Biomimetic Mineralization of Cytochrome c Improves the Catalytic Efficiency and Confers a Functional Multi-Enzyme Composite
Abstract
1. Introduction
2. Results and Discussion
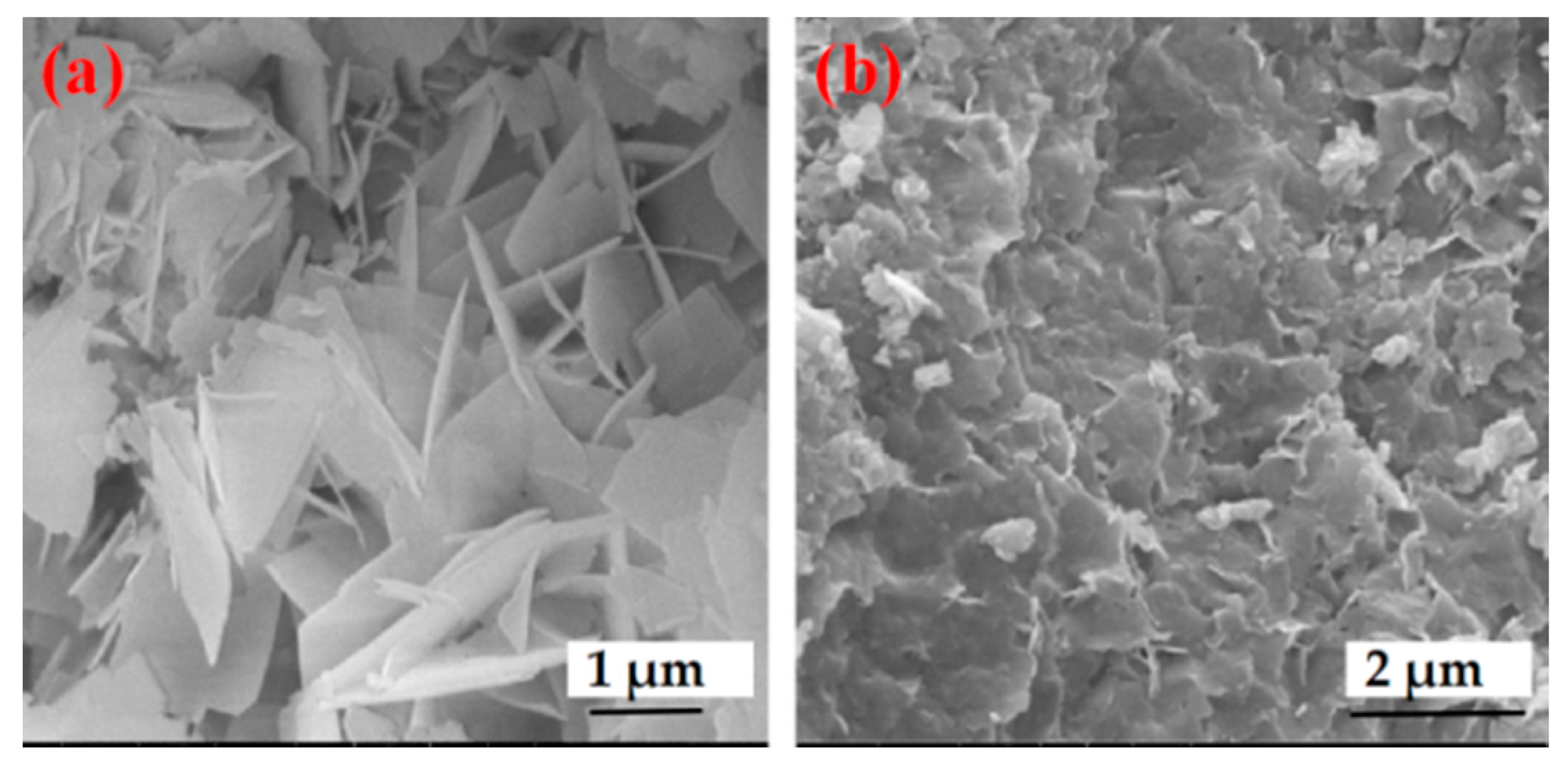
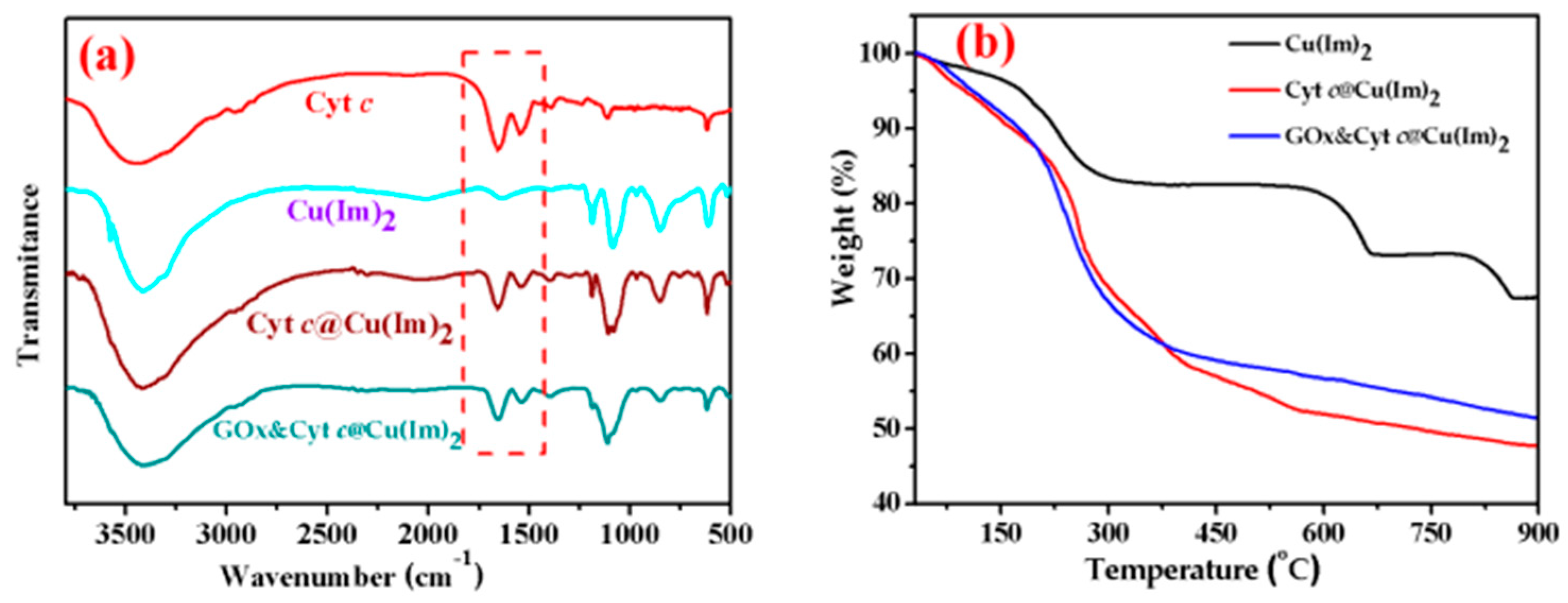
2.1. Preparation and Characterization of Enzyme@Cu(Im)2
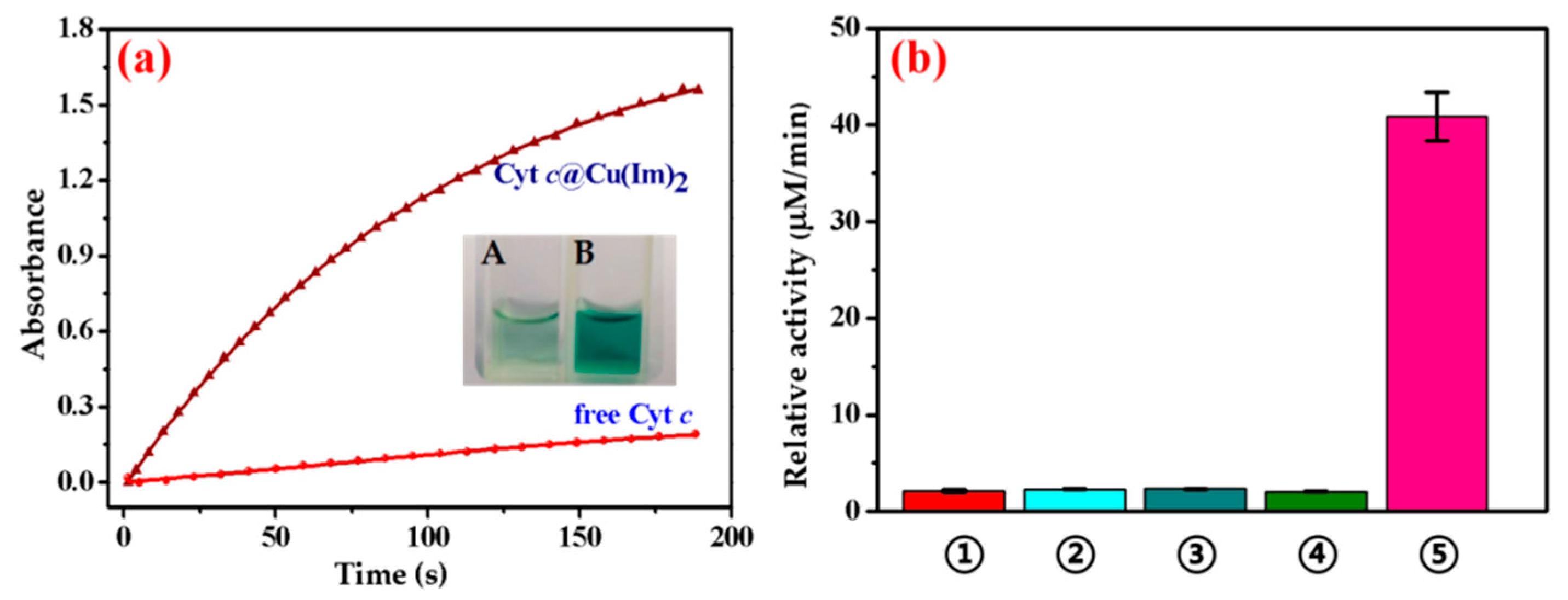
2.2. Peroxidase Activity Assay of Free Cyt c and Cyt c@Cu(Im)2
2.3. Peroxidase Activity Assay of Free GOx and Cyt c, and GOx-Cyt c@Cu(Im)2
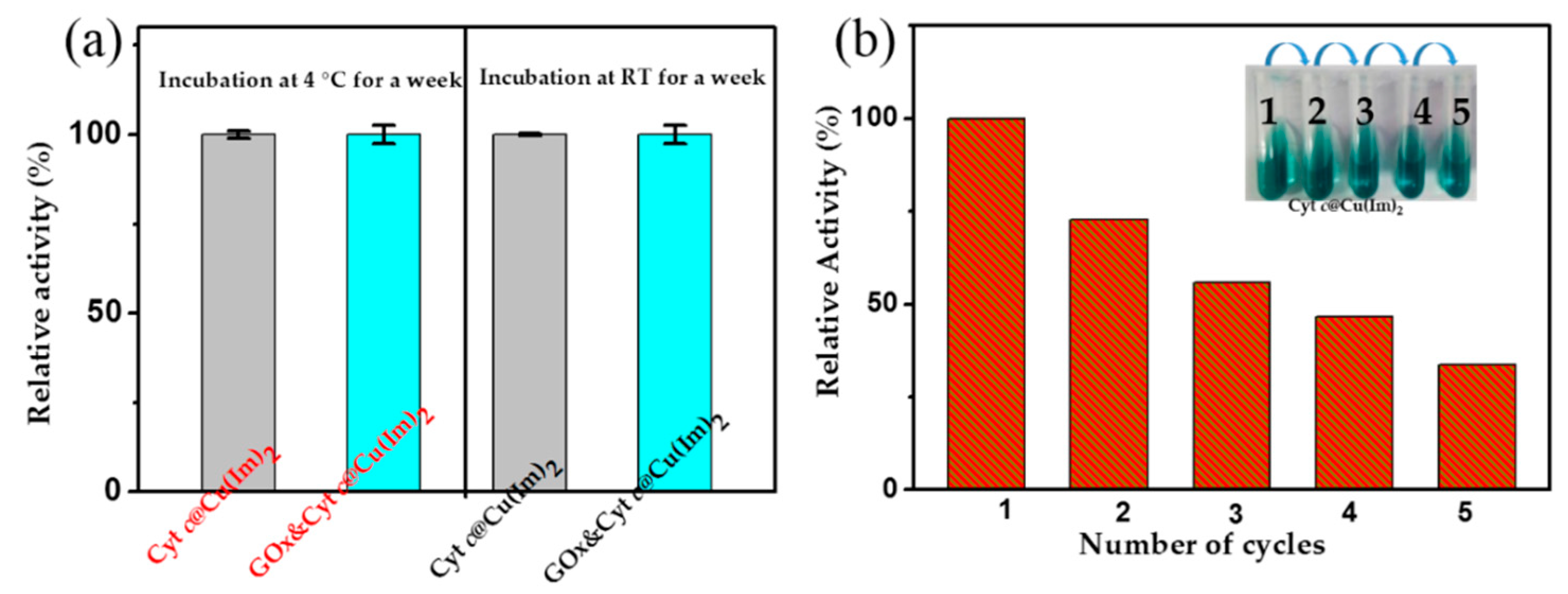
2.4. Stability and Reusability
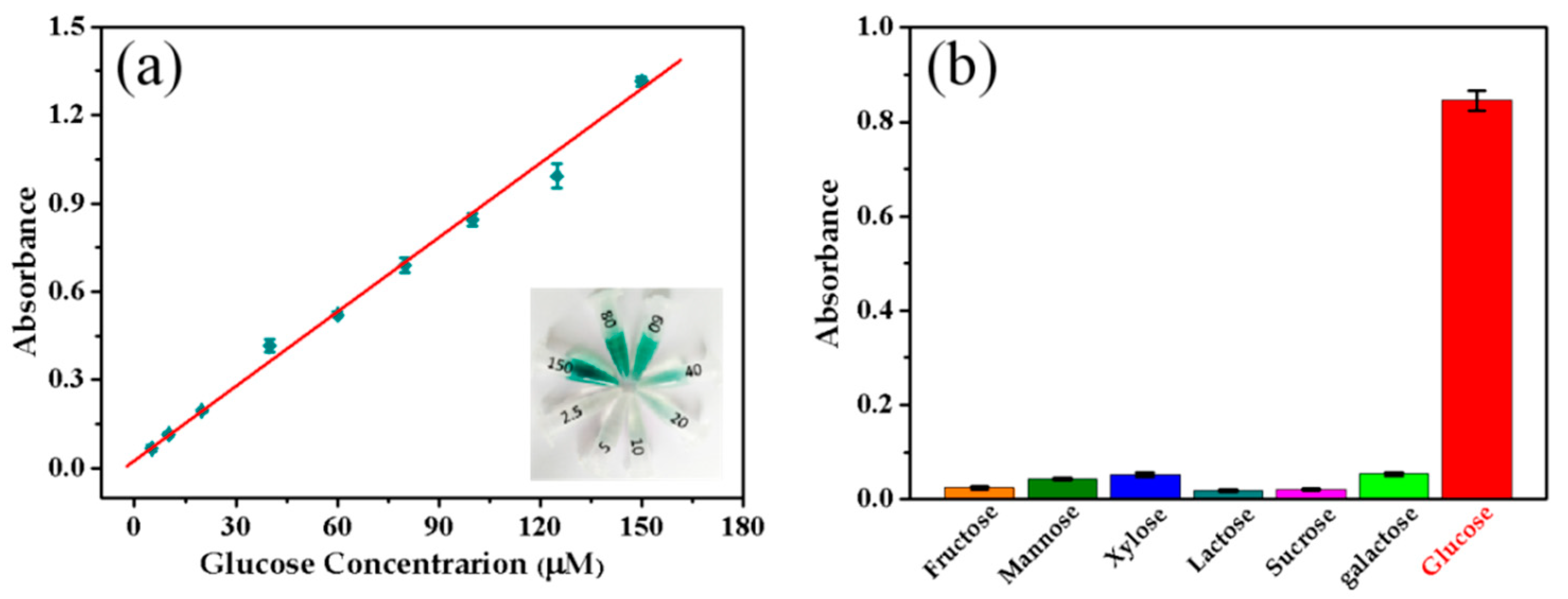
2.5. Glucose Detection
3. Materials and Methods
3.1. Materials and Instruments
3.2. Preparation of Enzyme@ Cu(Im)2
3.3. Fluorescent Labeling of Enzymes
3.4. Peroxidase Activity Assay
3.5. Enzyme Stability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lu, Y.; Yeung, N.; Sieracki, N.; Marshall, N.M. Design of functional metalloproteins. Nature 2009, 460, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Poulos, T.L. Heme enzyme structure and function. Chem. Rev. 2014, 114, 3919–3962. [Google Scholar] [CrossRef] [PubMed]
- Mirts, E.N.; Bhagi-Damodaran, A.; Lu, Y. Understanding and Modulating Metalloenzymes with Unnatural Amino Acids, Non-Native Metal Ions, and Non-Native Metallocofactors. Acc. Chem. Res. 2019, 52, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S.; Lin, Y.-W. Design of artificial metalloproteins/metalloenzymes by tuning noncovalent interactions. J. Biol. Inorg. Chem. 2018, 23, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W. Rational design of metalloenzymes: From single to multiple active sites. Coord. Chem. Rev. 2017, 336, 1–27. [Google Scholar] [CrossRef]
- Yin, L.-L.; Yuan, H.; Liu, C.; He, B.; Gao, S.-Q.; Wen, G.-B.; Tan, X.; Lin, Y.-W. A Rationally Designed Myoglobin Exhibits a Catalytic Dehalogenation Efficiency More than 1000-Fold That of a Native Dehaloperoxidase. ACS Catalysis 2018, 8, 9619–9624. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, H.; Liao, F.; Wei, C.-W.; Du, K.-J.; Gao, S.-Q.; Tan, X.; Lin, Y.-W. Unique Tyr-heme double cross-links in F43Y/T67R myoglobin: An artificial enzyme with a peroxidase activity comparable to that of native peroxidases. Chem. Commun. 2019, 55, 6610–6613. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-W. Rational design of heme enzymes for biodegradation of pollutants towards a green future. Biotechnol. Appl. Biochem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Moon, S.Y.; Guelta, M.A.; Harvey, S.P.; Hupp, J.T.; Farha, O.K. Encapsulation of a Nerve Agent Detoxifying Enzyme by a Mesoporous Zirconium Metal-Organic Framework Engenders Thermal and Long-Term Stability. J. Am. Chem. Soc. 2016, 138, 8052–8055. [Google Scholar] [CrossRef]
- Majewski, M.B.; Howarth, A.J.; Li, P.; Wasielewski, M.R.; Hupp, J.T.; Farha, O.K. Enzyme encapsulation in metal–organic frameworks for applications in catalysis. CrystEngComm 2017, 19, 4082–4091. [Google Scholar] [CrossRef]
- Hu, Y.; Dai, L.; Liu, D.; Du, W.; Wang, Y. Progress & prospect of metal-organic frameworks (MOFs) for enzyme immobilization (enzyme/MOFs). Renew. Sustain. Energy Rev. 2018, 91, 793–801. [Google Scholar]
- Khoshnevisan, K.; Vakhshiteh, F.; Barkhi, M.; Baharifar, H.; Poor-Akbar, E.; Zari, N.; Stamatis, H.; Bordbar, A.-K. Immobilization of cellulase enzyme onto magnetic nanoparticles: Applications and recent advances. Mol. Catal. 2017, 442, 66–73. [Google Scholar] [CrossRef]
- Gascón, V.; Carucci, C.; Jiménez, M.B.; Blanco, R.M.; Sánchez-Sánchez, M.; Magner, E. Rapid In Situ Immobilization of Enzymes in Metal-Organic Framework Supports under Mild Conditions. ChemCatChem 2017, 9, 1182–1186. [Google Scholar] [CrossRef]
- Naseri, M.; Pitzalis, F.; Carucci, C.; Medda, L.; Fotouhi, L.; Magner, E.; Salis, A. Lipase and Laccase Encapsulated on Zeolite Imidazolate Framework: Enzyme Activity and Stability from Voltammetric Measurements. ChemCatChem 2018, 10, 5425–5433. [Google Scholar] [CrossRef]
- Liang, K.; Ricco, R.; Doherty, C.M.; Styles, M.J.; Bell, S.; Kirby, N.; Mudie, S.; Haylock, D.; Hill, A.J.; Doonan, C.J.; et al. Biomimetic mineralization of metal-organic frameworks as protective coatings for biomacromolecules. Nature Commun. 2015, 6, 7240. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ding, Y.; Li, S.; Jiang, Y.; Liu, Z.; Ge, J. Highly active, stable and self-antimicrobial enzyme catalysts prepared by biomimetic mineralization of copper hydroxysulfate. Nanoscale 2016, 8, 17440–17445. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Ricco, R.; Maddigan, N.K.; Dickinson, R.P.; Xu, H.; Li, Q.; Sumby, C.J.; Bell, S.G.; Falcaro, P.; Doonan, C.J. Control of Structure Topology and Spatial Distribution of Biomacromolecules in Protein@ZIF-8 Biocomposites. Chem. Mater. 2018, 30, 1069–1077. [Google Scholar] [CrossRef]
- Maddigan, N.K.; Tarzia, A.; Huang, D.M.; Sumby, C.J.; Bell, S.G.; Falcaro, P.; Doonan, C.J. Protein surface functionalisation as a general strategy for facilitating biomimetic mineralisation of ZIF-8. Chem. Sci. 2018, 9, 4217–4223. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Bertini, I.; Cavallaro, G.; Rosato, A. Cytochrome c: Occurrence and functions. Chem. Rev. 2006, 106, 90–115. [Google Scholar] [CrossRef]
- Ying, T.; Wang, Z.H.; Lin, Y.W.; Xie, J.; Tan, X.; Huang, Z.X. Tyrosine-67 in cytochrome c is a possible apoptotic trigger controlled by hydrogen bonds via a conformational transition. Chem. Commun. 2009, 4512–4514. [Google Scholar] [CrossRef]
- Sun, M.-H.; Liu, S.-Q.; Du, K.-J.; Nie, C.-M.; Lin, Y.-W. A spectroscopic study of uranyl-cytochrome b5/cytochrome c interactions. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2014, 118, 130–137. [Google Scholar] [CrossRef]
- Wang, X.; Wei, C.; Su, J.-H.; He, B.; Wen, G.-B.; Lin, Y.-W.; Zhang, Y. A Chiral Ligand Assembly That Confers One-Electron O2 Reduction Activity for a Cu2+-Selective Metallohydrogel. Angew. Chem. Int. Ed. 2018, 57, 3504–3508. [Google Scholar] [CrossRef]
- Wu, X.; Yang, C.; Ge, J. Green synthesis of enzyme/metal-organic framework composites with high stability in protein denaturing solvents. Bioresour. Biopro. 2017, 4, 24. [Google Scholar] [CrossRef]
- Lyu, F.; Zhang, Y.; Zare, R.N.; Ge, J.; Liu, Z. One-pot synthesis of protein-embedded metal-organic frameworks with enhanced biological activities. Nano Lett. 2014, 14, 5761–5765. [Google Scholar] [CrossRef]
- Li, Z.; Xia, H.; Li, S.; Pang, J.; Zhu, W.; Jiang, Y. In situ hybridization of enzymes and their metal-organic framework analogues with enhanced activity and stability by biomimetic mineralisation. Nanoscale 2017, 9, 15298–15302. [Google Scholar] [CrossRef]
- Sun, J.; Ge, J.; Liu, W.; Lan, M.; Zhang, H.; Wang, P.; Wang, Y.; Niu, Z. Multi-enzyme co-embedded organic-inorganic hybrid nanoflowers: Synthesis and application as a colorimetric sensor. Nanoscale 2014, 6, 255–262. [Google Scholar] [CrossRef]
- Garny, S.; Beeton-Kempen, N.; Gerber, I.; Verschoor, J.; Jordaan, J. The co-immobilization of P450-type nitric oxide reductase and glucose dehydrogenase for the continuous reduction of nitric oxide via cofactor recycling. Enzyme Microb. Technol. 2016, 85, 71–81. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, D.; Wu, C.; Yao, K.; Li, Z.; Shi, N.; Wen, F.; Gates, I.D. Co-immobilization of cellulase and lysozyme on amino-functionalized magnetic nanoparticles: An activity-tunable biocatalyst for extraction of lipids from microalgae. Bioresour. Technol. 2018, 263, 317–324. [Google Scholar] [CrossRef]
- Jin, X.; Li, S.; Long, N.; Zhang, R. A robust and stable nano-biocatalyst by co-immobilization of chloroperoxidase and horseradish peroxidase for the decolorization of azo dyes. J. Chem. Technol. Biotechnol. 2018, 93, 489–497. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Rivas, B.D.L.; Muñoz, R.; Guisán, J.M.; López-Gallego, F. Rational Co-Immobilization of Bi-Enzyme Cascades on Porous Supports and their Applications in Bio-Redox Reactions with In Situ Recycling of Soluble Cofactors. ChemCatChem 2012, 4, 1279–1288. [Google Scholar] [CrossRef]
- Yu, M.; Liu, D.; Sun, L.; Li, J.; Chen, Q.; Pan, L.; Shang, J.; Zhang, S.; Li, W. Facile fabrication of 3D porous hybrid sphere by co-immobilization of multi-enzyme directly from cell lysates as an efficient and recyclable biocatalyst for asymmetric reduction with coenzyme regeneration in situ. Int. J. Biol. Macromol. 2017, 103, 424–434. [Google Scholar] [CrossRef]
- He, G.; Hu, W.; Li, C.M. Spontaneous interfacial reaction between metallic copper and PBS to form cupric phosphate nanoflower and its enzyme hybrid with enhanced activity. Colloids Surf. B. 2015, 135, 613–618. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeoliticimidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef]
- Kitagishi, H.; Shimoji, D.; Ohta, T.; Kamiya, R.; Kudo, Y.; Onoda, A.; Hayashi, T.; Weiss, J.; Wytko, J.A.; Kano, K. A water-soluble supramolecular complex that mimics the heme/copper hetero-binuclear site of cytochrome c oxidase. Chem. Sci. 2018, 9, 1989–1995. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Hou, M.; Li, X.; Wu, X.; Ge, J. Immobilization on Metal-Organic Framework Engenders High Sensitivity for Enzymatic Electrochemical Detection. ACS Appl. Mater. Inter. 2017, 9, 13831–13836. [Google Scholar] [CrossRef]
- Shi, L.; Cai, X.; Li, H.; He, H.; Zhao, H.; Lan, M. ZIF-67 Derived Porous Carbon from Calcination and Acid Etching Process as an Enzyme Immobilization Platform for Glucose Sensing. Electroanalysis 2018, 30, 466–473. [Google Scholar] [CrossRef]
- Aghayan, M.; Mahmoudi, A.; Nazari, K.; Dehghanpour, S.; Sohrabi, S.; Sazegar, M.R.; Mohammadian-Tabrizi, N. Fe(III) porphyrin metal–organic framework as an artificial enzyme mimics and its application in biosensing of glucose and H2O2. J. Porous Mat. 2019. [Google Scholar] [CrossRef]


| Sample | Mass Loss (%) T ˂ 200 °C | Mass Loss (%) 200 °C ˂ T ˂ 370 °C | Total Mass Loss (%) |
|---|---|---|---|
| Cu(Im)2 | 6.99 | 10.48 | 32.46 |
| Cyt c@Cu(Im)2 | 12.53 | 25.39 | 52.33 |
| GOx-Cyt c@Cu(Im)2 | 12.61 | 25.76 | 49.58 |
| Sample | kcat (s−1) | Km (mM) | kcat/Km (M−1s−1) |
|---|---|---|---|
| Cyt c | 0.75 ± 0.17 | 21 ± 5 | 35.7 |
| Cyt c@Cu(Im)2 | 7.05 ± 0.67 | 10 ± 1 | 705 |
| Sample | kcat (s−1) | Km (mM) | kcat/Km (M−1s−1) |
|---|---|---|---|
| GOx-Cyt c | 0.09 ± 0.01 | 1.30 ± 0.14 | 69.2 |
| GOx-Cyt c@Cu(Im)2 | 2.64 ± 0.24 | 6.39 ± 0.57 | 413.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, X.-Q.; Wei, C.-W.; Xu, J.-K.; Wang, X.-J.; Gao, S.-Q.; Lin, Y.-W. Biomimetic Mineralization of Cytochrome c Improves the Catalytic Efficiency and Confers a Functional Multi-Enzyme Composite. Catalysts 2019, 9, 648. https://doi.org/10.3390/catal9080648
Gong X-Q, Wei C-W, Xu J-K, Wang X-J, Gao S-Q, Lin Y-W. Biomimetic Mineralization of Cytochrome c Improves the Catalytic Efficiency and Confers a Functional Multi-Enzyme Composite. Catalysts. 2019; 9(8):648. https://doi.org/10.3390/catal9080648
Chicago/Turabian StyleGong, Xiao-Qing, Chuan-Wan Wei, Jia-Kun Xu, Xiao-Juan Wang, Shu-Qin Gao, and Ying-Wu Lin. 2019. "Biomimetic Mineralization of Cytochrome c Improves the Catalytic Efficiency and Confers a Functional Multi-Enzyme Composite" Catalysts 9, no. 8: 648. https://doi.org/10.3390/catal9080648
APA StyleGong, X.-Q., Wei, C.-W., Xu, J.-K., Wang, X.-J., Gao, S.-Q., & Lin, Y.-W. (2019). Biomimetic Mineralization of Cytochrome c Improves the Catalytic Efficiency and Confers a Functional Multi-Enzyme Composite. Catalysts, 9(8), 648. https://doi.org/10.3390/catal9080648





