Photodegradation of Herbicide Imazapyr and Phenol over Mesoporous Bicrystalline Phases TiO2: A Kinetic Study
Abstract
1. Introduction
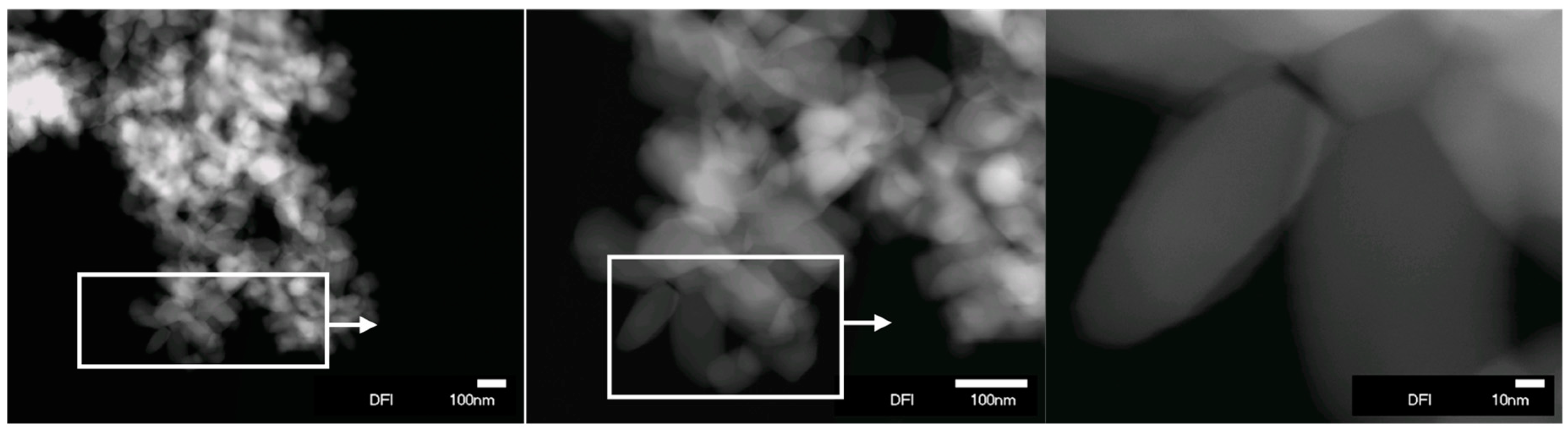
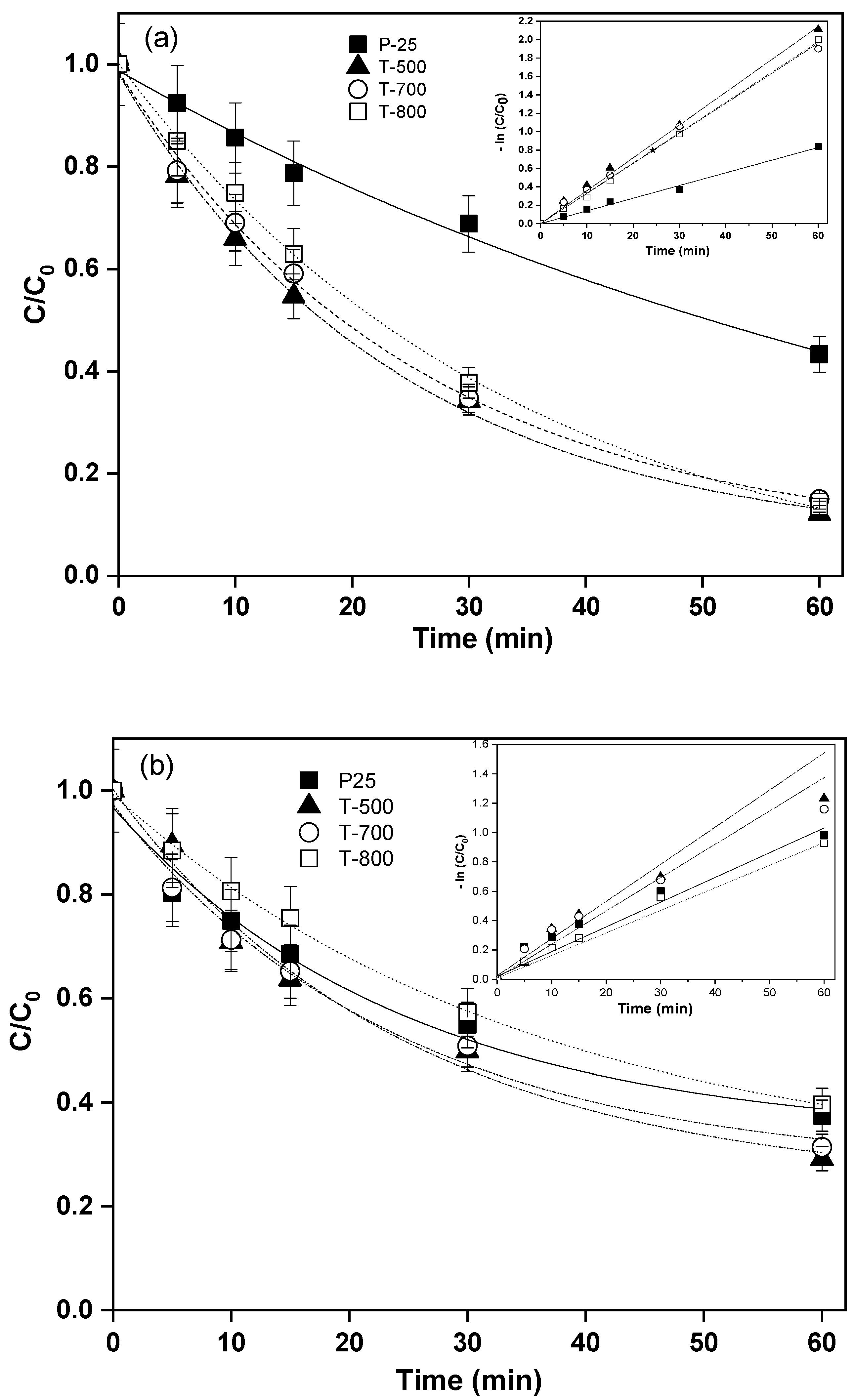
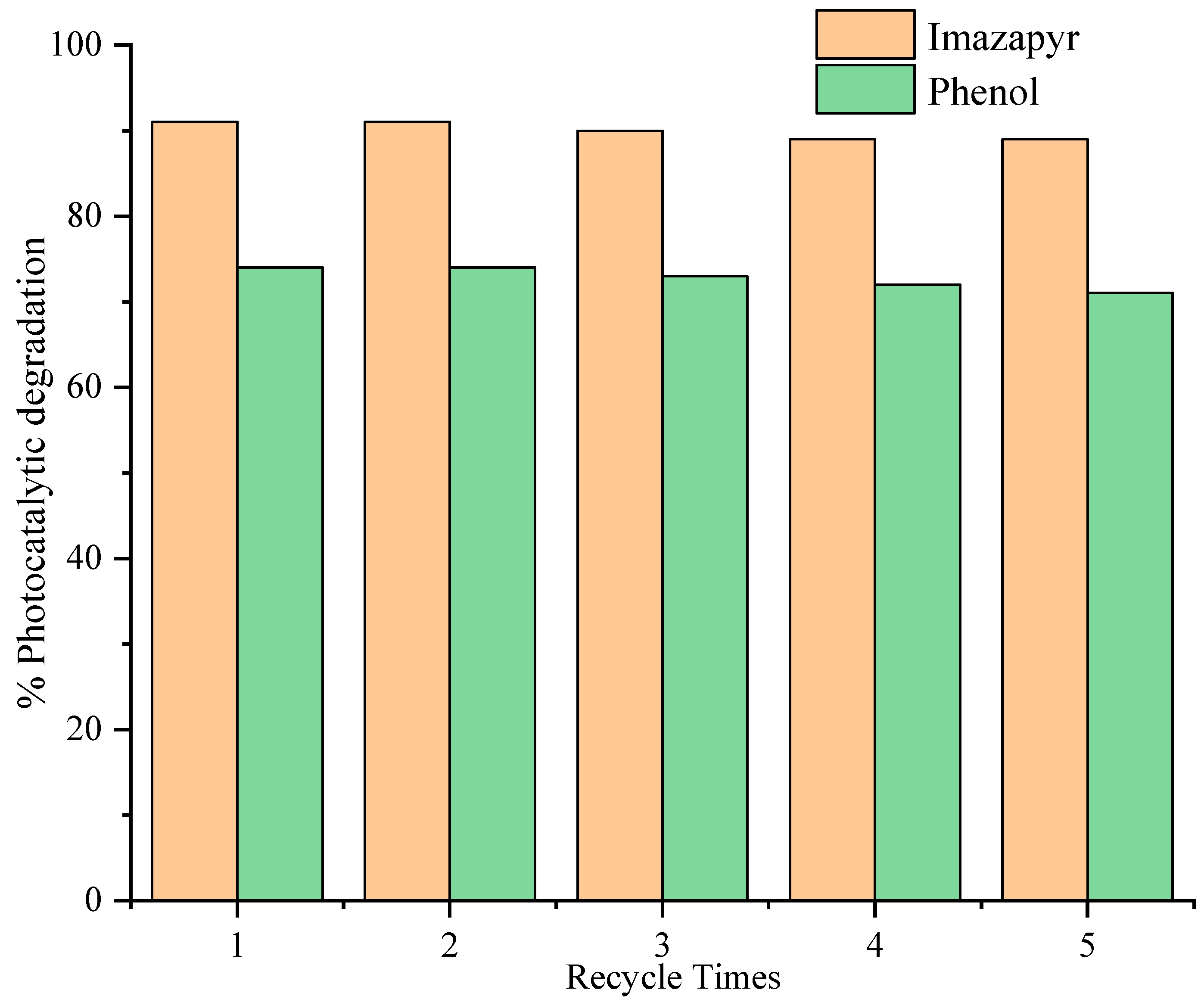
2. Results
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, J.Z.Y.; Maroto-Valer, M.M. A review of nanostructured non-titania photocatalysts and hole scavenging agents for CO2 photoreduction processes. J. Mater. Chem. A 2019, 7, 9368–9385. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Higashimoto, S. Titanium-dioxide-based visible-light-sensitive photocatalysis: Mechanistic insight and applications. Catalysts 2019, 9, 201. [Google Scholar] [CrossRef]
- Friedmann, D.; Mendive, C.; Bahnemann, D. TiO2 for water treatment: Parameters ffecting the kinetics and mechanisms of photocatalysis. Appl. Catal. B Environ. 2010, 99, 398–406. [Google Scholar] [CrossRef]
- Minero, C.; Maurino, V.; Vione, D. Photocatalytic mechanisms and reaction pathways drawn from kinetic and probe molecules. In Photocatalysis and Water Purification; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 53–72. [Google Scholar]
- Khedr, T.M.; El-Sheikh, S.M.; Ismail, A.A.; Bahnemann, D.W. Highly efficient solar light-assisted TiO2 nanocrystalline for photodegradation of ibuprofen drug. Opt. Mater. 2019, 88, 117–127. [Google Scholar] [CrossRef]
- Khedr, T.M.; El-Sheikh, S.M.; Ismail, A.A.; Bahnemann, D.W. Photodegradation of 4-aminoantipyrine over nano-titania heterojunctions using solar and LED irradiation sources. J. Environ. Chem. Eng. 2019, 7, 102797. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Titanium dioxide (anatase and rutile): Surface chemistry, liquid-solid interface chemistry, and scientific synthesis of supported catalysts. Chem. Rev. 2014, 114, 9754–9823. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, J.; Tian, B. Microemulsion-mediated solvothermal synthesis and photocatalytic properties of crystalline titania with controllable phases of anatase and rutile. J. Hazard. Mater. 2011, 192, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Gawel, A.; Rosen, D.; Krause, M.; Latif, A.A.; Griebel, J.; Prager, A.; Schulze, A. Low-temperature synthesis of anatase/rutile/brookite TiO2 nanoparticles on a polymer membrane for photocatalysis. Catalysts 2017, 7, 209. [Google Scholar] [CrossRef]
- Retamoso, C.; Escalona, N.; González, M.; Barrientos, L.; Allende-González, P.; Stancovich, S.; Serpell, R.; Fierro, J.L.G.; Lopez, M. Effect of particle size on the photocatalytic activity of modified rutile sand (TiO2) for the discoloration of methylene blue in water. J. Photochem. Photobiol. A Chem. 2019, 378, 136–141. [Google Scholar] [CrossRef]
- Bettini, L.G.; Dozzi, M.V.; Della Foglia, F.; Chiarello, G.L.; Selli, E.; Lenardi, C.; Piseri, P.; Milani, P. Mixed-Phase Nanocrystalline TiO2 Photocatalysts Produced by Flame Spray Pyrolysis. Appl. Catal. B Environ. 2015, 178, 226–232. [Google Scholar] [CrossRef]
- Kisch, H.; Bahnemann, D. Best practice in photocatalysis: Comparing rates or apparent quantum yields? J. Phys. Chem. Lett. 2015, 6, 1907–1910. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.A.; Bahnemann, D. Metal-free porphyrin-sensitized mesoporous titania films For visible-light indoor air oxidation. ChemSusChem 2010, 3, 1057–1062. [Google Scholar] [CrossRef]
- Robben, L.; Ismail, A.A.; Lohmeier, S.J.; Feldhoff, A.; Bahnemann, D.W.; Buhl, J.-C. Facile synthesis of highly ordered mesoporous and well crystalline TiO2: Impact of different gas atmosphere and calcination temperatures on structural properties. Chem. Mater. 2012, 24, 1268–1275. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W.; Robben, L.; Yarovyi, V.; Wark, M. Palladium doped porous titania photocatalysts: Impact of mesoporous order and crystallinity. Chem. Mater. 2010, 22, 108–116. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W.; Bannat, I.; Wark, M. Gold nanoparticles on mesoporous interparticle networks of titanium dioxide nanocrystals for enhanced photonic efficiencies. J. Phys. Chem. C 2009, 113, 7429–7435. [Google Scholar] [CrossRef]
- Ismail, A.A.; Bahnemann, D.W. One-step synthesis of mesoporous platinum/titania nanocomposites as photocatalyst with enhanced photocatalytic activity for methanol oxidation. Green Chem. 2011, 13, 428. [Google Scholar] [CrossRef]
- Ismail, A.; Bahnemann, D. Mesostructured Pt/TiO2 nanocomposites as highly active photocatalysts for the photooxidation of dichloroacetic acid. J. Phys. Chem. C 2011, 5784–5791. [Google Scholar] [CrossRef]
- Faycal Atitar, M.; Ismail, A.A.; Al-Sayari, S.A.; Bahnemann, D.; Afanasev, D.; Emeline, A.V. Mesoporous TiO2 nanocrystals as efficient photocatalysts: Impact of calcination temperature and phase transformation on photocatalytic performance. Chem. Eng. J. 2015, 264, 417–424. [Google Scholar] [CrossRef]
- Agrios, A.G.; Pichat, P. Recombination rate of photogenerated charges versus surface area: Opposing effects of TiO2 sintering temperature on photocatalytic removal of phenol, anisole, and pyridine in water. J. Photochem. Photobiol. A Chem. 2006, 180, 130–135. [Google Scholar] [CrossRef]
- Enríquez, R.; Agrios, A.G.; Pichat, P. Probing multiple effects of TiO2 sintering temperature on photocatalytic activity in water by use of a series of organic pollutant molecules. Catal. Today 2007, 120, 196–202. [Google Scholar] [CrossRef]
- Murakami, S.-Y.; Kominami, H.; Kera, Y.; Ikeda, S.; Noguchi, H.; Uosaki, K.; Ohtani, B. Evaluation of electron-hole recombination properties of titanium (IV) oxide particles with high photocatalytic activity. Res. Chem. Intermed. 2007, 33, 285–296. [Google Scholar] [CrossRef]
- Bacsa, R.; Kiwi, J. Effect of rutile phase on the photocatalytic properties of nanocrystalline titania during the degradation of p-coumaric acid. Appl. Catal. B Environ. 1998, 16, 19–29. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L.; Barbieriková, Z.; Brezová, V. Influence of crystallinity and OH surface density on the photocatalytic activity of TiO2 powders. J. Photochem. Photobiol. A Chem. 2014, 273, 59–67. [Google Scholar] [CrossRef]
- Sclafani, A.; Palmisano, L.; Schiavello, M. Influence of the preparation methods of TiO2 on the photocatalytic degradation of phenol in aqueous dispersion. J. Phys. Chem. 1990, 94, 829–832. [Google Scholar] [CrossRef]
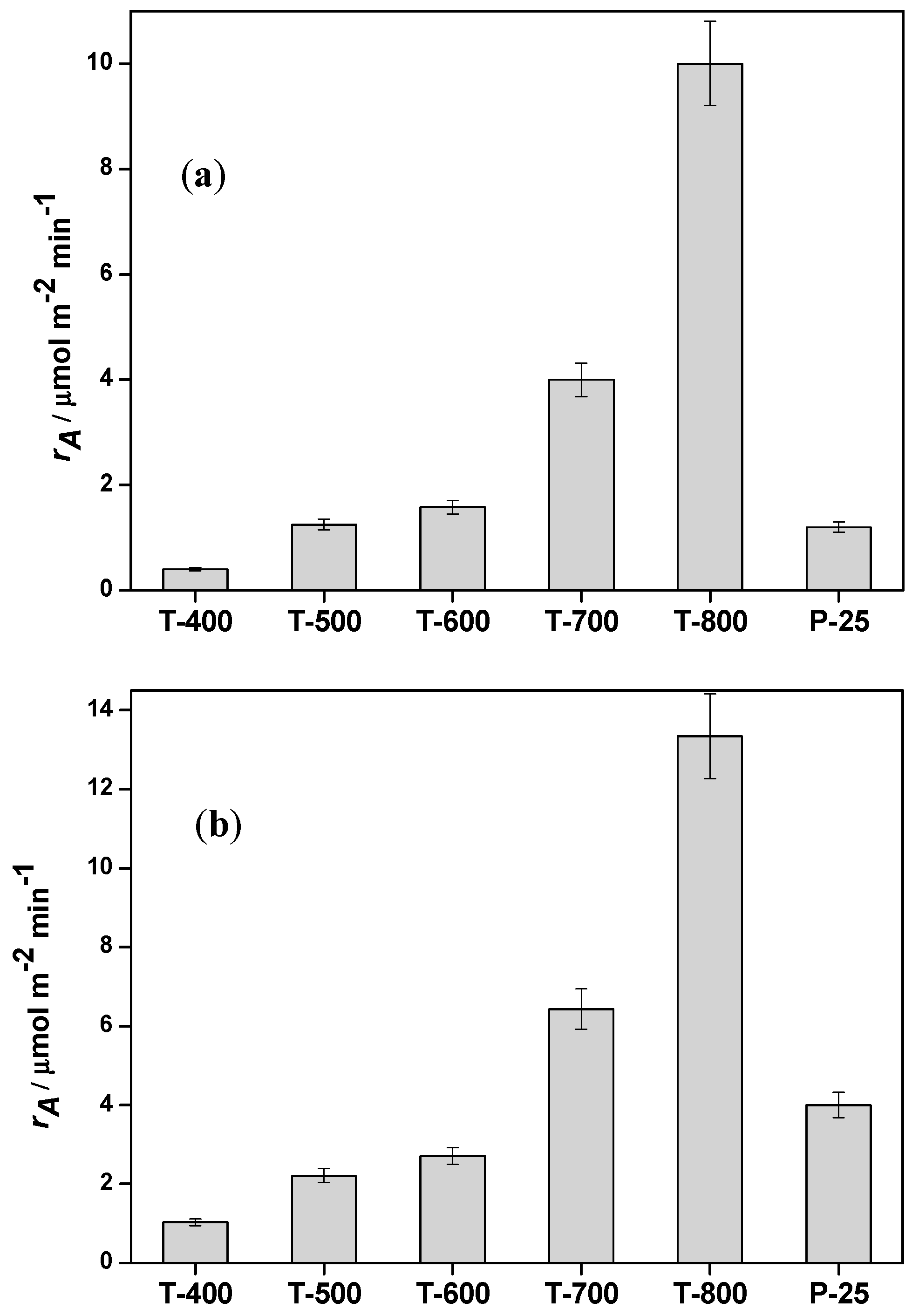
| Sample | Anatase (%) | Rutile (%) | ABET/m2g−1 | kapp/min−1 (Imazapyr) | kapp/min−1 (Phenol) | rA,0/µmol m−2 min−1 (Imazapyr) | rA,0/µmol m−2 min−1 (Phenol) |
|---|---|---|---|---|---|---|---|
| T-400 | 54 | 46 | 165 | 0.0134 | 0.0136 | 0.40 | 1.03 |
| T-500 | 73 | 27 | 120 | 0.0344 | 0.0199 | 1.25 | 2.21 |
| T-600 | 52 | 48 | 70 | 0.024 | 0.0148 | 1.58 | 2.71 |
| T-700 | 47 | 53 | 35 | 0.0313 | 0.0182 | 4.0 | 6.43 |
| T-800 | 18 | 82 | 15 | 0.0335 | 0.0152 | 10 | 13.34 |
| P-25 | 80 | 20 | 50 | 0.0136 | 0.0152 | 1.2 | 4.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faycal Atitar, M.; Ismail, A.A.; Dillert, R.; Bahnemann, D.W. Photodegradation of Herbicide Imazapyr and Phenol over Mesoporous Bicrystalline Phases TiO2: A Kinetic Study. Catalysts 2019, 9, 640. https://doi.org/10.3390/catal9080640
Faycal Atitar M, Ismail AA, Dillert R, Bahnemann DW. Photodegradation of Herbicide Imazapyr and Phenol over Mesoporous Bicrystalline Phases TiO2: A Kinetic Study. Catalysts. 2019; 9(8):640. https://doi.org/10.3390/catal9080640
Chicago/Turabian StyleFaycal Atitar, Mohamed, Adel. A. Ismail, Ralf Dillert, and Detlef W. Bahnemann. 2019. "Photodegradation of Herbicide Imazapyr and Phenol over Mesoporous Bicrystalline Phases TiO2: A Kinetic Study" Catalysts 9, no. 8: 640. https://doi.org/10.3390/catal9080640
APA StyleFaycal Atitar, M., Ismail, A. A., Dillert, R., & Bahnemann, D. W. (2019). Photodegradation of Herbicide Imazapyr and Phenol over Mesoporous Bicrystalline Phases TiO2: A Kinetic Study. Catalysts, 9(8), 640. https://doi.org/10.3390/catal9080640






