Pyrolysis Kinetics of Hydrochars Produced from Brewer’s Spent Grains
Abstract
1. Introduction
2. Results and Discussion
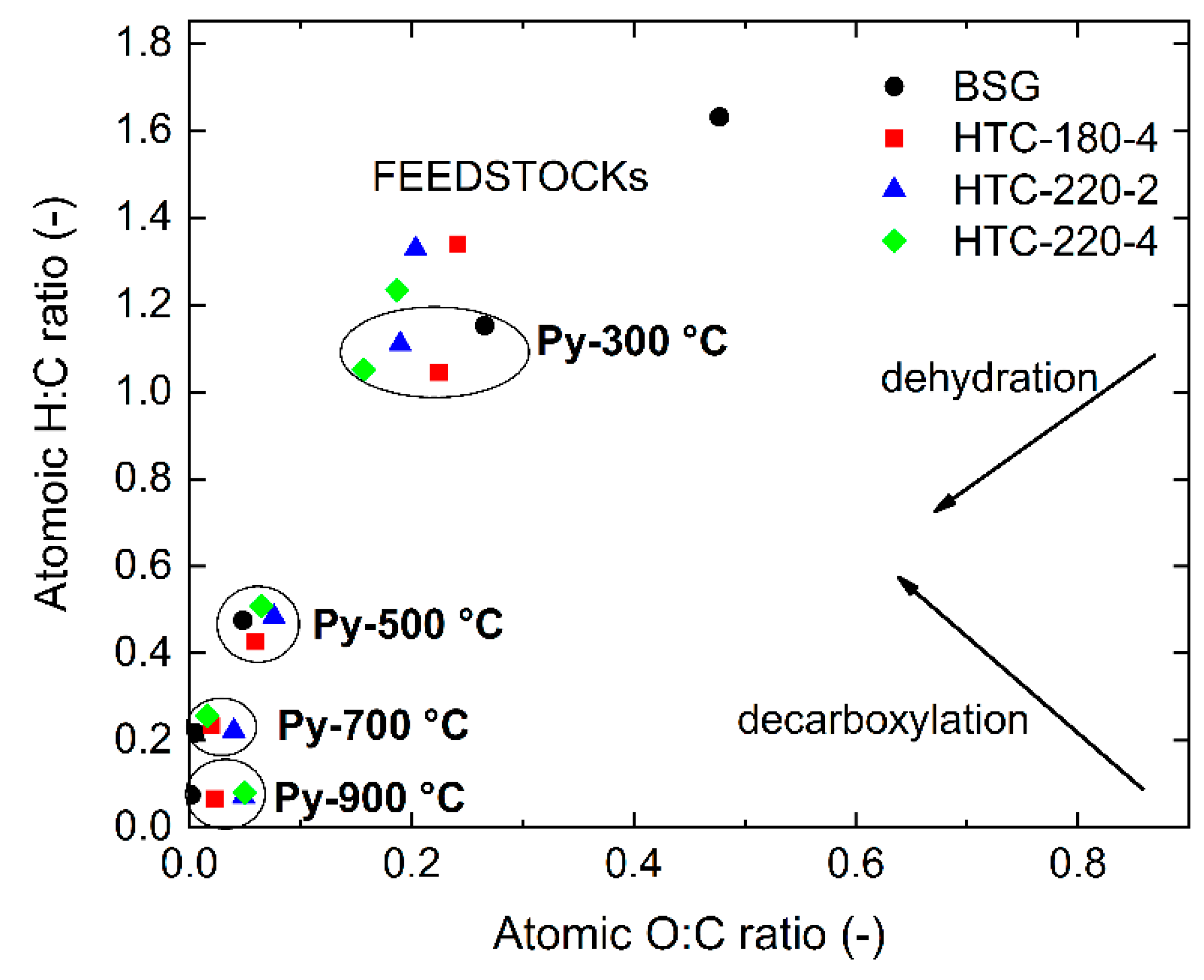
2.1. Feedstocks Characteristics
2.2. Pyrolysis Char Characteristic
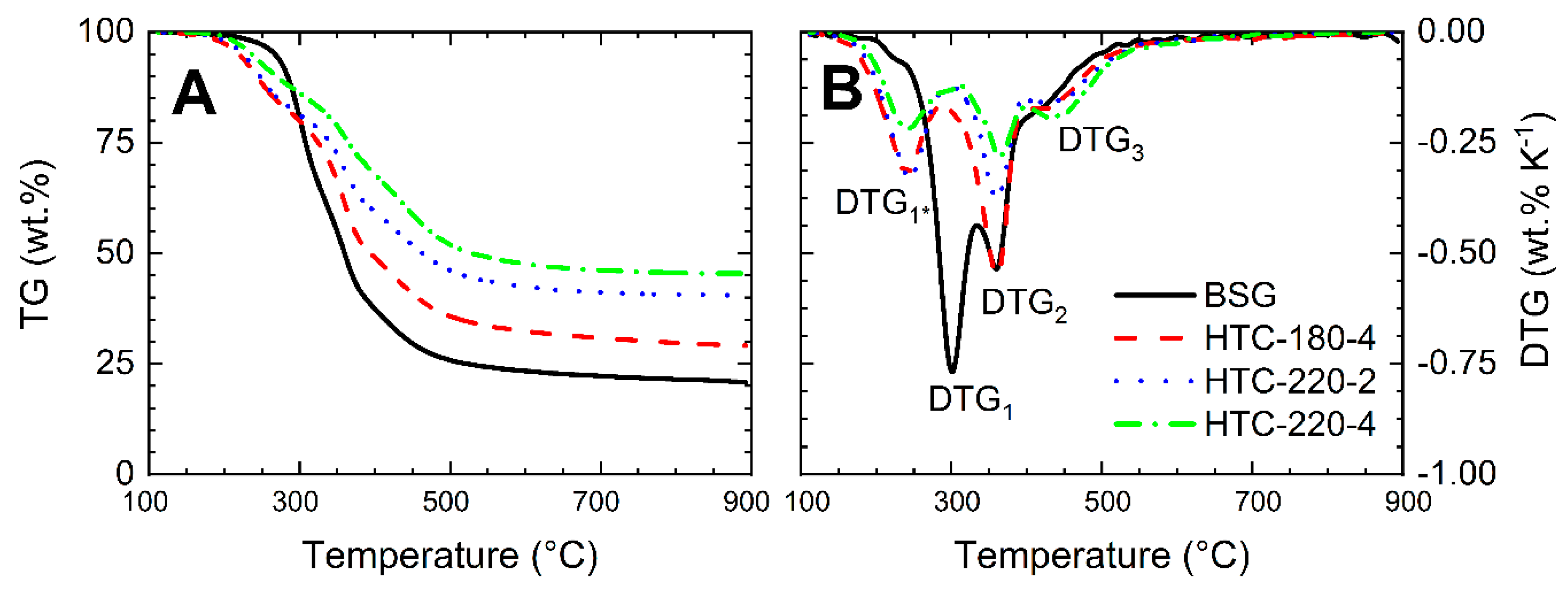
2.3. Analysis of TG–DTG Curves
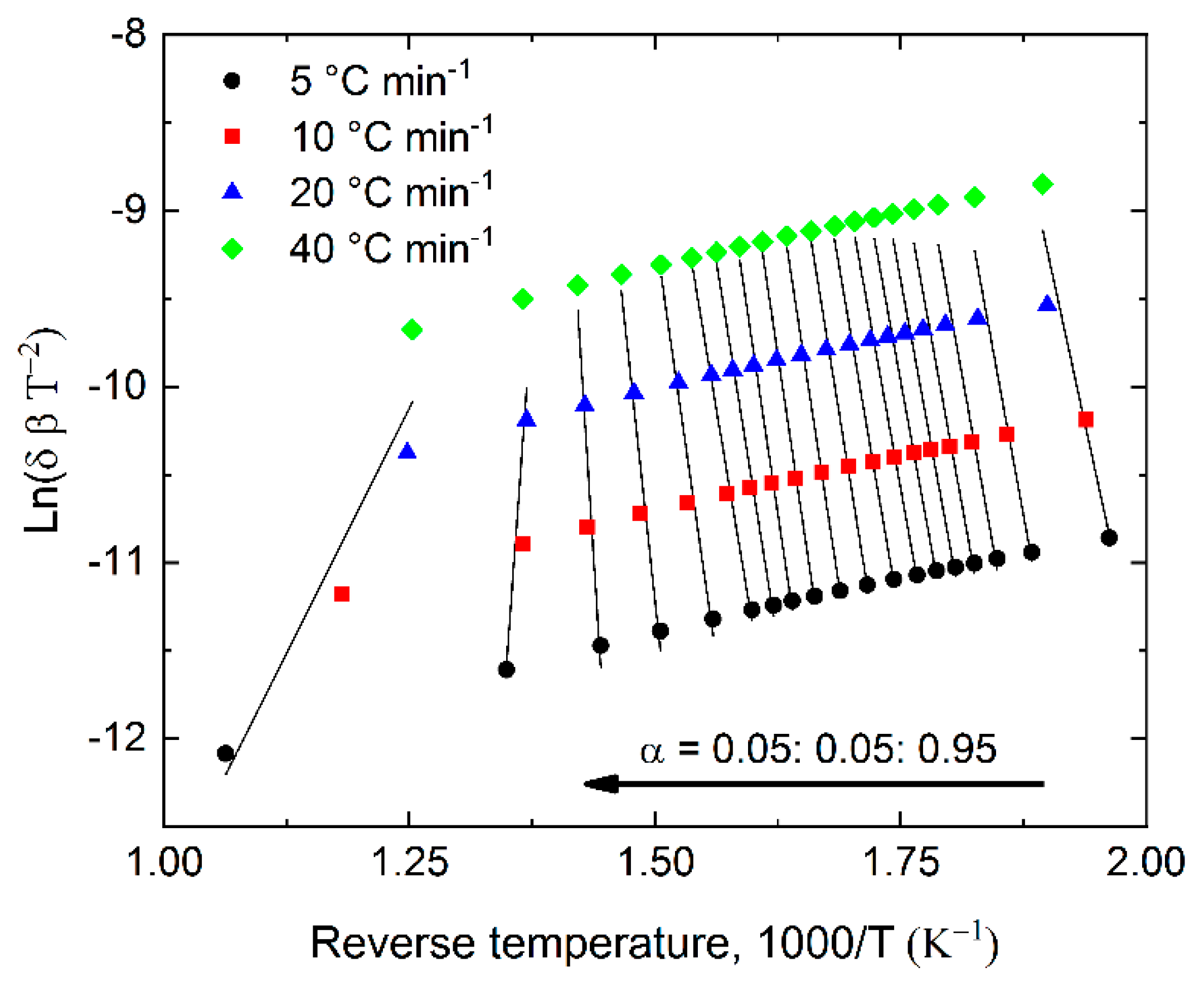
2.4. Pyrolysis Kinetics
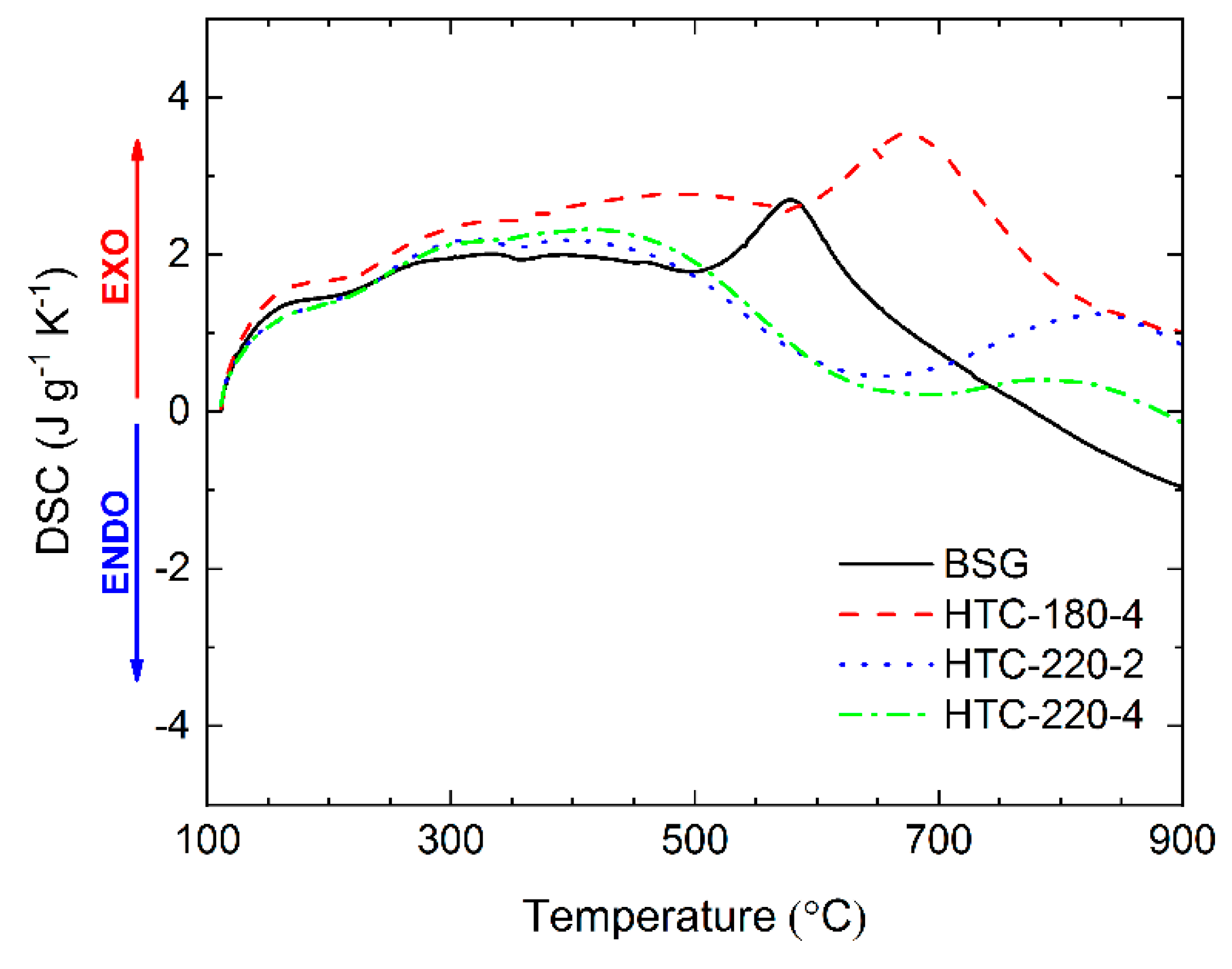
2.5. Heat Flow during Pyrolysis Reaction
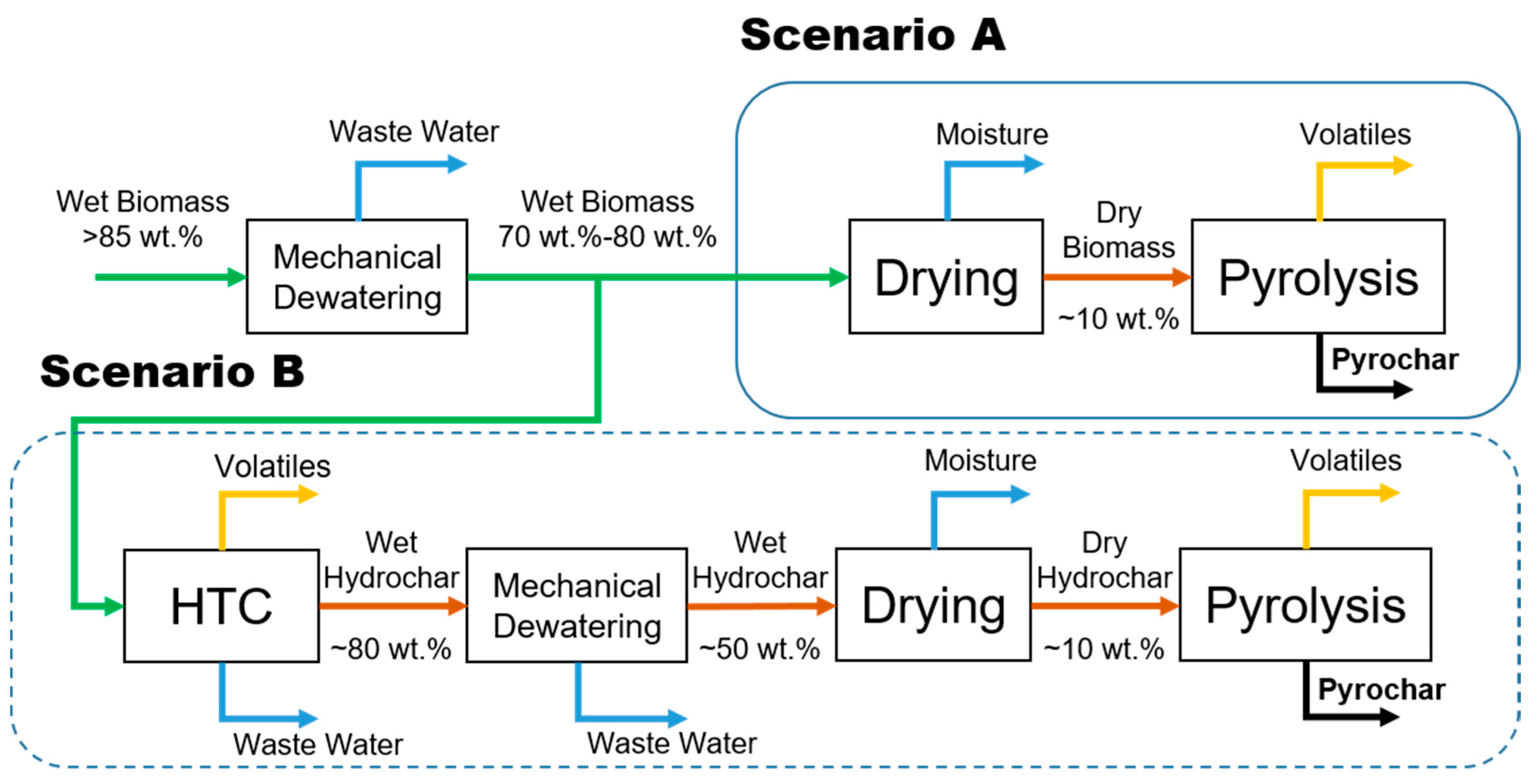
3. Materials and Methods
3.1. Materials
3.2. Pyrolysis Procedure
3.3. Proximate and Ultimate Analysis
3.4. Mathematical Model for Pyrolysis Kinetics
3.5. Calculation of Pyrolysis Kinetics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Material | Heating Rate °C min−1 | T1, T1,* °C | DTG1 DTG1,* % °C−1 | T2 °C | DTG2 % °C−1 | T3, T3* °C | DTG3 % °C−1 | Final Residue wt.% |
|---|---|---|---|---|---|---|---|---|
| BSG | 5 | 281.2 | −0.687 | 340.5 | −0.523 | 367.5 | −0.198 | 18.98 |
| 10 | 289.4 | −0.736 | 349.6 | −0.503 | 378.3 | −0.202 | 19.24 | |
| 20 | 298.6 | −0.776 | 357.9 | −0.515 | 388.3 | −0.211 | 20.99 | |
| 40 | 304.4 | −0.887 | 358.3 | −0.630 | 402.5 | −0.228 | 20.44 | |
| average | 293.4 | −0.771 | 351.6 | −0.543 | 384.2 | −0.210 | 19.91 | |
| HTC-180-4 | 5 | 211.2 | −0.285 | 339.8 | −0.592 | 404.2 | −0.169 | 24.97 |
| 10 | 223.9 | −0.267 | 349.4 | −0.556 | 412.1 | −0.165 | 29.78 | |
| 20 | 236.0 | −0.320 | 358.6 | −0.543 | 422.3 | −0.172 | 29.37 | |
| 40 | 251.6 | −0.317 | 362.2 | −0.633 | 419.9 | −0.183 | 29.08 | |
| average | 230.7 | −0.297 | 352.5 | −0.581 | 414.6 | −0.172 | 28.30 | |
| HTC-220-2 | 5 | 213.0 | −0.268 | 340.1 | −0.411 | 410.0 | −0.168 | 38.71 |
| 10 | 225.8 | −0.276 | 353.3 | −0.414 | 415.8 | −0.162 | 39.54 | |
| 20 | 243.3 | −0.325 | 359.5 | −0.370 | 427.0 | −0.167 | 41.32 | |
| 40 | 269.1 | −0.326 | 378.3 | −0.426 | 423.7 | −0.155 | 43.17 | |
| average | 237.8 | −0.298 | 357.8 | −0.405 | 419.1 | −0.163 | 40.68 | |
| HTC-220-4 | 5 | 209.2 | −0.171 | 342.8 | −0.298 | 410.3 | −0.193 | 44.62 |
| 10 | 221.3 | −0.175 | 353.9 | −0.302 | 427.4 | −0.194 | 45.60 | |
| 20 | 243.9 | −0.216 | 361.8 | −0.279 | 436.4 | −0.192 | 46.13 | |
| 40 | 269.4 | −0.236 | 378.9 | −0.340 | 441.1 | −0.176 | 46.66 | |
| average | 236.0 | −0.199 | 359.4 | −0.305 | 428.8 | −0.189 | 45.75 |
| α− | EA kJ mol−1 | R2− | A s−1 | TA K × 103 | EA kJ mol−1 | R2− | A s−1 | TA K × 103 |
|---|---|---|---|---|---|---|---|---|
| Material | BSG | HTC-180-4 | ||||||
| 0.05 | 186.50 | 0.81 | 1.17 × 1012 | 22.43 | 62.89 | 0.79 | 9.05 × 102 | 7.56 |
| 0.10 | 227.85 | 0.88 | 1.66 × 1018 | 27.41 | 79.24 | 0.87 | 2.72 × 104 | 9.53 |
| 0.15 | 240.19 | 0.93 | 1.34 × 1020 | 28.89 | 84.80 | 0.91 | 8.50 × 104 | 10.20 |
| 0.20 | 242.52 | 0.95 | 3.54 × 1020 | 29.17 | 82.86 | 0.89 | 5.68 × 104 | 9.97 |
| 0.25 | 243.15 | 0.97 | 4.64 × 1020 | 29.25 | 76.83 | 0.79 | 1.57 × 104 | 9.24 |
| 0.30 | 246.34 | 0.98 | 9.45 × 1020 | 29.63 | 88.18 | 0.74 | 1.37 × 105 | 10.61 |
| 0.35 | 252.30 | 0.98 | 3.44 × 1021 | 30.35 | 116.50 | 0.76 | 3.77 × 107 | 14.01 |
| 0.40 | 267.98 | 0.98 | 9.68 × 1022 | 32.23 | 154.98 | 0.81 | 7.6 × 1010 | 18.64 |
| 0.45 | 288.12 | 0.98 | 8.09 × 1024 | 34.65 | 190.25 | 0.87 | 9.41 × 1013 | 22.88 |
| 0.50 | 305.07 | 0.98 | 3.14 × 1026 | 36.69 | 208.11 | 0.90 | 3.57 × 1015 | 25.03 |
| 0.55 | 317.36 | 0.98 | 3.92 × 1027 | 38.17 | 213.69 | 0.93 | 1.12 × 1016 | 25.70 |
| 0.60 | 323.89 | 0.98 | 1.60 × 1028 | 38.96 | 210.42 | 0.93 | 6.01 × 1015 | 25.31 |
| 0.65 | 315.99 | 0.98 | 2.96 × 1027 | 38.01 | 198.37 | 0.91 | 5.78 × 1014 | 23.86 |
| 0.70 | 338.78 | 0.96 | 3.64 × 1029 | 40.75 | 167.24 | 0.77 | 1.15 × 1012 | 20.12 |
| 0.75 | 482.71 | 0.82 | 4.30 × 1043 | 58.06 | 170.34 | 0.72 | 1.38 × 1012 | 20.49 |
| 0.80 | 331.10 | 0.59 | 3.34 × 1069 | 39.82 | 191.85 | 0.74 | 5.42 × 1013 | 23.08 |
| 0.85 | 239.62 | 0.59 | 1.72 × 10166 | 28.82 | 204.37 | 0.77 | 2.15 × 1014 | 24.58 |
| average | 285.26 | 0.90 | − | 34.31 | 147.11 | 0.83 | − | 17.69 |
| Material | HTC-220-2 | HTC-220-4 | ||||||
| 0.05 | 85.19 | 0.98 | 9.98 × 104 | 10.25 | 78.39 | 0.99 | 4.25 × 104 | 9.43 |
| 0.10 | 87.22 | 0.99 | 1.30 × 105 | 10.49 | 82.98 | 0.99 | 1.10 × 105 | 9.98 |
| 0.15 | 86.77 | 0.99 | 1.15 × 105 | 10.44 | 91.69 | 0.98 | 6.47 × 105 | 11.03 |
| 0.20 | 89.62 | 0.98 | 2.06 × 105 | 10.78 | 112.46 | 0.95 | 4.14 × 107 | 13.53 |
| 0.25 | 104.21 | 0.96 | 4.43 × 106 | 12.53 | 142.34 | 0.91 | 1.56 × 1010 | 17.12 |
| 0.30 | 137.92 | 0.93 | 5.40 × 109 | 16.59 | 167.08 | 0.92 | 2.07 × 1012 | 20.10 |
| 0.35 | 170.20 | 0.93 | 3.53 × 1012 | 20.47 | 183.23 | 0.95 | 5.01 × 1013 | 22.04 |
| 0.40 | 188.88 | 0.96 | 1.14 × 1014 | 22.72 | 189.07 | 0.97 | 1.60 × 1014 | 22.74 |
| 0.45 | 193.33 | 0.98 | 2.05 × 1014 | 23.25 | 191.74 | 0.98 | 2.75 × 1014 | 23.06 |
| 0.50 | 190.65 | 0.98 | 1.09 × 1014 | 22.93 | 194.98 | 0.97 | 5.26 × 1014 | 23.45 |
| 0.55 | 189.12 | 0.98 | 7.75 × 1013 | 22.75 | 208.77 | 0.94 | 7.90 × 1015 | 25.11 |
| 0.60 | 191.91 | 0.98 | 1.39 × 1014 | 23.08 | 235.17 | 0.88 | 1.40 × 1018 | 28.29 |
| 0.65 | 205.44 | 0.89 | 5.49 × 1015 | 24.71 | 251.28 | 0.89 | 3.31 × 1019 | 30.22 |
| 0.70 | 222.05 | 0.76 | 3.69 × 1017 | 26.71 | 258.15 | 0.91 | 1.29 × 1020 | 31.05 |
| 0.75 | 244.16 | 0.74 | 5.03 × 1019 | 29.37 | 263.15 | 0.92 | 3.51 × 1020 | 31.65 |
| 0.80 | 255.68 | 0.73 | 1.31 × 1021 | 30.75 | 273.82 | 0.92 | 2.88 × 1021 | 32.93 |
| 0.85 | 239.95 | 0.63 | 2.18 × 1021 | 28.86 | 279.40 | 0.90 | 8.81 × 1021 | 33.61 |
| average | 169.55 | 0.90 | − | 20.39 | 188.45 | 0.94 | − | 22.67 |
| Reaction Mechanism | Integral Form, g(α) | Range I α = 0.10−0.40 | Range II α = 0.45−0.85 | ||
|---|---|---|---|---|---|
| EA kJ mol−1 | R2− | EA kJ mol−1 | R2− | ||
| Reaction Order | |||||
| Zero order F0 | α | 77.58 | 0.98 | 9.39 | 0.74 |
| First order F1 | −ln (1−α) | 89.76 | 0.98 | 26.11 | 0.95 |
| Second order F2 | [1/(1−α)] −1 | 103.21 | 0.99 | 51.11 | 0.99 |
| Third order F3 | [1/(1−α)2] −1 | 117.93 | 0.99 | 83.16 | 0.99 |
| Diffusion | |||||
| One-dimensional diffusion D1 | α 2 | 164.46 | 0.98 | 29.44 | 0.88 |
| Two-dimensional diffusion D2 | (1−α) ln (1−α) +α | 172.17 | 0.99 | 38.12 | 0.92 |
| Three-dimensional diffusion D3 | [1− (1−α)1/3]2 | 180.42 | 0.99 | 49.91 | 0.95 |
| Ginstling–Brouns D4 | 1− (2α/3) − (1−α)2/3 | 174.92 | 0.99 | 41.98 | 0.93 |
| Geometrical Contraction Models | |||||
| Contracting area (cylinder) R2 | 1− (1−α)1/2 | 83.51 | 0.99 | 10.76 | 0.86 |
| Contracting volume (sphere) R3 | 1− (1−α)1/3 | 85.56 | 0.99 | 13.60 | 0.90 |
| Nucleation Models | |||||
| Power law P1 | α 1/4 | 12.41 | 0.95 | −5.65 | 0.93 |
| Power law P2 | α 1/3 | 19.65 | 0.97 | −3.98 | 0.79 |
| Power law P3 | α 1/2 | 34.13 | 0.98 | −0.64 | 0.05 |
| Power law P4 | α 3/2 | 48.62 | 0.98 | 2.70 | 0.34 |
| Avrami–Erofeev (m = 2) A2 | [−ln (1−α)]1/2 | 40.22 | 0.99 | 7.72 | 0.85 |
| Avrami–Erofeev (m = 3) A3 | [−ln (1−α)]1/3 | 23.71 | 0.98 | 1.59 | 0.34 |
| Avrami–Erofeev (m = 4) A4 | [−ln (1−α)]1/4 | 15.46 | 0.97 | −1.47 | 0.42 |
| Average EA Obtained Using KAS Method | 245.76 | − | 326.96 | − | |
References
- Statista. Beer Production Worldwide From 1998 to 2016. Stat. Portal. 2017. Available online: https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed on 8 May 2018).
- Barth-Haas Group. The Barth Report. 2017. Available online: https://www.barthhaasgroup.com/images/mediacenter/downloads/pdfs/412/barthbericht20162017en.pdf (accessed on 20 January2019).
- Balogun, A.O.; Sotoudehniakarani, F.; McDonald, A.G. Thermo-kinetic, spectroscopic study of brewer’s spent grains and characterisation of their pyrolysis products. J. Anal. Appl. Pyrolysis 2017, 127, 8–16. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. A comparative analysis of pretreatment strategies on the properties and hydrolysis of brewers’ spent grain. Bioresour. Technol. 2018, 248, 272–279. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.L.; O’Callaghan, Y.C.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Brewers’ spent grain; Bioactivity of phenolic component, its role in animal nutrition and potential for incorporation in functional foods: A review. Proc. Nutr. Soc. 2013, 72, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Niemi, P.; Tamminen, T.; Smeds, A.; Viljanen, K.; Ohra-Aho, T.; Holopainen-Mantila, U.; Faulds, C.B.; Poutanen, K.; Buchert, J. Characterization of Lipids and Lignans in Brewer’s Spent Grain and Its Enzymatically Extracted Fraction. J. Agric. Food Chem. 2012, 60, 9910–9917. [Google Scholar] [CrossRef] [PubMed]
- Poerschmann, J.; Weiner, B.; Wedwitschka, H.; Baskyr, I.; Koehler, R.; Kopinke, F.-D. Characterization of biocoals and dissolved organic matter phases obtained upon hydrothermal carbonization of brewer’s spent grain. Bioresour. Technol. 2014, 164, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Mallen, E.; Najdanovic-Visak, V. Brewers’ spent grains: Drying kinetics and biodiesel production. Bioresour. Technol. Rep. 2018, 1, 16–23. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Kruse, A.; Dahmen, N. Water—A magic solvent for biomass conversion. J. Supercrit. Fluids 2015, 96, 36–45. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Qian, F.; Zhang, S.; Chen, J. Investigation on the Physical and Chemical Properties of Hydrochar and Its Derived Pyrolysis Char for Their Potential Application: Influence of Hydrothermal Carbonization Conditions. Energy Fuels 2015, 29, 5222–5230. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, S.; Ge, S.; Chen, X.; Ge, X.; Chen, M. Hydrothermal carbonization of medical wastes and lignocellulosic biomass for solid fuel production from lab-scale to pilot-scale. Energy 2017, 118, 312–323. [Google Scholar] [CrossRef]
- Titirici, M.-M.; White, R.J.; Falco, C.; Sevilla, M.; Solis, M.S. Black perspectives for a green future: Hydrothermal carbons for environment protection and energy storage. Energy Environ. Sci. 2012, 5, 6796. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Ro, K.S.; Libra, J.A.; Kammann, C.I.; Berge, N.; Yang, J.; Cao, X.; Lima, I.; Li, L.; Li, Y.; Chen, N.; et al. Effects of Biomass Types and Carbonization Conditions on the Chemical Characteristics of Hydrochars. J. Agric. Food Chem. 2013, 61, 9401–9411. [Google Scholar] [CrossRef]
- Kruse, A.; Zevaco, T.A. Properties of Hydrochar as Function of Feedstock, Reaction Conditions and Post-Treatment. Energies 2018, 11, 674. [Google Scholar] [CrossRef]
- Zhao, X.; Becker, G.; Faweya, N.; Correa, C.R.; Yang, S.; Xie, X.; Kruse, A. Biomass Conversion and Biorefinery Fertilizer and Activated Carbon Production by Hydrothermal Carbonization of Digestate. Biomass Convers. Biorefin. 2018, 8, 423–436. [Google Scholar] [CrossRef]
- Ulbrich, M.; Preßl, D.; Fendt, S.; Gaderer, M.; Spliethoff, H. Impact of HTC reaction conditions on the hydrochar properties and CO2 gasification properties of spent grains. Fuel Process. Technol. 2017, 167, 663–669. [Google Scholar] [CrossRef]
- Garlapalli, R.K.; Wirth, B.; Reza, M.T. Pyrolysis of hydrochar from digestate: Effect of hydrothermal carbonization and pyrolysis temperatures on pyrochar formation. Bioresour. Technol. 2016, 220, 168–174. [Google Scholar] [CrossRef]
- Breulmann, M.; Van Afferden, M.; Müller, R.A.; Schulz, E.; Fühner, C. Process conditions of pyrolysis and hydrothermal carbonization affect the potential of sewage sludge for soil carbon sequestration and amelioration. J. Anal. Appl. Pyrolysis 2017, 124, 256–265. [Google Scholar] [CrossRef]
- Arauzo, P.J.; Olszewski, M.P.; Kruse, A. Hydrothermal Carbonization Brewer’s Spent Grains with the Focus on Improving the Degradation of the Feedstock. Energies 2018, 11, 3226. [Google Scholar] [CrossRef]
- Huang, R.; Fang, C.; Lu, X.; Jiang, R.; Tang, Y. Transformation of Phosphorus during (Hydro)thermal Treatments of Solid Biowastes: Reaction Mechanisms and Implications for P Reclamation and Recycling. Environ. Sci. Technol. 2017, 51, 10284–10298. [Google Scholar] [CrossRef]
- Heilmann, S.M.; Molde, J.S.; Timler, J.G.; Wood, B.M.; Mikula, A.L.; Vozhdayev, G.V.; Colosky, E.C.; Spokas, K.; Valentas, K.J. Phosphorus Reclamation through Hydrothermal Carbonization of Animal Manures. Environ. Sci. Technol. 2014, 48, 10323–10329. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Influence of temperature on nitrogen fate during hydrothermal carbonization of food waste. Bioresour. Technol. 2018, 247, 182–189. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Xinag, J.; Sun, L.; Yang, T.; Zhang, A.; Wang, Y.; Chen, G. Effects of Pyrolysis Temperature on Characteristics of Porosity in Biomass Chars. In Proceedings of the 2009 International Conference on Energy and Environment Technology, Guilin, Chian, 16–18 October 2009. [Google Scholar]
- Yang, W.; Wang, H.; Zhang, M.; Zhu, J.; Zhou, J.; Wu, S. Fuel properties and combustion kinetics of hydrochar prepared by hydrothermal carbonization of bamboo. Bioresour. Technol. 2016, 205, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Bach, Q.-V.; Tran, K.-Q. Dry and Wet Torrefaction of Woody Biomass—A Comparative Studyon Combustion Kinetics. Energy Procedia 2015, 75, 150–155. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Sun, K.; Jiang, J.C. Preparation and characterization of activated carbon from rubber-seed shell by physical activation with steam. Biomass Bioenergy 2010, 34, 539–544. [Google Scholar] [CrossRef]
- Mochidzuki, K.; Soutric, F.; Tadokoro, K.; Antal, M.J.; Toth, M.; Zelei, B.; Várhegyi, G. Electrical and Physical Properties of Carbonized Charcoals. Ind. Eng. Chem. Res. 2003, 42, 5140–5151. [Google Scholar] [CrossRef]
- Herou, S.; Schlee, P.; Jorge, A.B.; Titirici, M. Biomass-derived electrodes for flexible supercapacitors. Curr. Opin. Green Sustain. Chem. 2018, 9, 18–24. [Google Scholar] [CrossRef]
- Olszewski, M.; Kempegowda, R.S.; Skreiberg, Ø.; Wang, L.; Løvås, T. Techno-Economics of Biocarbon Production Processes under Norwegian Conditions. Energy Fuels 2017, 31, 14338–14356. [Google Scholar] [CrossRef]
- Olszewski, M.; Arauzo, P.; Wądrzyk, M.; Kruse, A. Py-GC-MS of hydrochars produced from brewer’s spent grains. J. Anal. Appl. Pyrolysis 2019, 140, 255–263. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Yuan, X.; Xi, Y.; Huang, Z.; Tan, M.; Li, C. The effects of temperature and color value on hydrochars’ properties in hydrothermal carbonization. Bioresour. Technol. 2018, 249, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.T.; Wirth, B.; Lüder, U.; Werner, M. Behavior of selected hydrolyzed and dehydrated products during hydrothermal carbonization of biomass. Bioresour. Technol. 2014, 169, 352–361. [Google Scholar] [CrossRef]
- Shen, J.; Igathinathane, C.; Yu, M.; Pothula, A.K.; Cannayen, I. Biomass pyrolysis and combustion integral and differential reaction heats with temperatures using thermogravimetric analysis/differential scanning calorimetry. Bioresour. Technol. 2015, 185, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Ricci, L.; Umiltà, E.; Righetti, M.C.; Messina, T.; Zurlini, C.; Montanari, A.; Bronco, S.; Bertoldo, M. On the thermal behavior of protein isolated from different legumes investigated by DSC and TGA. J. Sci. Food Agric. 2018, 98, 5368–5377. [Google Scholar] [CrossRef] [PubMed]
- Fermoso, J.; Mašek, O. Thermochemical decomposition of coffee ground residues by TG-MS: A kinetic study. J. Anal. Appl. Pyrolysis 2018, 130, 358–367. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, W.; Cen, K. A Modeling Study on Cellulose Particle Pyrolysis Under Fluidized-Bed Conditions. In Progress in Thermochemical Biomass Conversion; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Grønli, M.G.; Melaaen, M.C. Mathematical Model for Wood PyrolysisComparison of Experimental Measurements with Model Predictions. Energy Fuels 2000, 14, 791–800. [Google Scholar] [CrossRef]
- Haykiri-Acma, H.; Yaman, S.; Küçükbayrak, S. Effect of heating rate on the pyrolysis yields of rapeseed. Renew. Energy 2006, 31, 803–810. [Google Scholar] [CrossRef]
- Anca-Couce, A. Reaction mechanisms and multi-scale modelling of lignocellulosic biomass pyrolysis. Prog. Energy Combust. Sci. 2016, 53, 41–79. [Google Scholar] [CrossRef]
- Corbetta, M.; Bennadji, H.; Smith, K.; Serapiglia, M.J.; Gauthier, G.; Melkior, T.; Frassoldati, A.; Ranzi, E.; Fisher, E.M. Pyrolysis of Centimeter-Scale Woody Biomass Particles: Kinetic Modeling and Experimental Validation. Energy Fuels 2014, 28, 3884–3898. [Google Scholar] [CrossRef]
- Park, W.C.; Atreya, A.; Baum, H.R. Experimental and theoretical investigation of heat and mass transfer processes during wood pyrolysis. Combust. Flame 2010, 157, 481–494. [Google Scholar] [CrossRef]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Chun, L.; Junmao, T. Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer. Bioresour. Technol. 2010, 101, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Fermoso, J.; Pizarro, P.; Coronado, J.M.; Serrano, D.P. Transportation Biofuels via the Pyrolysis Pathway: Status and Prospects BT. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2017; pp. 1–33. [Google Scholar]
- Islam, M.A.; Asif, M.; Hameed, B. Pyrolysis kinetics of raw and hydrothermally carbonized Karanj (Pongamia pinnata) fruit hulls via thermogravimetric analysis. Bioresour. Technol. 2015, 179, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, B. Investigation of thermodynamic parameters in the pyrolysis conversion of biomass and manure to biochars using thermogravimetric analysis. Bioresour. Technol. 2013, 146, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S.; Mehmood, M.A.; Taqvi, S.T.H.; Elkamel, A.; Liu, C.-G.; Xu, J.; Rahimuddin, S.A.; Gull, M. Pyrolysis, kinetics analysis, thermodynamics parameters and reaction mechanism of Typha latifolia to evaluate its bioenergy potential. Bioresour. Technol. 2017, 245, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Mei, Y.; Zhang, L.; Liu, R.; Cai, J. Kinetics and reaction chemistry of pyrolysis and combustion of tobacco waste. Fuel 2015, 156, 71–80. [Google Scholar] [CrossRef]
- Braga, R.M.; Melo, D.M.A.; Aquino, F.M.; Freitas, J.C.O.; Melo, M.A.F.; Barros, J.M.F.; Fontes, M.S.B. Characterization and comparative study of pyrolysis kinetics of the rice husk and the elephant grass. J. Therm. Anal. Calorim. 2014, 115, 1915–1920. [Google Scholar] [CrossRef]
- Lin, F.; Waters, C.L.; Mallinson, R.G.; Lobban, L.L.; Bartley, L.E. Relationships between Biomass Composition and Liquid Products Formed via Pyrolysis. Front. Energy Res. 2015, 3, 1518. [Google Scholar] [CrossRef]
- Kawamoto, H.; Horigoshi, S.; Saka, S. Pyrolysis reactions of various lignin model dimers. J. Wood Sci. 2007, 53, 168–174. [Google Scholar] [CrossRef]
- Fan, Y.; Hornung, U.; Dahmen, N.; Kruse, A. Hydrothermal liquefaction of protein-containing biomass: Study of model compounds for Maillard reactions. Biomass Convers. Biorefin. 2018, 8, 909–923. [Google Scholar] [CrossRef]
- Peterson, A.A.; Lachance, R.P.; Tester, J.W. Kinetic Evidence of the Maillard Reaction in Hydrothermal Biomass Processing: Glucose−Glycine Interactions in High-Temperature, High-Pressure Water. Ind. Eng. Chem. Res. 2010, 49, 2107–2117. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.-M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273. [Google Scholar] [CrossRef]
- Svante, A. Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte. Z. Phys. Chem. 1889, 4, 96. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z. Phys. Chem. 1889, 4, 226–248. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Eduardo, F.; João, C.; José, G.; Joaquim, S.; Fernando, R.; Ana, V. Spent Brewery Grains for Improvement of Thermal Insulation of Ceramic Bricks. J. Mater. Civ. Eng. 2013, 25, 1638–1646. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Scharler, R. Modelling heat of reaction in biomass pyrolysis with detailed reaction schemes. Fuel 2017, 206, 572–579. [Google Scholar] [CrossRef]
- Gomez, C.; Velo, E.; Barontini, F.; Cozzani, V. Influence of Secondary Reactions on the Heat of Pyrolysis of Biomass. Ind. Eng. Chem. Res. 2009, 48, 10222–10233. [Google Scholar] [CrossRef]
- Rath, J.; Wolfinger, M.; Steiner, G.; Krammer, G.; Barontini, F.; Cozzani, V. Heat of wood pyrolysis. Fuel 2003, 82, 81–91. [Google Scholar] [CrossRef]
- Anca-Couce, A.; Zobel, N.; Berger, A.; Behrendt, F. Smouldering of pine wood: Kinetics and reaction heats. Combust. Flame 2012, 159, 1708–1719. [Google Scholar] [CrossRef]
- Bryś, A.; Bryś, J.; Ostrowska-Ligęza, E.; Kaleta, A.; Górnicki, K.; Głowacki, S.; Koczoń, P. Wood biomass characterization by DSC or FT-IR spectroscopy. J. Therm. Anal. Calorim. 2016, 126, 27–35. [Google Scholar] [CrossRef]
- Antal, M.J.; Grønli, M. The Art, Science, and Technology of Charcoal Production†. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Galgano, A.; Di Blasi, P.C.; Branca, D.C.; Galgano, D.A. On the Experimental Evidence of Exothermicity in Wood and Biomass Pyrolysis. Energy Technol. 2016, 5, 19–29. [Google Scholar] [CrossRef]
- Cai, J.; Xu, D.; Dong, Z.; Yu, X.; Yang, Y.; Banks, S.W.; Bridgwater, A.V. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: Case study of corn stalk. Renew. Sustain. Energy Rev. 2018, 82, 2705–2715. [Google Scholar] [CrossRef]
- Carrier, M.; Knoetze, J.H.; Auret, L.; Bridgwater, A. Using Apparent Activation Energy as a Reactivity Criterion for Biomass Pyrolysis. Energy Fuels 2016, 30, 7834–7841. [Google Scholar] [CrossRef]
- Channiwala, S.; Parikh, P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Liang, Y.-G.; Cheng, B.; Si, Y.-B.; Cao, D.-J.; Jiang, H.-Y.; Han, G.-M.; Liu, X.-H. Thermal decomposition kinetics and characteristics of Spartina alterniflora via thermogravimetric analysis. Renew. Energy 2014, 68, 111–117. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 2018, 4, 323–328. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Joint convention of four electrical institutes. Res. Rep. Chiba Inst. Technol. Sci. Technol. 1971, 16, 22–31. [Google Scholar]
- Damartzis, T.; Vamvuka, D.; Sfakiotakis, S.; Zabaniotou, A.; Damartzis, T. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA). Bioresour. Technol. 2011, 102, 6230–6238. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H. Investigation of Thermodynamic Parameters in the Thermal Decomposition of Plastic Waste. Waste Lube Oil Compd. 2010, 44, 5313–5317. [Google Scholar]
- Ebrahimi-Kahrizsangi, R.; Abbasi, M. Evaluation of reliability of Coats-Redfern method for kinetic analysis of non-isothermal TGA. Trans. Nonferrous Met. Soc. China 2008, 18, 217–221. [Google Scholar] [CrossRef]
- Sherchenkov, A.; Kozyukhin, S.; Babich, A. Estimation of kinetic parameters for the phase change memory materials by DSC measurements. J. Therm. Anal. Calorim. 2014, 117, 1509–1516. [Google Scholar] [CrossRef]

| Analysis | BSG | HTC-180-4 | HTC-220-2 | HTC-220-4 | ||||
|---|---|---|---|---|---|---|---|---|
| Moisture, wt.% | 3.75 | ± 0.65 | 4.06 | ± 0.41 | 3.42 | ± 0.75 | 3.25 | ± 0.83 |
| Proximate analysis, db, wt.% | ||||||||
| Ash | 4.32 | ± 0.05 | 4.40 | ± 0.05 | 4.40 | ± 0.13 | 4.83 | ± 0.13 |
| Volatile Matter | 79.22 | ± 0.07 | 70.59 | ± 0.17 | 68.06 | ± 0.57 | 63.93 | ± 1.03 |
| Fixed Carbon | 16.46 | ± 0.76 | 25.01 | ± 0.24 | 27.54 | ± 0.63 | 31.25 | ± 0.57 |
| Ultimate analysis, daf, wt.% | ||||||||
| C | 53.50 | ± 0.40 | 66.29 | ± 0.33 | 68.82 | ± 0.75 | 70.17 | ± 0.77 |
| H | 7.27 | ± 0.09 | 7.39 | ± 0.04 | 7.62 | ± 0.05 | 7.21 | ± 0.06 |
| N | 4.89 | ± 0.27 | 4.54 | ± 0.06 | 4.61 | ± 0.06 | 4.67 | ± 0.14 |
| S | 0.30 | ± 0.02 | 0.46 | ± 0.01 | 0.45 | ± 0.01 | 0.45 | ± 0.02 |
| O | 34.04 | ± 0.80 | 21.33 | ± 0.45 | 18.51 | ± 0.65 | 17.51 | ± 1.00 |
| HHV, daf, MJ kg−1 | 23.53 | ± 0.16 | 29.54 | ± 0.11 | 30.89 | ± 0.25 | 31.06 | ± 0.23 |
| Pyrolysis Temperature (°C) | Feedstock | Char Yield * | Ash | VM | FC | HHV MJ kg−1 |
|---|---|---|---|---|---|---|
| wt.% | ||||||
| db | daf | |||||
| 300 | BSG | 78.5 | 5.31 | 56.45 | 38.24 | 27.26 |
| HTC-180-4 | 79.4 | 5.31 | 51.13 | 43.56 | 29.49 | |
| HTC-220-2 | 81.0 | 5.26 | 43.59 | 51.15 | 30.99 | |
| HTC-220-4 | 85.8 | 5.44 | 40.05 | 54.51 | 31.12 | |
| 500 1 | BSG | 25.9 | 13.08 | 6.89 | 80.03 | 32.22 |
| HTC-180-4 | 35.8 | 11.78 | 7.50 | 80.72 | 31.59 | |
| HTC-220-2 | 46.2 | 9.21 | 8.83 | 81.96 | 31.11 | |
| HTC-220-4 | 52.0 | 8.98 | 6.21 | 84.80 | 31.88 | |
| 700 | BSG | 22.3 | 18.67 | 0.29 | 81.03 | 33.09 |
| HTC-180-4 | 30.9 | 13.64 | 2.63 | 83.74 | 32.34 | |
| HTC-220-2 | 41.2 | 10.34 | 3.79 | 85.87 | 31.48 | |
| HTC-220-4 | 46.2 | 10.11 | 0.43 | 89.46 | 33.13 | |
| 900 | BSG | 22.0 | 18.92 | − | 81.08 | 32.72 |
| HTC-180-4 | 28.3 | 14.90 | − | 85.10 | 31.94 | |
| HTC-220-2 | 37.4 | 11.39 | − | 88.61 | 30.73 | |
| HTC-220-4 | 45.8 | 10.20 | − | 89.80 | 30.75 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewski, M.P.; Arauzo, P.J.; Maziarka, P.A.; Ronsse, F.; Kruse, A. Pyrolysis Kinetics of Hydrochars Produced from Brewer’s Spent Grains. Catalysts 2019, 9, 625. https://doi.org/10.3390/catal9070625
Olszewski MP, Arauzo PJ, Maziarka PA, Ronsse F, Kruse A. Pyrolysis Kinetics of Hydrochars Produced from Brewer’s Spent Grains. Catalysts. 2019; 9(7):625. https://doi.org/10.3390/catal9070625
Chicago/Turabian StyleOlszewski, Maciej P., Pablo J. Arauzo, Przemyslaw A. Maziarka, Frederik Ronsse, and Andrea Kruse. 2019. "Pyrolysis Kinetics of Hydrochars Produced from Brewer’s Spent Grains" Catalysts 9, no. 7: 625. https://doi.org/10.3390/catal9070625
APA StyleOlszewski, M. P., Arauzo, P. J., Maziarka, P. A., Ronsse, F., & Kruse, A. (2019). Pyrolysis Kinetics of Hydrochars Produced from Brewer’s Spent Grains. Catalysts, 9(7), 625. https://doi.org/10.3390/catal9070625






