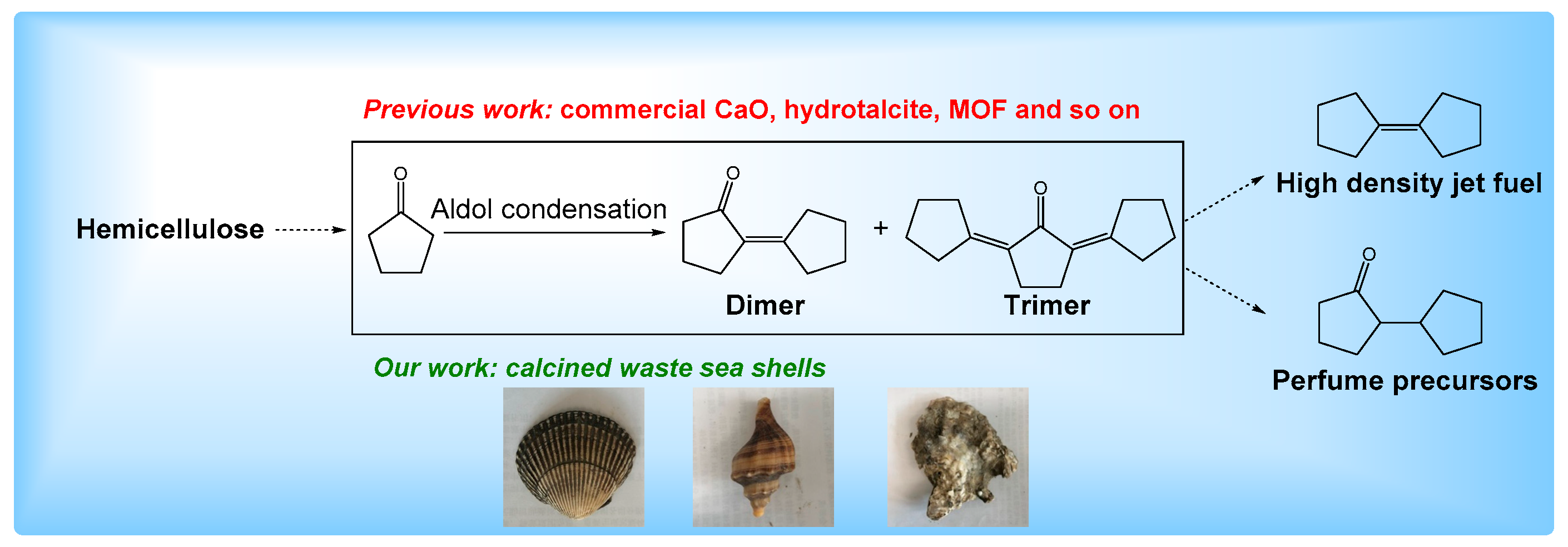
Waste Seashells as a Highly Active Catalyst for Cyclopentanone Self-Aldol Condensation
Abstract
1. Introduction
2. Results and Discussion
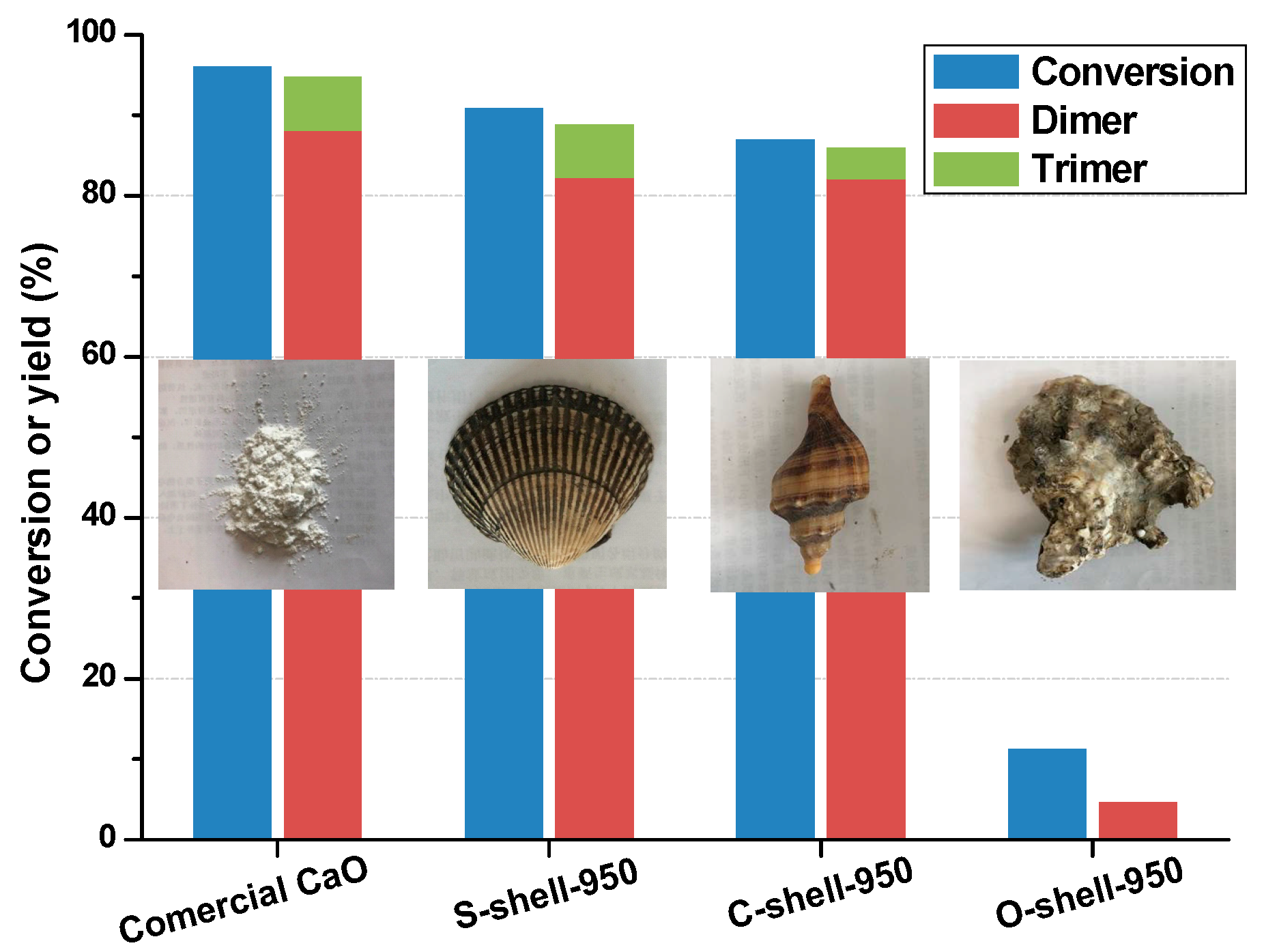
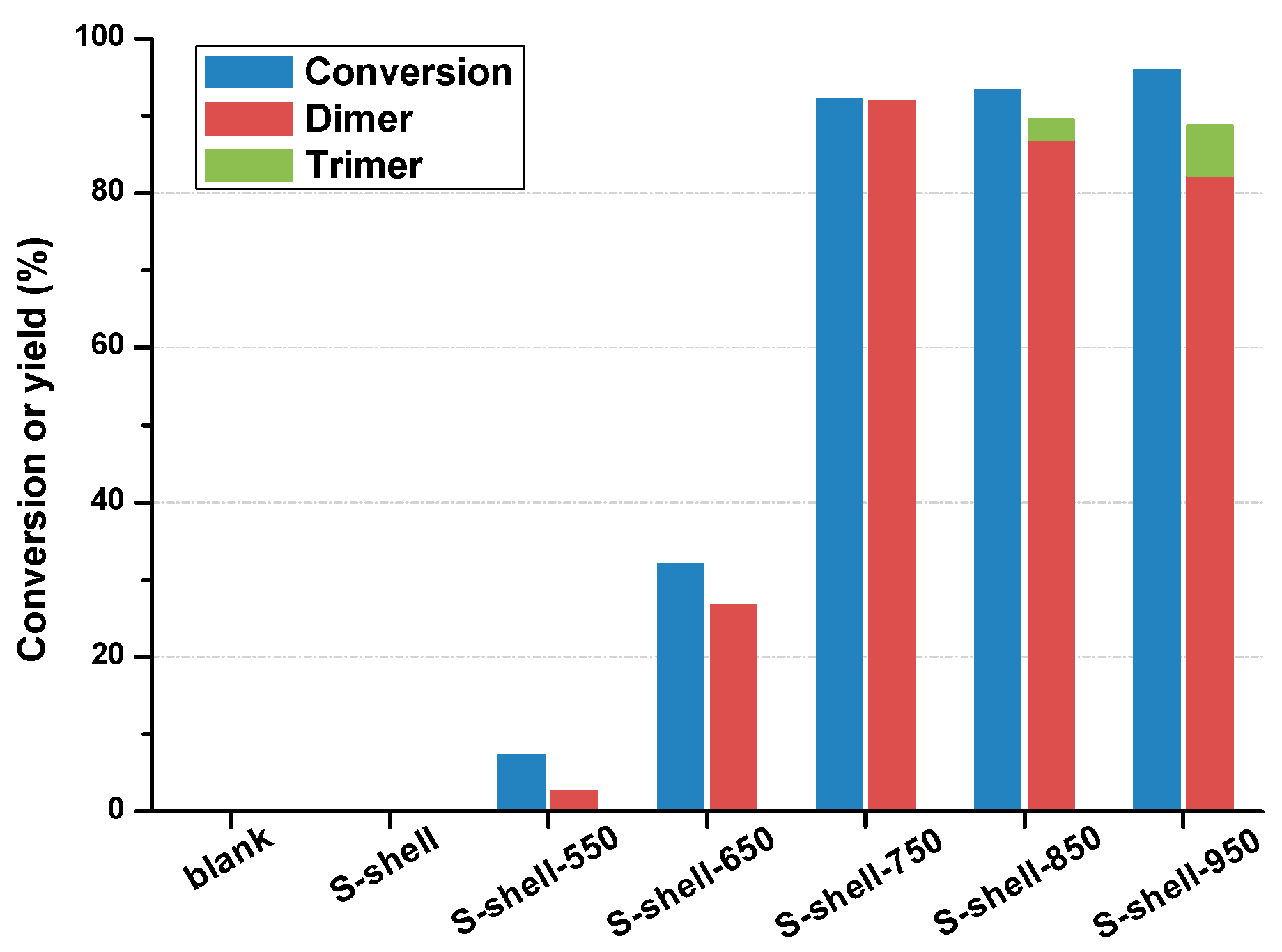
2.1. Catalytic Activity
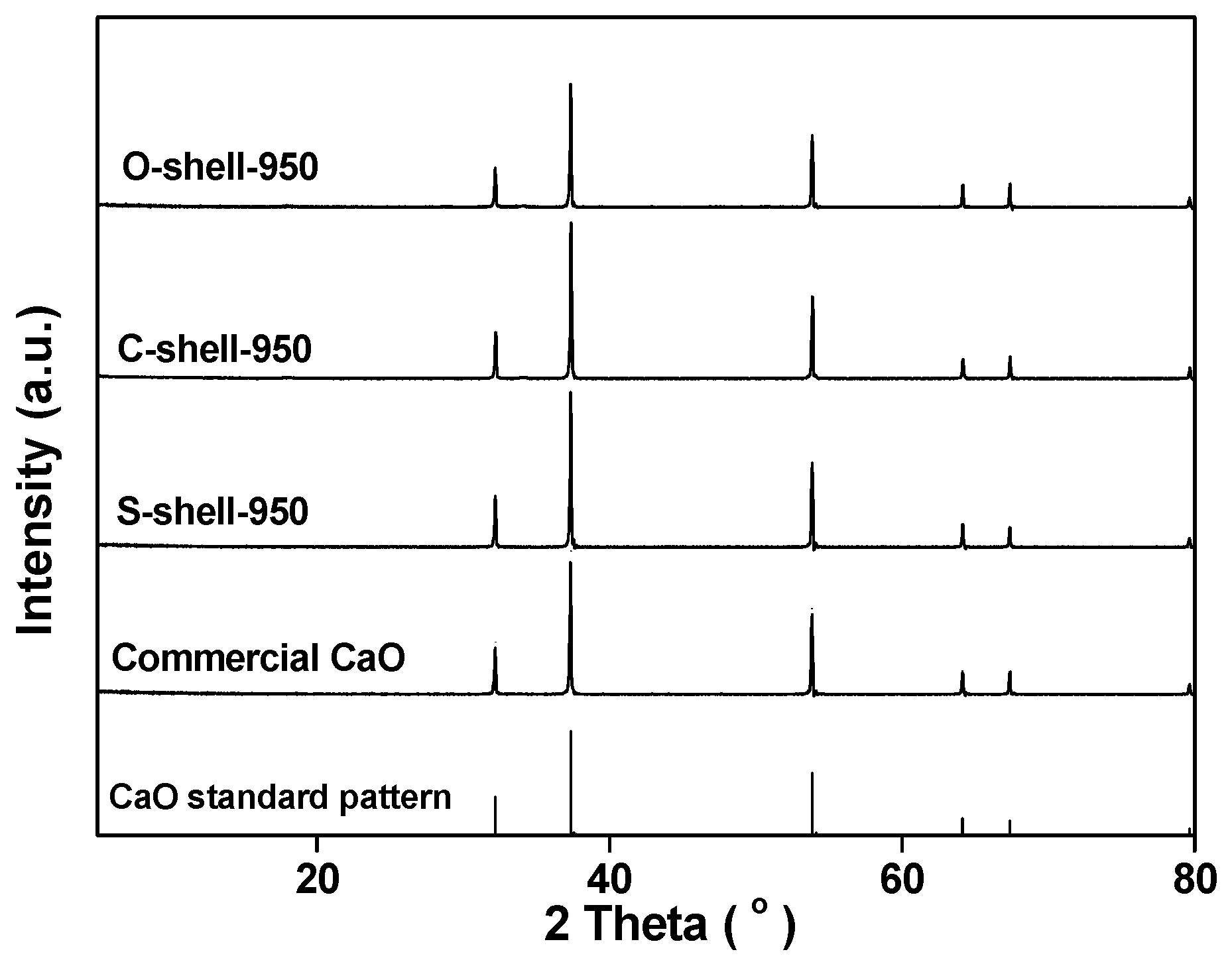
2.2. Catalyst Characterization
3. Materials and Methods
3.1. Materials and Catalyst Preparation
3.2. Characterization of Catalysts
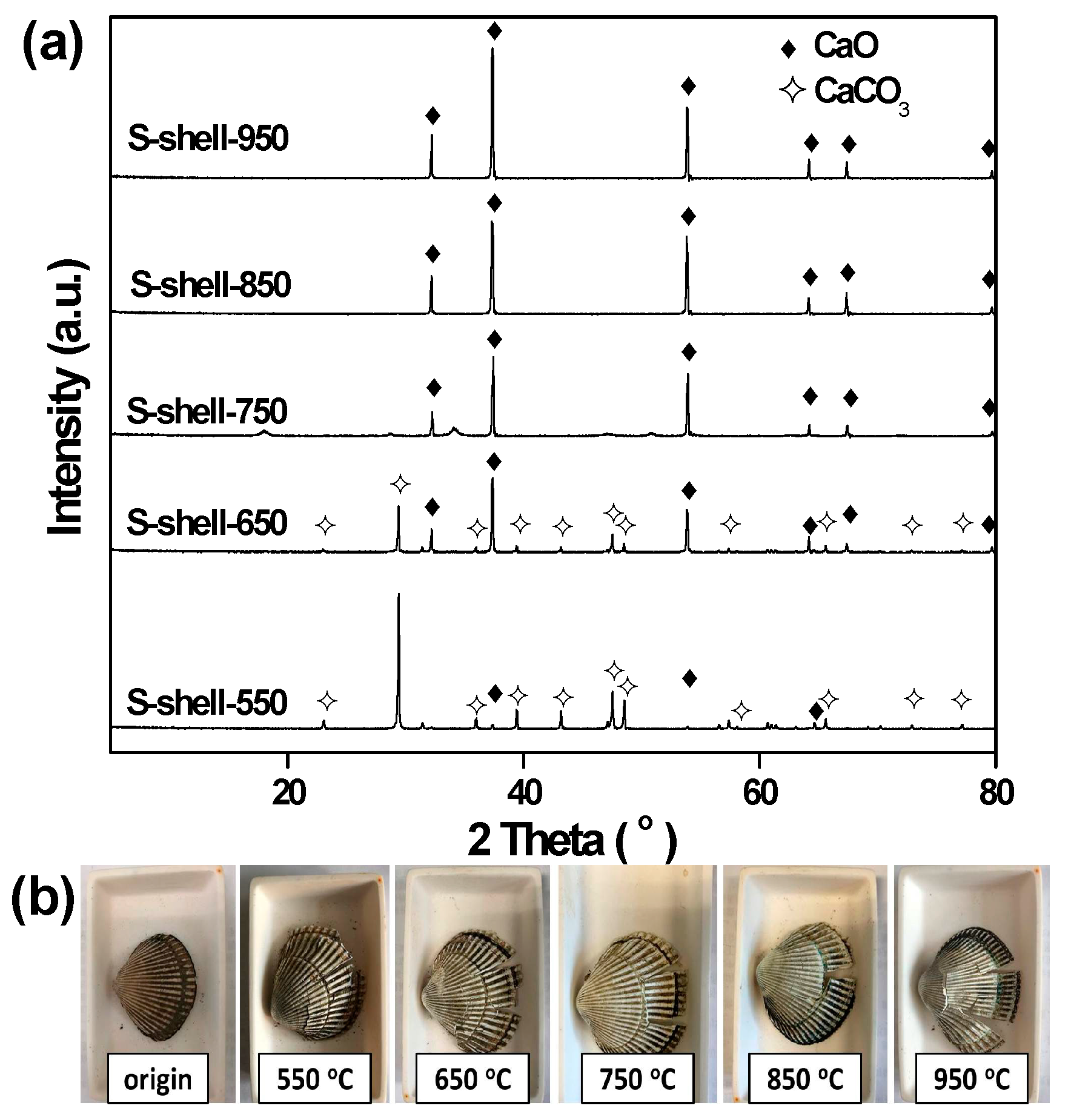
3.2.1. X-ray Diffraction (XRD)
3.2.2. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
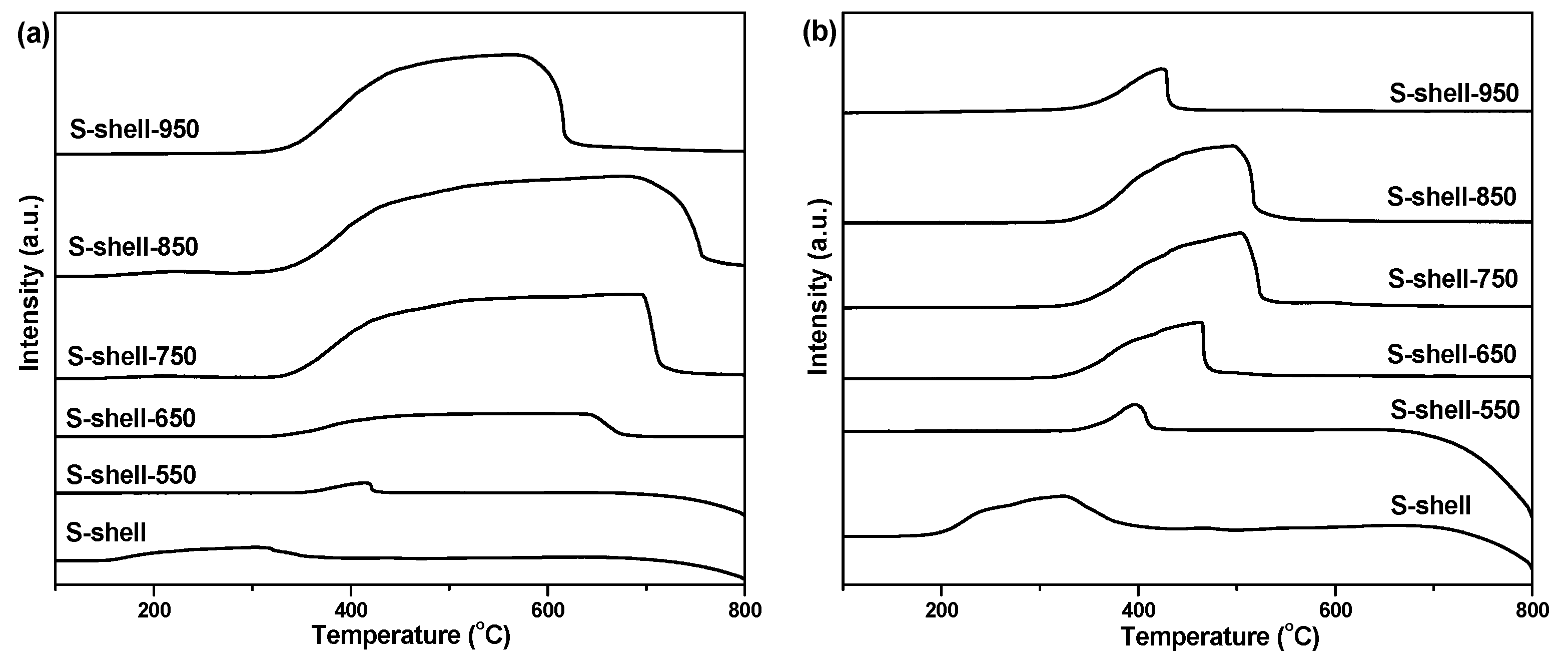
3.2.3. Physical Adsorption
3.2.4. Chemi-Sorption
3.2.5. Contact Angle
3.2.6. Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Mika, L.T.; Csefalvay, E.; Nemeth, A. Catalytic conversion of carbohydrates to initial platform chemicals: Chemistry and sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef] [PubMed]
- Zang, H.; Wang, K.; Zhang, M.; Xie, R.; Wang, L.; Chen, E.Y.X. Catalytic coupling of biomass-derived aldehydes into intermediates for biofuels and materials. Catal. Sci. Technol. 2018, 8, 1777–1798. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, M.; Zhang, J.; Zhang, Z.; An, J.; Du, S.; An, H.; Fan, F.; Liu, X.; Zhai, P.; et al. Selective production of phase-separable product from a mixture of biomass-derived aqueous oxygenates. Nat. Commun. 2018, 9, 5183. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Z.; Liu, Y.; Li, G.; Wang, A.; Cong, Y.; Zhang, T.; Wang, F.; Li, N. Synthesis of 1,4-cyclohexanedimethanol, 1,4-cyclohexanedicarboxylic acid and 1,2-cyclohexanedicarboxylates from formaldehyde, crotonaldehyde and acrylate/fumarate. Angew. Chem. Int. Edit 2018, 57, 6901–6905. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y.; Zhang, S.; Gupta, K.M.; Hülsey, M.J.; Asakura, H.; Liu, L.; Han, Y.; Karp, E.M.; Beckham, G.T.; et al. Catalytic amino acid production from biomass-derived intermediates. Proc. Natl. Acad. Sci. USA 2018, 115, 5093–5098. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, H.; Agblevor, F.A. Hydrodeoxygenation of Aqueous-Phase Catalytic Pyrolysis Oil to Liquid Hydrocarbons Using Multifunctional Nickel Catalyst. Ind. Eng. Chem. Res. 2018, 57, 13257–13268. [Google Scholar] [CrossRef]
- Kaminski, T.; Sheng, Q.; Husein, M.M. Hydrocracking of Athabasca Vacuum Residue Using Ni-Mo-Supported Drill Cuttings. Catalysts 2019, 9, 216. [Google Scholar] [CrossRef]
- Fang, R.; Liu, H.; Luque, R.; Li, Y. Efficient and selective hydrogenation of biomass-derived furfural to cyclopentanone using Ru catalysts. Green Chem. 2015, 17, 4183–4188. [Google Scholar] [CrossRef]
- Yang, Y.; Du, Z.; Huang, Y.; Lu, F.; Wang, F.; Gao, J.; Xu, J. Conversion of furfural into cyclopentanone over Ni–Cu bimetallic catalysts. Green Chem. 2013, 15, 1932–1940. [Google Scholar] [CrossRef]
- Yang, J.; Li, N.; Li, G.; Wang, W.; Wang, A.; Wang, X.; Cong, Y.; Zhang, T. Synthesis of renewable high-density fuels using cyclopentanone derived from lignocellulose. Chem. Commun. 2014, 50, 2572–2574. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Li, G.; Wang, W.; Cong, Y.; Wang, X.; Huber, G.W.; Li, N.; Wang, A.; Zhang, T. Dual-bed catalyst system for the direct synthesis of high density aviation fuel with cyclopentanone from lignocellulose. AIChE J. 2016, 62, 2754–2761. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Mifsud, M.; Velty, A. New one-pot multistep process with multifunctional catalysts: Decreasing the E factor in the synthesis of fine chemicals. Green Chem. 2010, 12, 99–107. [Google Scholar] [CrossRef]
- Deng, Q.; Nie, G.; Pan, L.; Zou, J.-J.; Zhang, X.; Wang, L. Highly selective self-condensation of cyclic ketones using MOF-encapsulating phosphotungstic acid for renewable high-density fuel. Green Chem. 2015, 17, 4473–4481. [Google Scholar] [CrossRef]
- Ngo, D.T.; Sooknoi, T.; Resasco, D.E. Improving stability of cyclopentanone aldol condensation MgO-based catalysts by surface hydrophobization with organosilanes. Appl. Catal. B Environ. 2018, 237, 835–843. [Google Scholar] [CrossRef]
- News, C.F. Shellfish Consumption of China. Available online: http://www.shuichan.cc/news_view-375425.html (accessed on 29 July 2019).
- Nakatani, N.; Takamori, H.; Takeda, K.; Sakugawa, H. Transesterification of soybean oil using combusted oyster shell waste as a catalyst. Biores. Technol. 2009, 100, 1510–1513. [Google Scholar] [CrossRef] [PubMed]
- Viriya-Empikul, N.; Krasae, P.; Puttasawat, B.; Yoosuk, B.; Chollacoop, N.; Faungnawakij, K. Waste shells of mollusk and egg as biodiesel production catalysts. Biores. Technol. 2010, 101, 3765–3767. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Kim, Y.-M.; Jae, J.; Lee, S.M.; Jung, S.-C.; Park, Y.-K. The use of calcined seashell for the prevention of char foaming/agglomeration and the production of high-quality oil during the pyrolysis of lignin. Renew. Energy 2019, 144, 147–152. [Google Scholar] [CrossRef]
- Granados, M.L.; Poves, M.D.Z.; Alonso, D.M.; Mariscal, R.; Galisteo, F.C.; Moreno-Tost, R.; Santamaría, J.; Fierro, J.L.G. Biodiesel from sunflower oil by using activated calcium oxide. Appl. Catal. B Environ. 2007, 73, 317–326. [Google Scholar] [CrossRef]
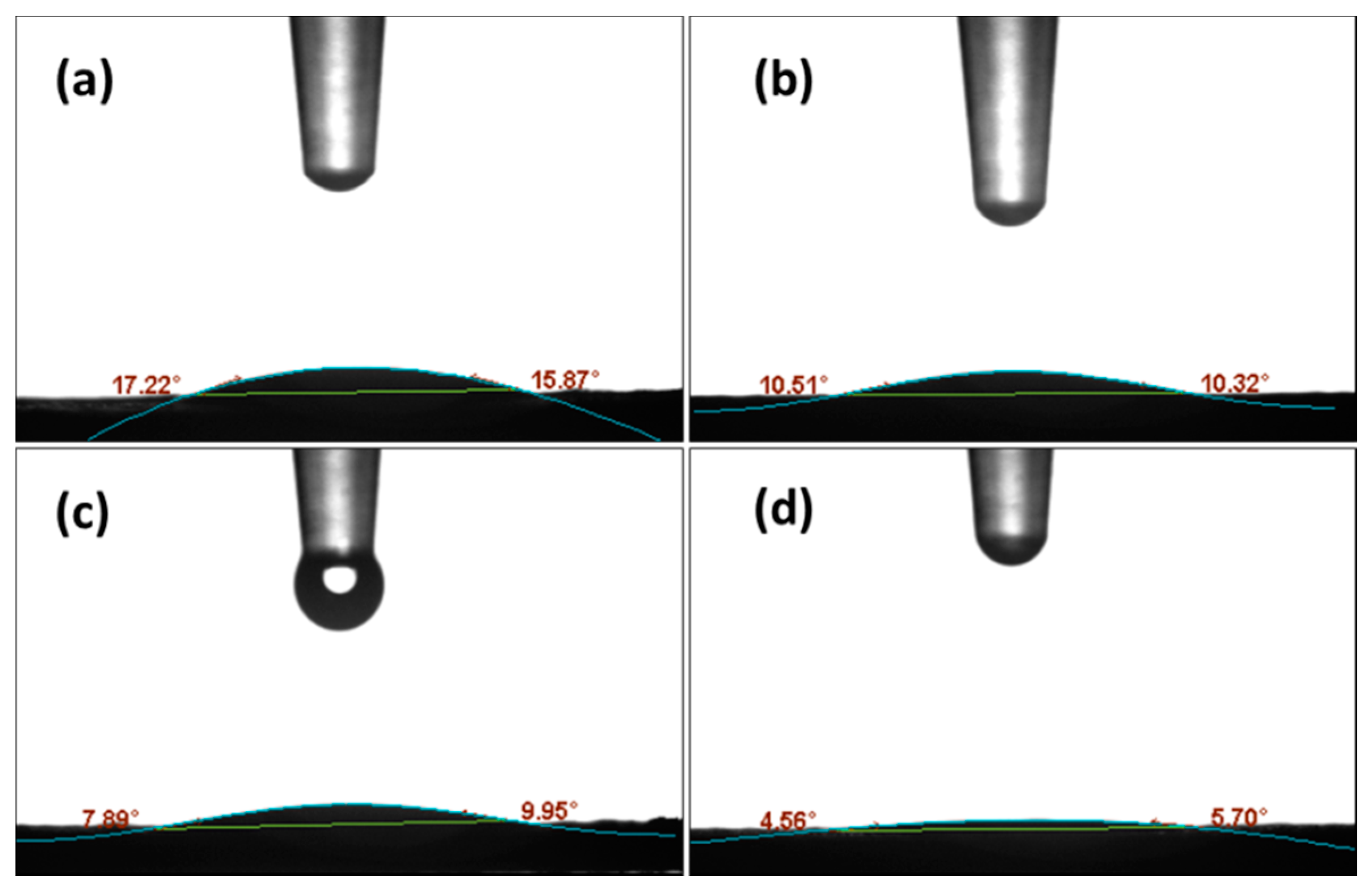
| Catalyst | SBET (m2 g−1) 1 | CaO Content (%) 2 | CA Mean(°) 3 |
|---|---|---|---|
| Commercial CaO | 9.69 | 98.00 | 16.55 |
| S-shell-950 | 8.00 | 97.78 | 10.42 |
| C-shell-950 | 11.80 | 80.34 | 8.92 |
| O-shell-950 | 15.73 | 76.14 | 5.13 |
| Catalysts | Base Sites Amount (μmol g−1) | Acid Sites Amount (μmol g−1) |
|---|---|---|
| S-shell | ||
| S-shell-550 | 1.93 | 2.04 |
| S-shell-650 | 72.79 | 9.47 |
| S-shell-750 | 496.83 | 16.48 |
| S-shell-850 | 744.34 | 14.92 |
| S-shell-950 | 428.32 | 5.34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, X.; Xu, Q.; Wang, X.; Li, N.; Jia, H.; Shi, H.; Niu, M.; Zhang, J.; Ping, Q. Waste Seashells as a Highly Active Catalyst for Cyclopentanone Self-Aldol Condensation. Catalysts 2019, 9, 661. https://doi.org/10.3390/catal9080661
Sheng X, Xu Q, Wang X, Li N, Jia H, Shi H, Niu M, Zhang J, Ping Q. Waste Seashells as a Highly Active Catalyst for Cyclopentanone Self-Aldol Condensation. Catalysts. 2019; 9(8):661. https://doi.org/10.3390/catal9080661
Chicago/Turabian StyleSheng, Xueru, Qianqian Xu, Xing Wang, Na Li, Haiyuan Jia, Haiqiang Shi, Meihong Niu, Jian Zhang, and Qingwei Ping. 2019. "Waste Seashells as a Highly Active Catalyst for Cyclopentanone Self-Aldol Condensation" Catalysts 9, no. 8: 661. https://doi.org/10.3390/catal9080661
APA StyleSheng, X., Xu, Q., Wang, X., Li, N., Jia, H., Shi, H., Niu, M., Zhang, J., & Ping, Q. (2019). Waste Seashells as a Highly Active Catalyst for Cyclopentanone Self-Aldol Condensation. Catalysts, 9(8), 661. https://doi.org/10.3390/catal9080661






