP
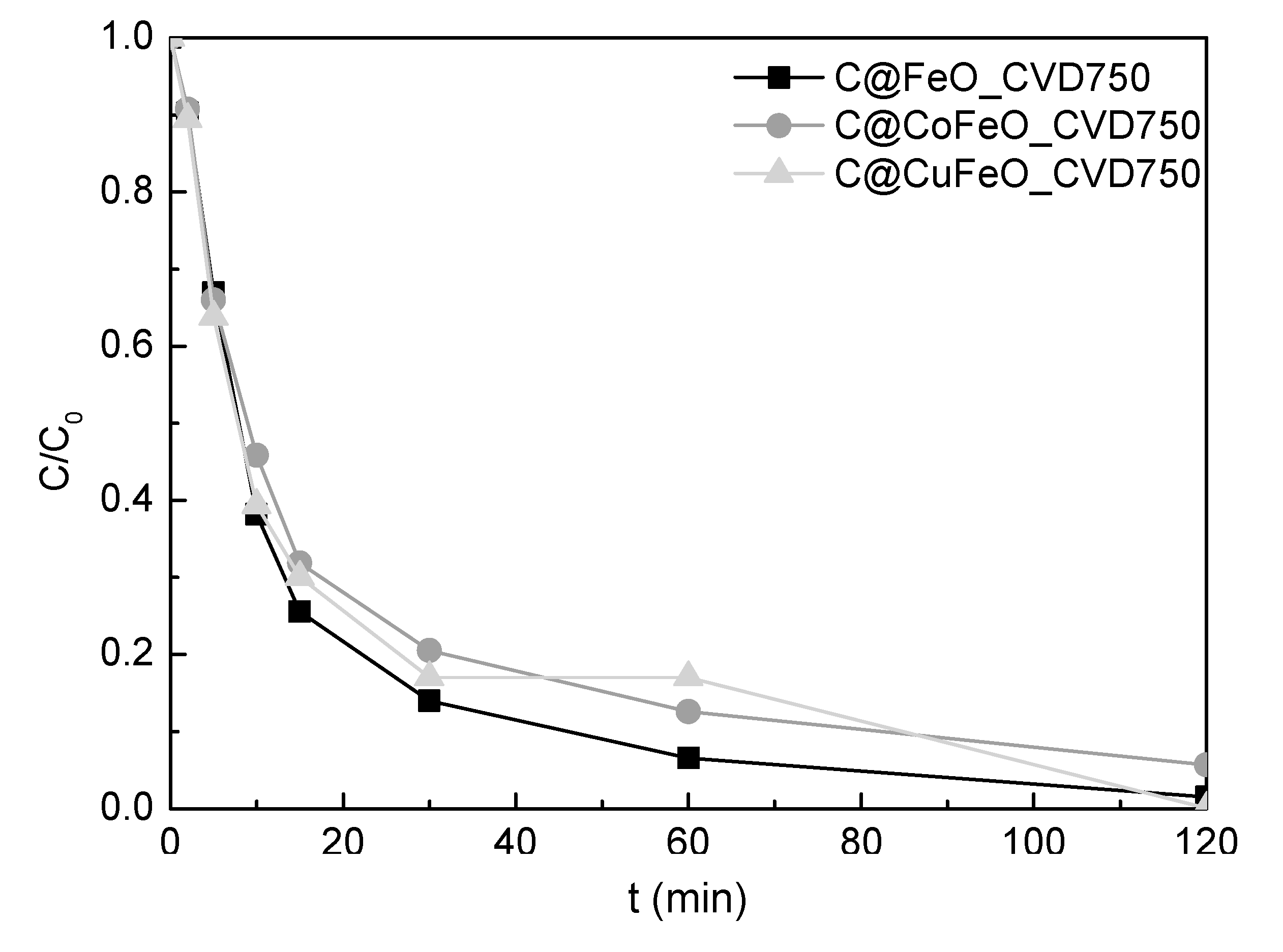
2.3. OMA Removal by Carbon Coated MNP by Using 750 °C CVD
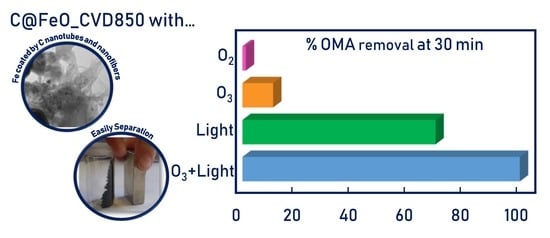
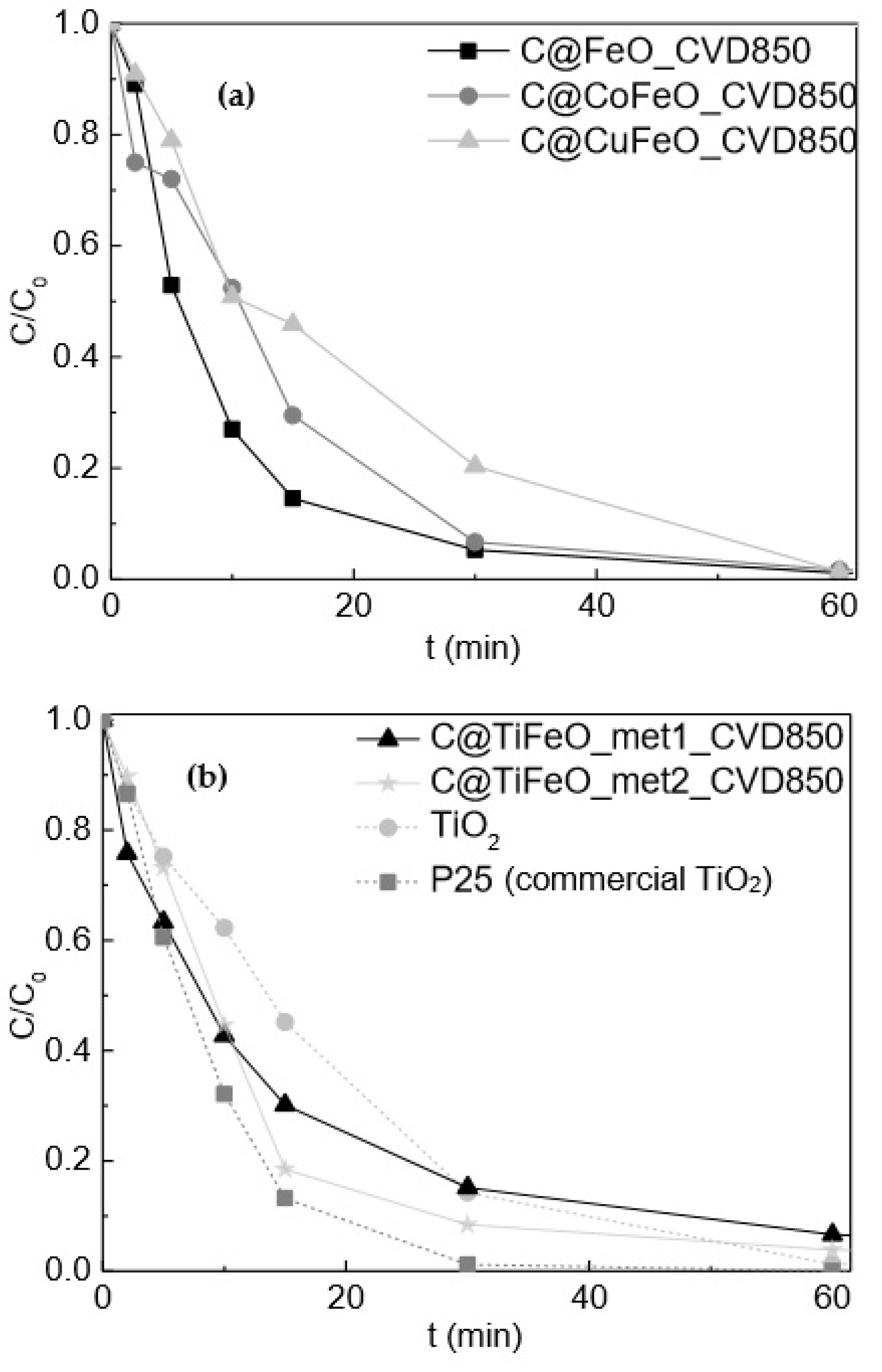
2.4. OMA Removal by Carbon Coated MNP by Using 850 °C CVD
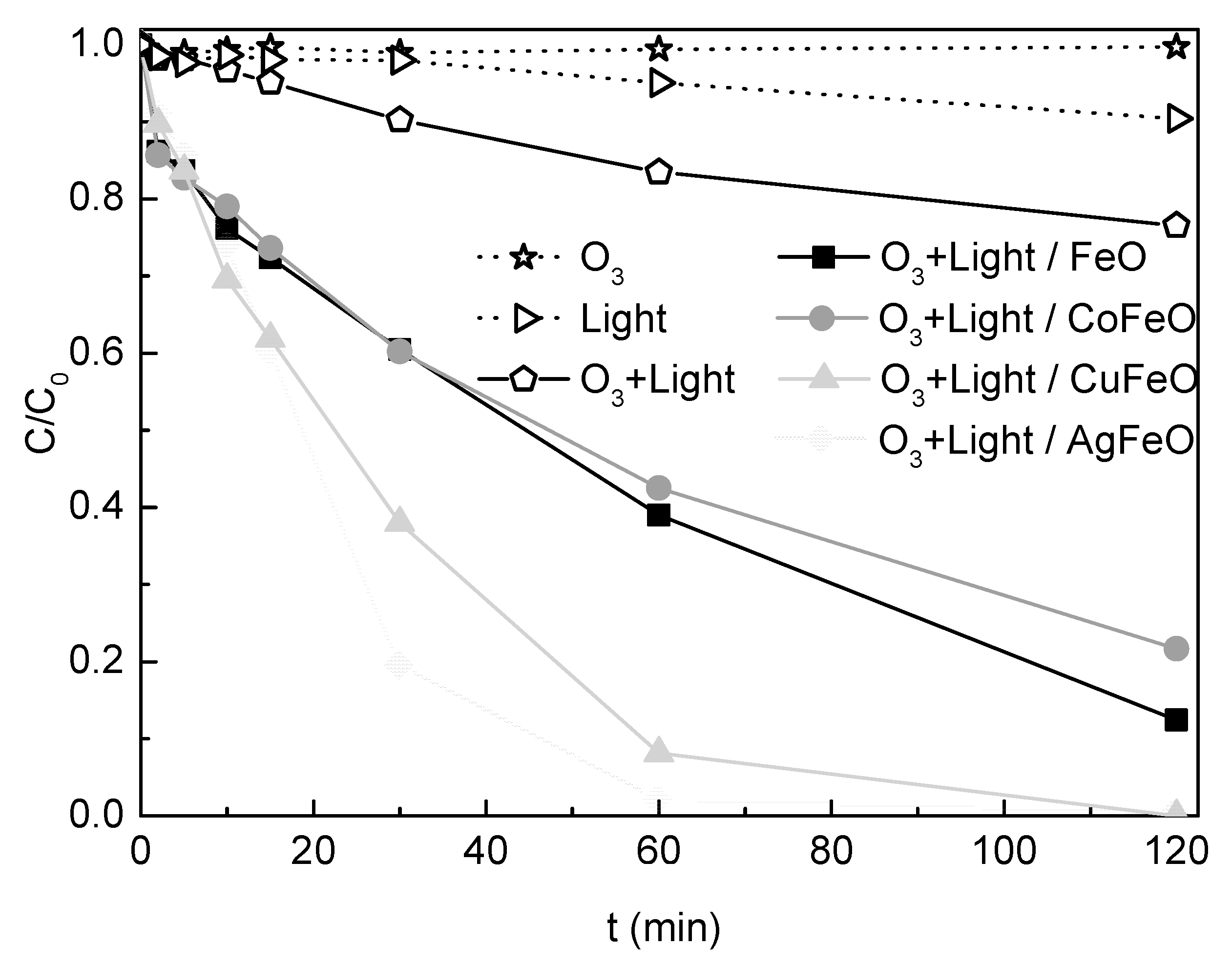
2.5. Testing the Removal Efficiency under Different Conditions
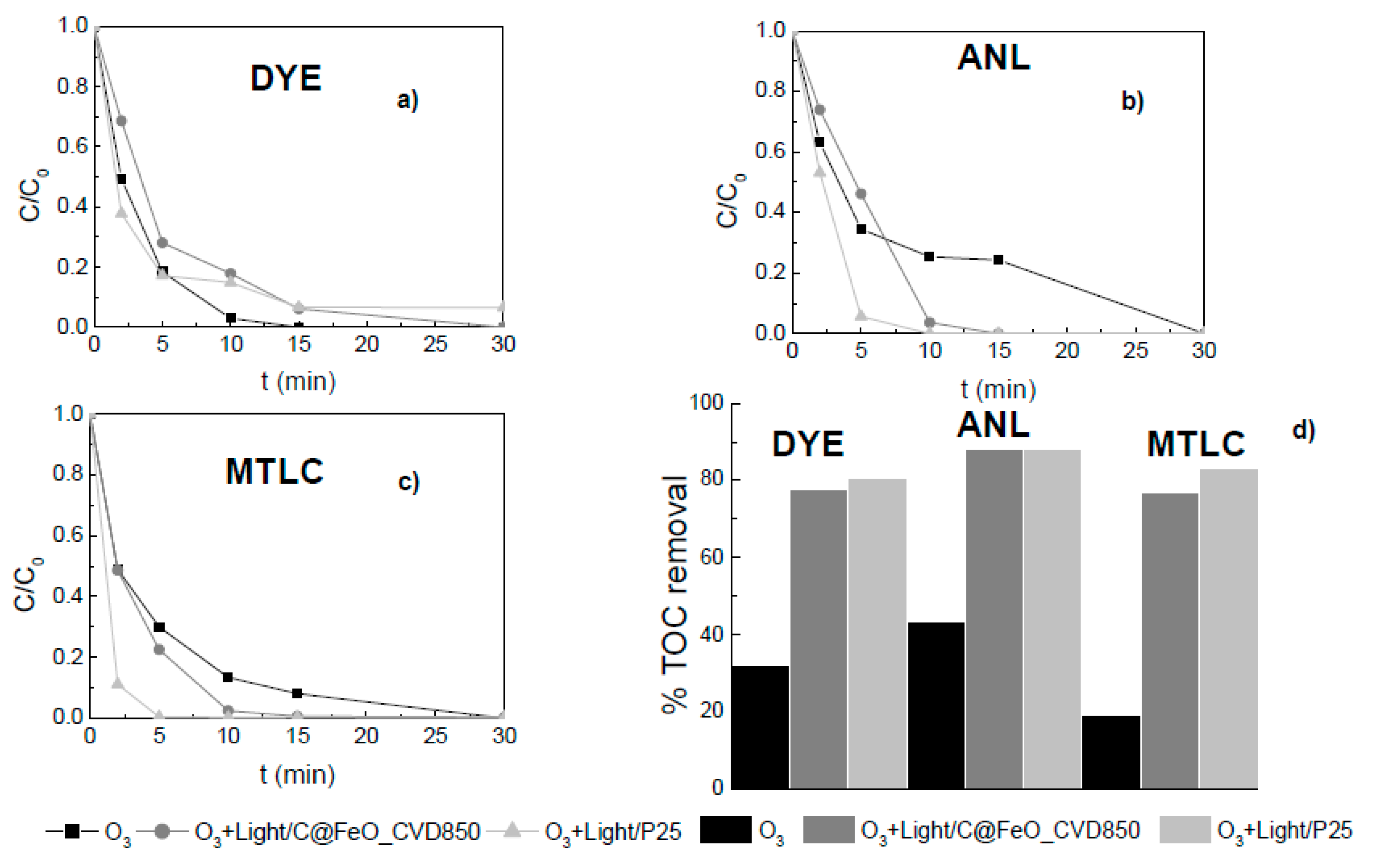
2.6. Removal of Other Pollutants by PCO
3. Materials and Methods
3.1. Catalyst Preparation and Characterisation
3.2. Photocatalytic Ozonation Reactions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mehrjouei, M.; Müller, S.; Möller, D. A review on photocatalytic ozonation used for the treatment of water and wastewater. Chem. Eng. J. 2015, 263, 209–219. [Google Scholar] [CrossRef]
- Chávez, A.M.; Ribeiro, A.R.; Moreira, N.F.F.; Silva, A.M.T.; Rey, A.; Álvarez, P.M.; Beltrán, F.J. Removal of Organic Micropollutants from a Municipal Wastewater Secondary Effluent by UVA-LED Photocatalytic Ozonation. Catalysts 2019, 9, 472. [Google Scholar] [CrossRef]
- Mills, A.; Lee, S.K. A web-based overview of semiconductor photochemistry-based current commercial applications. J. Photochem. Photobiol. A Chem. 2002, 152, 233–247. [Google Scholar] [CrossRef]
- Chen, X.; Gambhir, S.S.; Cheon, J. Theranostic Nanomedicine. Acc. Chem. Res. 2011, 44, 841. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, G.; Eden, H.S.; Ai, H.; Chen, X. Surface-Engineered Magnetic Nanoparticle Platforms for Cancer Imaging and Therapy. Acc. Chem. Res. 2011, 44, 883–892. [Google Scholar] [CrossRef]
- Suber, L.; Marchegiani, G.; Olivetti, E.; Celegato, F.; Coisson, M.; Tiberto, P.M.; Allia, P.; Barrera, G.; Pilloni, L.; Barba, L.; et al. Pure magnetic hard fct FePt nanoparticles: Chemical synthesis, structural and magnetic properties correlations. Mater. Chem. Phys. 2014, 144, 186–193. [Google Scholar] [CrossRef]
- Long, Y.; Xie, M.; Niu, J.; Wang, P.; Ma, J. Preparation of acid–base bifunctional core–shell structured Fe3O4@SiO2 nanoparticles and their cooperative catalytic activity. Appl. Surf. Sci. 2013, 277, 288–292. [Google Scholar] [CrossRef]
- Xu, X.; Wu, H.; Li, Z.; Sun, X.; Wang, Z. Iron oxide-silver magnetic nanoparticles as simple heterogeneous catalysts for the direct inter/intramolecular nucleophilic substitution of π-activated alcohols with electron-deficient amines. Tetrahedron 2015, 71, 5254–5259. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalyst 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Hu, J.; Chen, G.; Lo, I.M.C. Selective Removal of Heavy Metals from Industrial Wastewater Using Maghemite Nanoparticle: Performance and Mechanisms. J. Environ. Eng. 2006, 132, 709–715. [Google Scholar] [CrossRef]
- Ali, Q.; Ahmed, W.; Lal, S.; Sen, T. Novel Multifunctional Carbon Nanotube Containing Silver and Iron Oxide Nanoparticles for Antimicrobial Applications in Water Treatment. Mater. Today Proc. 2017, 4, 57–64. [Google Scholar] [CrossRef]
- Funari, V.; Mantovani, L.; Vigliotti, L.; Tribaudino, M.; Dinelli, E.; Braga, R. Superparamagnetic iron oxides nanoparticles from municipal solid waste incinerators. Sci. Total Environ. 2018, 621, 687–696. [Google Scholar] [CrossRef]
- Pereira, L.; Dias, P.; Soares, O.; Ramalho, P.; Pereira, M.; Alves, M.; Pereira, M.F. Synthesis, characterization and application of magnetic carbon materials as electron shuttles for the biological and chemical reduction of the azo dye Acid Orange 10. Appl. Catal. B Environ. 2017, 212, 175–184. [Google Scholar] [CrossRef]
- Mahmoodi, N.M. Photocatalytic ozonation of dyes using copper ferrite nanoparticle prepared by co-precipitation method. Desalination 2011, 279, 332–337. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Bashiri, M.; Moeen, S.J. Synthesis of nickel–zinc ferrite magnetic nanoparticle and dye degradation using photocatalytic ozonation. Mater. Res. Bull. 2012, 47, 4403–4408. [Google Scholar] [CrossRef]
- Yin, J.; Liao, G.; Zhou, J.; Huang, C.; Ling, Y.; Lu, P.; Li, L. High performance of magnetic BiFeO3 nanoparticle-mediated photocatalytic ozonation for wastewater decontamination. Sep. Purif. Technol. 2016, 168, 134–140. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R. Carbon as Catalyst. In Carbon Materials for Catalysis; Serp, P., Figueiredo, J.L., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 177–217. [Google Scholar]
- Cai, Y.; Qin, J.; Wei, F.; Xu, C.; Yao, Y.; Lu, F.; Wang, S. Magnetic ZnFe2O4–C3N4 Hybrid for Photocatalytic Degradation of Aqueous Organic Pollutants by Visible Light. Ind. Eng. Chem. Res. 2014, 53, 17294–17302. [Google Scholar]
- Hussain, I.; Zhang, Y.; Li, M.; Huang, S.; Hayat, W.; He, L.; Du, X.; Liu, G.; Du, M. Heterogeneously degradation of aniline in aqueous solution using persulfate catalyzed by magnetic BiFeO3 nanoparticles. Catal. Today 2018, 310, 130–140. [Google Scholar] [CrossRef]
- De Oliveira, J.S.; Salla, J.D.; Kuhn, R.C.; Jahn, S.L.; Foletto, E.L. Catalytic Ozonation of Melanoidin in Aqueous Solution over CoFe2O4 Catalyst. Mater. Res. Ibero Am. J. 2019, 22, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Shao, X.; Zhang, Q.; Ma, J.; Ge, H. Preparation and photocatalytic application of magnetic Fe2O3/SBA-15 nanomaterials. J. Mol. Liq. 2018, 260, 304–312. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, H.; Zhang, C.; Feng, J.; Pu, S.; Ren, Y.; Wang, Y. Enhanced catalytic ozonation treatment of dibutyl phthalate enabled by porous magnetic Ag-doped ferrospinel MnFe2O4 materials: Performance and mechanism. Chem. Eng. J. 2018, 354, 42–52. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.L.; Pal, U.; Villanueva, M.S. Structural, Magnetic, and Catalytic Evaluation of Spinel Co, Ni, and Co–Ni Ferrite Nanoparticles Fabricated by Low-Temperature Solution Combustion Process. ACS Omega 2018, 3, 14986–15001. [Google Scholar] [CrossRef]
- Vinosel, V.M.; Anand, S.; Janifer, M.A.; Pauline, S.; Dhanavel, S.; Praveena, P.; Stephen, A. Enhanced photocatalytic activity of Fe3O4/SnO2 magnetic nanocomposite for the degradation of organic dye. J. Mater. Sci. Mater. Electron. 2019, 30, 9663–9677. [Google Scholar] [CrossRef]
- Shi, J.; Feng, S.; Chen, T.; Wu, F.; Guo, W.; Li, Y.; Zhao, P. Novel rattle-type magnetic Fe3O4@Ag@H-BiOCl photocatalyst with enhanced visible light-driven photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 10204–10213. [Google Scholar] [CrossRef]
- Orge, C.; Faria, J.; Pereira, M.; Pereira, M.F. Removal of oxalic acid, oxamic acid and aniline by a combined photolysis and ozonation process. Environ. Technol. 2014, 36, 1075–1083. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Lima, M.J.; Baptista, D.L.; Silva, A.M.; Silva, C.G.; Faria, J.L. Ag-loaded ZnO materials for photocatalytic water treatment. Chem. Eng. J. 2017, 318, 95–102. [Google Scholar] [CrossRef]
- Yao, Y.; Li, G.; Ciston, S.; Lueptow, R.M.; Gray, K.A. Photoreactive TiO2/carbon nanotube composites: Synthesis and reactivity. Environ. Sci. Technol. 2008, 42, 4952–4957. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical vapor deposition of carbon nanotubes: A review on growth mechanism and mass production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef]
- Orge, C.; Pereira, M.F.; Faria, J. Photocatalytic ozonation of model aqueous solutions of oxalic and oxamic acids. Appl. Catal. B Environ. 2015, 174, 113–119. [Google Scholar] [CrossRef]
- Orge, C.A.; Soares, O.S.G.; Faria, J.L.; Pereira, M.F.R. Synthesis of TiO2-Carbon Nanotubes through ball-milling method for mineralization of oxamic acid (OMA) by photocatalytic ozonation. J. Environ. Chem. Eng. 2017, 5, 5599–5607. [Google Scholar] [CrossRef]
- Orge, C.; Faria, J.; Pereira, M.; Pereira, M.F.; Orge, C. Photocatalytic ozonation of aniline with TiO2-carbon composite materials. J. Environ. Manag. 2017, 195, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Orge, C.; Pereira, M.; Faria, J.; Orge, C.; Pereira, M.F. Photocatalytic-assisted ozone degradation of metolachlor aqueous solution. Chem. Eng. J. 2017, 318, 247–253. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, G.; Liu, C.; Luo, S.; Xu, X.; Chen, L.; Wang, B. Magnetic TiO2-graphene composite as a high-performance and recyclable platform for efficient photocatalytic removal of herbicides from water. J. Hazard. Mater. 2013, 252, 115–122. [Google Scholar] [CrossRef] [PubMed]



| Sample | SBET (m2 g−1) (±10 m2 g−1) | % C † |
|---|---|---|
| FeO | 154 | 0 |
| CoFeO | 184 | 0 |
| Cu FeO | 235 | 0 |
| AgFeO | 134 | 0 |
| C@FeO_CVD750 | 63 * | 16 |
| C@FeO_CVD850 | 29 * | 35 |
| C@CoFeO_CVD750 | 29 * | 34 |
| C@CoFeO_CVD850 | 33 * | 43 |
| C@CuFeO_CVD750 | 27 * | 67 |
| C@CuFeO_CVD850 | 16 * | 63 |
| C@TiFeO_met1_CVD850 | 25 * | 20 |
| C@TiFeO_met2_CVD850 | 26 * | 33 |
| Sample | Phase (%vol/vol) | Crystallite Size (nm) ±6% |
|---|---|---|
| FeO | 100% magnetite (Fe3O4) | 20.5 |
| AgFe3O4 | 7.3% chlorargyrite (AgCl) | - |
| 92.6% magnetite (Fe3O4) | 8.2 | |
| C@FeO_CVD750 | 3% cementite (Fe3C) | 84 |
| 1.6% Fe α | 72 | |
| 95.4% graphite | 16 | |
| C@FeO_CVD850 | 3% cementite (Fe3C) | 52 |
| 1.5% Fe α | 73 | |
| 95.5% graphite | 27 | |
| C@TiFeO_met2_CVD850 | 14.1% cementite (Fe3C) | 72 |
| 2.3% Fe α | 10 | |
| 83.6% graphite | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orge, C.A.; Soares, O.S.G.P.; Ramalho, P.S.F.; Pereira, M.F.R.; Faria, J.L. Magnetic Nanoparticles for Photocatalytic Ozonation of Organic Pollutants. Catalysts 2019, 9, 703. https://doi.org/10.3390/catal9090703
Orge CA, Soares OSGP, Ramalho PSF, Pereira MFR, Faria JL. Magnetic Nanoparticles for Photocatalytic Ozonation of Organic Pollutants. Catalysts. 2019; 9(9):703. https://doi.org/10.3390/catal9090703
Chicago/Turabian StyleOrge, Carla A., O. Salomé G. P. Soares, Patrícia S. F. Ramalho, M. Fernando R. Pereira, and Joaquim L. Faria. 2019. "Magnetic Nanoparticles for Photocatalytic Ozonation of Organic Pollutants" Catalysts 9, no. 9: 703. https://doi.org/10.3390/catal9090703
APA StyleOrge, C. A., Soares, O. S. G. P., Ramalho, P. S. F., Pereira, M. F. R., & Faria, J. L. (2019). Magnetic Nanoparticles for Photocatalytic Ozonation of Organic Pollutants. Catalysts, 9(9), 703. https://doi.org/10.3390/catal9090703