Abstract
The availability of bound residues of polycyclic aromatic hydrocarbons (PAHs), in reference to their parent compounds, can be enhanced by microbial activity and chemical reactions, which pose severe risks for the ecosystems encompassing contaminated soils. Considerable attention has been raised on how to remove these bound residues from PAH-contaminated soils. This paper provides a novel application of Fenton oxidation in the removal of bound residues of model PAHs, such as naphthalene (NAP), acenaphthene (ACP), fluorene (FLU) and anthracene (ANT), from naturally contaminated soils. The citric acid-enhanced Fenton treatment resulted in the degradation of bound PAH residues that followed pseudo-first-order kinetics, with rate constants within 4.22 × 10−2, 1.25 × 10−1 and 2.72 × 10−1 h−1 for NAP, FLU, and ANT, respectively. The reactivity of bound PAH residues showed a correlation with their ionization potential (IP) values. Moreover, the degradation rate of bound PAH residues was significantly correlated with H2O2-Fe2+ ratio (m/m) and H2O2 concentrations. The highest removal efficiencies of bound PAH residues was up to 89.5% with the treatment of chelating agent oxalic acid, which was demonstrated to be superior to other acids, such as citric acid and hydrochloric acid. This study provides valuable insight into the feasibility of citric acid-Fenton and oxalic acid-Fenton treatments in rehabilitating bound PAH residues in contaminated soils.
1. Introduction
Polycyclic aromatic hydrocarbon (PAHs), a class of toxic organic pollutants, are composed of multiple fused aromatic rings in linear, angular or cluster arrangements. In addition, 16 PAHs have been listed by the US Environmental Protection Agency (US-EPA) as priority contaminants [1,2,3]. PAHs have generated extreme concern due to their toxicity, teratogenicity, carcinogenicity, and mutagenesis to humans and the wider environment. They are mainly released by incomplete combustion of fossil fuels or activities in the petrochemical industry, including crude oil refining, coal tar production, and asphalt production [4,5]. Owing to their high hydrophobicity and persistence, soils are the ultimate repository of PAHs with concentration up to hundreds of mg/kg in many countries [6,7]. Therefore, an urgent need globally to reduce the contamination risks to human and environmental health through remediating PAH-contaminated soils.
In aged soils, after exhaustive organic solvent extraction, PAHs are considered as bound residues [8]. Bound PAH residues exist in soils as parent compounds or their metabolites [9,10], which are closely related to soil organic matter (SOM) [11]. The formation of a bound residue is commonly considered as a detoxification process by permanently binding the contaminant to the soil. PAHs firmly attached within the soil matrix and organic matter can promote the formation of bound residues, with this process significantly reducing the PAH bioavailability [12,13]. Indeed, enzymatic activities or condensation reactions could promote the blinding of PAHs with SOM [14]. Certain condensation reactions could be mediated by soil clays and metal oxides [11]. Therefore, the formation of bound PAH residues could act as an environmental solution, which lowers the environmental risk posed by these contaminants [15,16]. However, a recent investigation has indicated that although the extractable fraction of PAHs was removed, the uptake, accumulation, and translocation of PAHs by ryegrass (Lolium multiflorum Lam.) was evident, thus, pointing towards the bioavailability of bound PAH residues in soil [8]. Additionally, the high bioavailability of bound PAH residues could be enhanced by low-molecular-weight organic acids (LMWOAs) and root exudates in contaminated soil [17]. Therefore, how to remove bound PAH residues in contaminated soils is an urgent concern currently.
Advanced oxidation processes (AOPs) are advantageous due to their effectiveness and a shorter duration in the treatment methods of remediating contaminated soils containing hydrophobic organic compounds (HOCs) [18,19]. Fenton oxidation is a classic AOP that generates hydroxyl radicals (•OH), which has a highly reactive and non-specific oxidation. Typically, the major reactions of Fenton are as follows. Hydrogen peroxide decomposes into hydroxyl radicals, in the presence of ferric ions, then, hydroxyl radical oxidizes organic material by hydrogen abstraction or hydroxyl addition [20]:
The applications of Fenton oxidation in PAH-contaminated soils has been shown to have great potential. For example, the oxidative degradation of PAHs by photo-Fenton [21], sono-Fenton [22] and electro-Fenton process [23] has been extensively studied, the considerable degradation in PAH removal was observed in their works. In recent years, the heterogeneous Fenton reaction using natural or synthetic substances as catalysts, and the Fenton reaction of various combinations of technologies including photo-electro-Fenton-like processes, microwave-cavitation-Fenton processes, and microwave-electro-Fenton-like processes, these technologies have become the research focus of Fenton oxidation. However, the formation of bound PAH residues in soils will hinder the transfer of PAHs from the solid matrix to the liquid phase and reduce the oxidation of PAHs [24]. Moreover, most investigations only focused on how to improving Fenton treatment to removal PAHs not bound PAH residues.
In a Fenton oxidation system, chelating agents (CAs) can bind iron and maintain in its dissolvent form. Different kinds of CAs have been explored including ethylenediaminetetraacetic acid (EDTA), citric acid (CA), oxalic acid (OA), humic acid (HA), malic acid (MA) and sodium citrate (SC) [25,26,27,28,29]. However, CAs, like EDTA poses environmental risks because of its persistence and their contribution to improving heavy metal mobility/bioavailability [30]. By contrast, citric acid and oxalic acid are excellent complexing agents that are easy to obtain, cheap and less toxic to the environment. In our previous investigation, LMWOAs (citric acid and oxalic acid) could promote the release of bound PAH residues. Citric acid and oxalic acid have three and two carboxyls and have strong complexing ability to iron. Moreover, these LMWOAs are readily derived from carbohydrates and can be applied for the extensive restoration of contaminated soil. Therefore, citric acid and oxalic acid were selected as CAs in this investigation.
Up to date, a dearth of information still exists on the removal of bound PAH residues in soils. In this study, the degradation kinetics and factors affecting the degradation were investigated. The efficiency of citric acid (CA) as a chelating agent in remediating bound PAH residues was also evaluated and compared with oxalic acid (OA). The findings from this investigation could provide a quick and effective treatment for the removal of bound PAH residues from contaminated soils.
2. Results
2.1. Degradation Kinetics of Bound PAH Residues in Soil by Fenton Oxidation Technique
The citric-Fenton reaction experiments were carried out in contaminated soils containing only bound PAH residues, and the degradation rate and kinetics of bound PAH residues were studied. Figure 1 showed that the Fenton oxidation of bound PAH residues such as naphthalene (NAP), acenaphthene (ACP), fluorene (FLU) and anthracene (ANT) in polluted soils. The degradation of bound PAH residues was represented in terms of Ct/C versus time (Figure 1). After 36 h, the degradation of bound PAH residues reached 83%, 27%, 100%, and 100%, respectively. The concentrations of bound PAH residues in soil with different reaction time were shown in Table S1. The degradation of bound PAH residues (NAP, FLU, and ANT) followed pseudo-first-order kinetics in the 36 h duration. However, the degradation of ACP appeared to change linearly within 36 h. After 36 h, the total degradation rate of bound PAHs residues was up to 89.5%. Hence, the citric-Fenton oxidation can be used for the removal of bound PAH residues in contaminated soils.
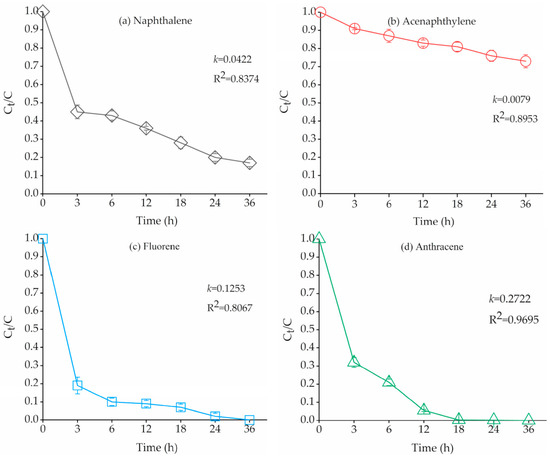
Figure 1.
Degradation of bound PAH residues during oxidation experiment. This degradation is represented in terms of Ct/C, where Ct is the concentration of bound PAH residues at a specific oxidation time, and C is the initial concentration. Experimental conditions were: solid = 3 g, H2O2: Fe2+ = 20:1 (m/m), H2O2 (27.9 × 103 mg/L), pH = 3, Determination coefficient (R2), rate constant (k) values calculated from pseudo-first-order kinetic model. Error bars represent standard errors.
The degradation reaction of bound PAH residues, in the presence of hydroxyl radicals, can be simplified by pseudo-first-order kinetics as shown in Equation (3)
where Ct is the concentration of bound PAH residues at a specific oxidation time. The pseudo-first-order kinetics fitting figure of bound PAH residues degradation was listed in Figure S1. To determine the degradation rate of individual PAHs during the Fenton oxidation period, the pseudo-first-order kinetic reaction rate constant (k, h−1) was used to characterize the reactivity. From Figure 2, the preferred oxidation reaction (ANT > FLU > NAP > ACP) was observed in the citric-Fenton reaction. In bound PAH residues, the kinetic reaction rate constants of ANT were 2.17, 6.45 and 34.46 fold higher than that of FLU, NAP and ACP, respectively.

Figure 2.
The pseudo-first-order rate constant (k) and Ionization potential (IP) of bound PAH residues. The correlation between pseudo-first-order rate constant (k) and Ionization potential (IP) for the 4 abovementioned bound PAH residues.
The correlation between the reactive activity of PAHs and their ionization potential (IP) has been reported [31]. The aromatic ring as a nucleophile in the Fenton oxidation process and •OH as an electrophile. Additionally, reactions of hydroxyl radicals with PAHs, •OH attack ring carbons with their unpaired electrons, formation C-O bonds, the pi bonds of the aromatic systems are broken. Figure 2 showed that the k value of four bound PAH residues (i.e., NAP, ACP, FLU, and ANT) as a function of IP of the molecules during the Fenton oxidation process. The kinetic reaction rate constants are inversely proportional to the ionization potential, and the correlation coefficient (R2) is 0.9998. The PAHs degradation rate appears to be effect by hydrophobicity. High hydrophobicity may strongly impede the degradation of PAHs. A higher log of the octanol-water partition coefficient (logKow) indicates a higher hydrophobicity of PAHs (Table 1). However, there were some exceptions to this: for example, the oxidation of ANT and FLU (logKow: 4.53 and 4.12) was considerably higher than for NAP (logKow: 3.37). ANT and FLU possess higher IP than NAP and ACP. Therefore, the higher degradation rate of ANT and FLU can be explained by their higher reactivity, meaning that the IP of PAHs plays a vital role in the degradation of bound PAH residues.

Table 1.
Laboratory scale studies of Fenton treatments for PAH-contaminated soils.
2.2. Effects of Fenton Reagent Concentration on the Degradation of Bound PAH Residues
In an aqueous system, the degradation rate of Fenton oxidation HOC varies greatly with reaction conditions, the optimum conditions of Fenton oxidation in the slurry were similar to those in an aqueous system. At pH = 3, to observe the effect of Fenton reagent on bound PAH residues degradation, different H2O2: Fe2+ (m/m) and H2O2 concentrations were added. The addition amounts of citric acid, FeSO4, and H2O2 were shown in Tables S2 and S3.
In Figure 3, the degradation rates of four bound PAH residues at different H2O2: Fe2+ (m/m) and H2O2 concentrations were plotted. The degradation rate of four bound PAH residues increased with the increase of H2O2: Fe2+ (m/m). When H2O2: Fe2+ (m/m) was 10:1, the removal of NAP, ACP, FLU and ANT were only 37%, 5.4%, 76% and 68%; while the degradation of bound PAH residues was up to 90%, 27%, 100% and 100%, respectively, when the H2O2: Fe2+ (m/m) was 30:1. Expect for NAP and ANT complete degradation, the removal rate increased with the H2O2: Fe2+ (m/m) greater than 30:1, while the removal rate of ANT no longer increased(Figure 3a). H2O2 plays an important role in the oxidation system as a Fenton reagent because its addition quantity will determine the amount and rate of •OH generation, which directly impacts the removal rate of organic pollutants in soil. Additionally, the Fenton oxidation process can be catalyzed by native iron oxides and certain transition metals present within the soil matrix. Therefore, the contribution of soil as a catalyst in the degradation of bound PAH residues was explored, as shown in Figure S2. Without Fe2+, the contribution of soil as a catalyst for total degradation of bound PAH residues (NAP, ACP, FLU, and ANT) was 10.8%, 11.5%, 12.7% and 14.4% after 36 h, respectively. This likely suggested that soil, as a catalyst, plays a minor role in degradation. From Figure 3b, H2O2 concentration had a strong effect on the removal of bound PAH residues. When the H2O2 concentration was 27.9 × 103 mg/L, the degradation of NAP, ACP, FLU, and FLU reached 73%, 15%, 77% and 52%, while the increased concentration of H2O2 to 124 × 103 mg/L led to the removal of NAP, ACP, FLU and ANT at 100%, 46%, 94%, and 100%, respectively. In addition to NAP and ANT degradation, ACP removal rate no longer increased. The results indicated that higher H2O2: Fe2+ (m/m) can significantly promote the degradation of bound PAH residues and higher H2O2 concentration alone may restrain the removal the bound PAH residues in contaminated soil.
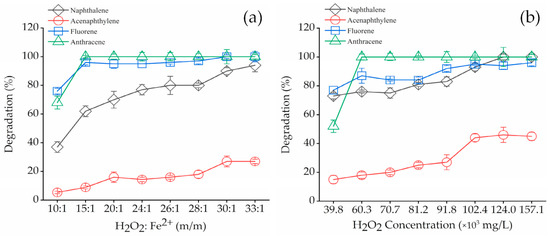
Figure 3.
Effects of Fenton reagent on the degradation of bound PAH residues. (a) Degradation of bound PAH residues on different H2O2: Fe2+ = 30:1 (m/m) (b) Degradation of bound PAH residues on different H2O2 concentration. (a) solid = 3 g, H2O2 (27.9 × 103 mg/L), pH = 3. (b) Solid = 3 g, H2O2: Fe2+ = 30:1 (m/m), pH = 3. Error bars represent standard errors.
2.3. Effect of pH Values on the Degradation of Bound PAH Residues
Fenton oxidation is more efficient in the acidic range, and the hydroxyl radicals have a fairly strong oxidation ability in acidic conditions, both in solution [32] and soil systems. Therefore, slurry pH directly affects the performance of Fenton oxidation degradation of a contaminant in the soil.
The effect of pH (1–13) on bound PAH residues degradation was studied, and results were obtained, as shown in Figure 4. The findings revealed that pH significantly influenced the degradation of bound PAH residues. The maximum degradation of bound PAH residues (NAP, FLU and ANT) was observed at pH 5, but the optimal pH for bound ACP residues degradation was 3. For example, when pH was 3, the removal rates of four bound PAH residues were 87%, 57%, 63%, and 100%, while their degradation rates were 89%, 37%, 65% and 100% at pH 5. In the control treatments, bound PAH residues were hardly degraded at initial different pH values without Fenton reagent, as shown in Figure S3. In the pH ranges of both 3 and 5, the removal rates of four bound PAH residues were all relatively high, while the degradation rates were decreased under extremely acidic, neutral, and alkaline conditions.
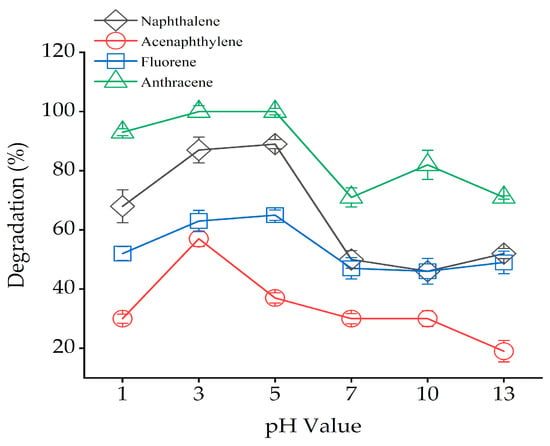
Figure 4.
Effect pH on bound PAH residues oxidation with Fenton reagent. Solid = 3 g, H2O2: Fe2+ = 30:1 (m/m), H2O2 (102.4 × 103 mg/L). Error bars represent standard errors.
2.4. Effects of the Ratio of Soil Mass to Water Volume on the Degradation of Bound PAH Residues
Under the predetermined reaction time, H2O2: Fe2+ (m/m), H2O2 concentration and pH, the influence of adjusting different solid: liquid (g/mL) on the removal of bound PAH residues were determined. The specific experimental conditions were shown in Table 2 (Sets 5). In Figure 5, the degradation of specific bound PAH residues in the soil was highest when the system was just muddy, i.e., only 1.6 mL of deionized water was added. With the increase in water content, the removal of PAHs decreased. When the solid: liquid ratio (g/mL) was 3:5 g/mL, the removal of the four bound PAH residues were 30%, 26%, 85% and 85%. This denoted that a higher solid: liquid ratio (g/mL) could dilute the concentration of H2O2 so that the removal rate of bound PAH residues was reduced. When the solid: liquid (g/mL) was low, hydroxyl radicals only exist in the aqueous phase, PAHs were adsorbed and isolated by soil matrix and could not react with •OH. The above results showed that, regardless of a very high/low solid-liquid ratio, the degradation rate of bound PAH residues would be seriously affected during the reaction.

Table 2.
Organic by-products and their corresponding parent PAHs, after Fenton oxidation of NAP, ACP, FLU, and ANT.
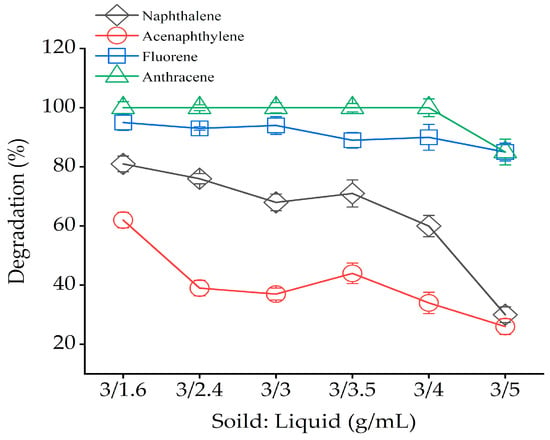
Figure 5.
Effect solid: liquid (g/mL) on bound PAH residues oxidation with Fenton reagent. Solid = 3 g, H2O2: Fe2+ = 30:1 (m/m), H2O2 (102.4 × 103 mg/L), pH = 3. Error bars represent standard errors.
2.5. H2O2 Decomposition and the Degradation Efficiency of Citric-Fenton System
The H2O2 decomposition was studied using optimal reagent dose (H2O2: Fe2+ ) equal to 30:1, the pH was adjusted to 3. The determination of the residual H2O2 concentration was performed at the same reaction time intervals, which were used in the oxidation experiments.
The residual concentration of H2O2 over time was analyzed to determine the decomposition rate. Figure 6a showed the evolution of bound PAH residues oxidation over time in soil, i.e., k = 0.16 h−1 (R2 = 0.78). The k value was lower than that reported in the previous study [33], this pointed to the absence of oxidizable matter (transition matter, soil organic matter, etc.) in soil.
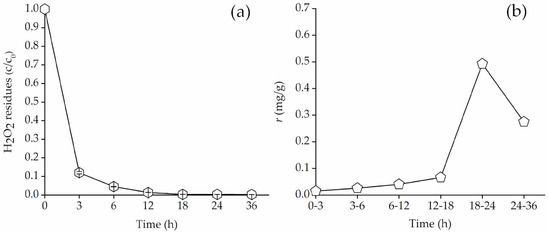
Figure 6.
(a) H2O2 residual (%) in Fenton oxidation systems versus reaction time (h) and (b) the amount of bound PAH residues removed per gram of H2O2 decomposed versus reaction time (h). Solid = 3 g, H2O2: Fe2+ = 30:1 (m/m), H2O2 (102.4 × 103 mg/L), pH = 3. Error bars represent standard errors.
The degradation efficiency (r, mg/g) of the citric-Fenton system at different time phases was calculated as follows:
where Mi is the amount of degradation of bound PAH residues, mi is the amount of H2O2 decomposition. Figure 6b illustrated the amount of bound PAH residues removed per gram of H2O2 decomposed. In the initial phase, the degradation efficiency of Fenton oxidation system was lower. The reaction of ferrous ions with hydrogen peroxide led to a strongly exothermic reaction, which promoted the rapid decomposition of H2O2; •OH oxidize soil organic matter; •OH failed to adequately contact with bound PAH residues. As the reaction progresses, •OH in the aqueous phase migrated deeper into the soil; the degradation efficiency was improved. After 24 h, the amount of bound PAH residues removed was little, and H2O2 was almost exhausted, so the overall degradation efficiency was reduced.
2.6. The Degradation Rate of Bound PAH reSidues under Different Acid Conditions
Inorganic acid is commonly used to create an acidic condition needed for catalytic oxidation. However, inorganic acids will reduce the soil quality after Fenton treatment, and will cause some safety hazards. Potassium oxalate can promote the degradation of PAHs in Fenton oxidation treatment. Therefore, different LMWOAs (citric and oxalic) and hydrochloric were selected to investigate their effects on the degradation of bound PAH residues with increasing K2C2O4. The experimental conditions are provided in Table 4 (Sets 6).
PAHs are rarely degraded through Fenton-like oxidation due to their low bioavailability in soils [34]. In the hydrochloric acid-Fenton system, the addition of K2C2O4 notably enhanced the degradation of bound PAH residues. In Figure 7, the removal rates of four bound PAH residues (NAP, ACP, FLU, and ANT) were 70%, 43%, 50%, and 72%, respectively, under hydrochloric acid condition. Using citric acid, almost 93%, 50%, 76% and 96% of degradation was observed. For the oxalic acid-Fenton system, the degradation of bound PAH residues was least 91%. This result indicated that the degradation of the three acids were significantly different, which can be summarized as follows: oxalic > citric > hydrochloric.
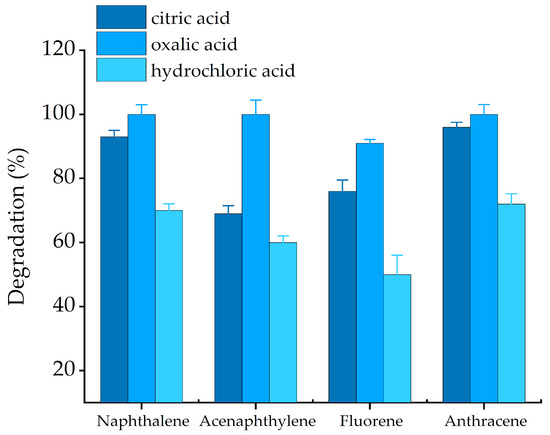
Figure 7.
Effect of chelating agents on bound PAH residues oxidation with Fenton reagent. Solid = 3 g, H2O2: Fe2+ =30:1 (m/m), H2O2 (102.4 × 103 mg/L), pH = 3. Error bars represent standard errors.
3. Discussion
This investigation delved into the removal of bound PAH residues in contaminated soil by Fenton oxidation. Sun et al. [35] reported that the degradation rate of pyrene decreased significantly after soil aged for 30 days, but when the soil was aged further to 60 and 180 days, there was no considerable change in the removal rate. In fact, in the case of low PAH bioavailability, magnetite-activated persulfate oxidation cannot effectively remove PAHs unless the bioavailability of PAHs in the soil was improved by pretreatment [36]. Table 1 summarized the relevant report on the Fenton removal of PAHs. In our study, the degradation rate of bound PAH residues by citric-Fenton and oxalic-Fenton was up to 89.5%. Compared with previous studies, the removal of bund PAH residues was more efficient. This comparison could contribute to the use of higher reagent ratio (H2O2: Fe2+ = 30:1 (m/m)) and lower bound PAH residues content in the soil.
The pseudo-first-order rate constant can directly reflect the degradation rate of contaminant. Virkutyte et al. [39] reported that sono-Fenton oxidation degradation of NAP at various ultrasound irradiations and the pseudo-first-order rate constant was 1.08 × 10−2 to 2.4 × 10−1 h−1, with this result being similar to our study. The measure of the pseudo-first-order rate constant for the degradation of ANT was 1.02 × 10−1 h−1 at H2O2 concentration of 4 M and 33.5 g/kg goethite [40]. They reported the pseudo-first-order rate constant of the same order of magnitude as in this study, but the pseudo-first-order rate constant stated in this study was twice as high as what they reported. This could be described by the small amount of the decomposed H2O2 into •OH catalyzed by Fe2+ dissolved from goethite. Yap et al. [18] studied the Fenton oxidation kinetic of ANT in ethyl lactate/water (EL/W) system, their pseudo-first-order rate constant were 1.47 and 2.72 h−1 for EL/W fc = 0.20 and EL/W fc = 0.33, respectively, with this result being same as ours. Valderrama et al. [41] reported that Fenton oxidation to removal PAHs from aged soil with creosote oil followed pseudo-first-order kinetic, and the pseudo-first-order rate constant was 2.6 × 10−2 to 3.9 × 10−2 h−1 for 3-aromatic ring PAH. The values reported in the literature and obtained in this investigation were slightly dissimilar, which could be caused by the different types of CAs. From the degradation kinetics, there is no significant difference between PAHs and bound PAH residues in the pseudo-first-order rate constant. According to the above results, the key to removing bound PAH residues is to improve its availability in the soil, such as thermal pretreatment or chemical pretreatment.
PAHs have abundant delocalized π electrons, which can strongly interact with electron-deficient by electron-donor-acceptor interactions [42]. Meanwhile, •OH have a very strong ability to gain electrons, thus can actively oxidize and degrade PAHs. The reaction rate of PAHs with •OH can be controlled by the IP of PAHs. IP is an essential characteristic of quantitative estimation of a molecular energy state in the redox process, and can fittingly characterize the reactivity and electron affinity [27]. Forsey et al. [43] studied the correlation between the reaction rate of pyrene, fluoranthene, phenanthrene and naphthalene, and their IP, and the reaction rates increased as IP decreased. Jonsson et al. [31] suggested that lower IP could explain higher degradation rate of PAHs in Fenton oxidation treatment. The abovementioned reported findings corresponded to this study, whereby bound PAH residues with lower IP are more readily degraded.
The relationship between the amount of reactant (H2O2: Fe2+ (m/m)) and the amount of contaminant is usually used to describe the minimum amount of oxidant required for an effective treatment. There are three mechanisms for H2O2 consumption in Fenton treatment, reaction with Fe2+ and Fe3+, reaction with the contaminant and •OH. The degradation rate of PAHs depends on the H2O2: Fe2+ (m/m) in Fenton treatment, regardless of the number of aromatic rings [44]. Higher H2O2: Fe2+ (m/m) can significantly improve the removal of bound PAH residues. Shih et al. [45] reported that the degradation rate of the chemical oxidation of PAHs in sediments increased with increasing amounts of oxidants (H2O2). In another report, when H2O2: Fe2+ (m/m) dropped from 100:1 to 50:1, the removal of PAHs decreased by 10–33%, higher H2O2: Fe2+ (m/m) was more conducive to the degradation of PAHs [46]. At lower reagent ration (H2O2: Fe2+), iron salt will accelerate the decomposition of H2O2, resulting in high temperature, leading to excessive consumption of •OH. Therefore, ferric ion should be kept as low as possible, avoiding the rapid decomposition of H2O2 and the formation of iron precipitation. Within higher H2O2 concentration, a considerable amount of •OH can be consumed by H2O2; this process is called quenching reaction (). In Figure 3, the four bound PAH residues reached maximum degradation at different H2O2: Fe2+ (m/m) and H2O2 concentrations. To minimize the ecological risk of bound PAH residues, the suggested degradation rate of bound PAH residues using citric-Fenton oxidation had the highest impact at H2O2: Fe2+ = 30:1 (m/m) and H2O2 concentration (102.4 × 103 mg/L).
The evolution of H2O2 was carried out, and the degradation rate of the citric-Fenton system was calculated under intensified operating conditions. In particular, the degradation of bound PAH residues still proceeded after the depletion of H2O2 (12 h). The same phenomenon has been reported by Valderrama et al. [41] and Yap et al. [18]. They contributed this to the combined effect of oxidation with reductive transformation by non-hydroxyl reactive species, including perhydroxyl radical (HO2•), superoxide radical anion (O2•−), and hydroperoxide anion (HO2−), or high valence iron cations. These species have less reactivity, more stable, and long lives. Therefore, they were able to degrade bound PAH residues even though H2O2 had exhausted.
Conventional Fenton oxidation had the highest degradation rate for contaminants around a pH of 3. Babuponnusami et al. [32] studied that 100% phenol degradation and 82.59% of COD removal was observed at pH 3 in Fenton oxidation. In this study, the maximum bound PAH residues (NAP, FLU and ANT) degradation was detected at pH 5, but the optimal pH for bound ACP residues degradation was 3. The ferric citrate generated increases the solubility of iron ions in the reaction system so that the activation process continues; this process can explain the pH level above the optimal pH (in conventional Fenton). From Figure 4, the increase in pH from 5 to 13 reduced the degradation of bound PAH residues in the Fenton oxidation treatment. This is due to the acidic condition, a prerequisite in keeping Fe(II) in the reaction system, and high oxidation potential of •OH. When pH is above the optimal pH, iron may precipitate to iron oxyhydroxides, leading to deactivation of the catalyst and further formation of [Fe(OH)4]− [47], the decomposition of H2O2 is hindered, and the adsorption of Fe2+ or Fe3+ increased by soil mineral and organic matter [30]. On the contrary, when pH is below the optimal pH, the consumption of •OH by hydrogen ion (H+) () and lower pH slowdowns the reaction between Fe3+ and H2O2 (). Thus, the addition of citric acid as a chelating agent increases the pH range required for Fenton treatment and reduces the risk to the soil of the lower pH required by conventional Fenton.
LMWOAs are natural products released by plant roots and are abundant in soils, especially near the rhizosphere. Previous studies have reported that the influence of LMWOAs on the desorption and bioavailability of PAHs, the addition of LMWOAs promoted the desorption of PAHs and increased the bioavailability of PAHs in soil [48]. Gao et al. [8] reported that the release of bound PAH residues is dominantly attributed to chemical processes by inhibiting microbial activity in the soil. They proposed that LMWOAs (citric acid and oxalic acid) could significantly enhance the release of bound PAH residues in soils. The addition of carboxylic acid to the Fenton reaction can form ferrous complexes, which could affect the generation of •OH, as well documented [49]. Oxalic acid contains two carboxyl groups, and it can chelate Fe(II) to form [Fe(C2O4)2]2−. The [Fe(C2O4)2]2− can catalyze H2O2 more efficiently to yield •OH than free ferrous ions by approximately 3–4 orders of magnitude [50]. Chen et al. [51] showed that Fe(II)-chelating agent complexes (Fe(II)-oxalic acid) is superior to other complexes (Fe(II)-EDTA, Fe(II)-sodium pyrophosphate and Fe(II)-potassium dihydrogen phosphate) on activating H2O2. Baba et al. [49] reported that the Fe(II)-oxalic acid complex could significantly accelerate the generation of •OH and iron redox than Fe(II)-citric acid complex in the Fenton reaction. When citric acid and oxalic acid were added to the Fenton’s reagent, the ratios of ferric ions forming complexes was above 99%; these complexes could significantly reduce iron precipitation. The above studies can explain PAH degradation by Fenton treatment using oxalic acid is more effective than citric acid. Compared with the inorganic acid system, the organic acid system has a stronger buffer capacity which may reduce the pH range of the system, while increasing ferric ions complexes, to enhance the degradation rate of bound PAH residues (Figure 6).
As mention above, the oxalic-Fenton and citric-Fenton could significantly promote the degradation rate of bound PAH residues. A problem that may raise during Fenton oxidation is the risk of organic by-products. Determination of organic by-products is crucial on the chance some may be of a comparable or greater hazard than the target pollutant. Table 2 gives the organic by-products of 4 PAHs found in previous studies. Lee et al. [52] studied that Fenton oxidation led to the formation of quinone in each PAH. The author found the oxidation positions of quinone corresponded with predicted positions where Frontier electron density was high. The organic by-products of PAHs mainly include oxygenated PAHs (oxy-PAHs). In many cases, oxy-PAHs can be more toxic but less potent in mutagenic effects than their parent compounds. Oxy-PAHs had been shown to induce oxidative stress, endocrine system disruptions, and cytotoxic effects for mammalian cell systems. Then, it is necessary to carry out a more detailed study of the formation mechanism of their organic by-products, as well as their toxicity.
4. Materials and Methods
4.1. Chemicals
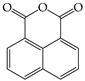
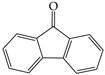
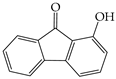
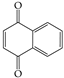
All experimental water was deionized water. Citric acid, oxalic acid, and potassium oxalate were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Methanol and acetonitrile were chromatographic grades. Organic solvents used for extraction were analytical grade, including dichloromethane and acetone. Citric acid (C6H8O7·H2O 10 g was dissolved in 100 mL water), oxalic acid (C2H2O4·2H2O 10 g was dissolved in 100 mL water), ferrous sulfate (FeSO4·7H2O 28 g was dissolved in 100 mL water), hydrogen peroxide (H2O2 30%), potassium oxalate (K2C2O4·H2O 184 g was dissolved in 100 mL), these reagents were easily oxidized and freshly prepared just before use. Sodium hydroxide (NaOH 6.0 mol/L), hydrochloric acid (HCL 6.0 mol/L). The basic physicochemical properties of the primary chemical reagents are listed in Table S4. Some properties of the model PAHs, including Naphthalene (NAP), Acenaphthene (ACP), Fluorene (FLU) and Anthracene (ANT), are listed in Table 3.

Table 3.
Physicochemical properties of the test PAHs.
4.2. Contaminated Soils Containing Bound PAH Residues
Contaminated soils were collected from a petrochemical plant located in Nanjing, China. Soil samples were air-dried for more than a week and ground through a 20-mesh sieve, storing it for later use.
The moisture content of the soil sample was 3.11% (w/w). Soil samples contained bound PAH residues were prepared as follow: pouring treated soil samples (3 g) into 25 mL glass centrifuge tube, a mixed solution of dichloromethane: acetone (1:1, vol/vol) were added (10 mL). The extraction of soils was carried out in an ultrasonic bath for 10 min, then, centrifuging the mixture under 2000 r/min for 20 min. Whereafter the supernatant was decanted, and the soils were reextracted with fresh solution and sonicated. Repeat the above steps at least six times. Filter the last supernatant with anhydrous sodium sulfate and concentrated by rotary evaporation, the volume was added to 2 mL with methanol and filtration with 0.22 microporous membrane. Finally, the supernatant was analyzed by an HPLC to ensure that no PAHs were detected. Soil samples were air-dried and ground through a 20-mesh sieve. After exhaustive solvent extraction Soil samples only contained bound PAH residues were prepared.
The determination of bound PAH residues in soils was referred to existing literature [6]. Soils (3 g) only contained bound PAH residues were placed in 25 mL glass centrifuge tube, adding 10 mL of 2 mol/L NaOH. The mixture was heated at 100 °C for 2 h, then centrifuged under 2000 r/min for 20 min. The supernatant was acidified with 6 mol/L HCl to a pH < 2. Soils liquid-liquid extraction was conducted with 10 mL dichloromethane, and the extraction was repeated at least 3 times. The organic solvent was dehydrated by permeating through anhydrous sodium sulfate, concentrated by rotary evaporation and volume to 2 mL with methanol and filtration with 0.22 microporous membrane. Lastly, solutions containing bound PAH residues were detected by High-Performance Liquid Chromatography (HPLC) analysis. The analysis of PAHs concentration was conducted using an HPLC system fitted on an ultraviolet detector, and a 4.6 × 150 mm alkyl reversed phase C18 column, using acetonitrile/water (Vacetonitrile/Vwater = 65/35) as mobile phase with a flow rate of 1 mL/min. The chromatographic column was prepared at 40 °C. Sample solutions (20 μL) were injected into the HPLC system using an autosampler. The concentration of bound PAH residues (NAP, ACP, FLU, and ANT) were 0.2733, 0.2083, 0.1879, and 0.1308 mg/kg in test soils, respectively.
4.3. Fenton Oxidation Treatment of Contaminated Soil
All the tests conducted in the presented research, together with their correlative experimental conditions were listed in Table 4. The detailed information on these tests is provided in the subsequent section.

Table 4.
Summary experimental conditions for Fenton oxidation of bound PAH residues in soil.
The additional number of experimental reagents in Citric-MF treatment was calculated by the following formula:
The volume of hydrogen peroxide:
A: the volume (mL) of H2O2 (30%); H: concentration (%) of aimed H2O2; Cv: acid (mL);
Wv: acid (mL); Sw: soil sample (g); SM: soil moisture content; Fr: H2O2: Fe2+ (m/m)
The volume of ferrous sulfate:
B: the volume (mL) of ferrous sulfate; A: the volume (mL) of H2O2 (30%); Fr: H2O2: Fe2+ (m/m)
All experimental runs were performed at room temperature and in the absence of light obtained by tin foil paper covering to avoid photolytic degradation. All batch experiments were performed in triplicates. All outcomes were calculated as a mean value of the 3 experiments and the standard deviation of the three replicates. Batch series were conducted by assigning one batch for each time point (3 h, 6 h, 12 h, 18 h, 24 h, and 36 h) to investigate the kinetic for bound PAH residues degradation. Test conditions are shown in Table 2 (Sets 1). 3 g of soil was poured into a 25 mL glass reaction bottle, then, adding 1.6 mL Milli-Q water containing 0.5% NaN3, NaN3 was used to inhibit microbial activity in soils, the volume of water to be added was determined to make the soil slurry. The initial soil pH was 5.87, adjusting pH to 3 with 0.15 mL citric acid. After stirring evenly through the magnetic oscillator, 0.72 mL FeSO4 catalyst and 1.58 mL H2O2 were added slowly in sequence. The bottles were incubated with shaker incubator at 160 rpm, 25 °C. At the start of the experiment (t = 0), bound PAH residues concentration were analyzed as described in Section 4.2. At a special time, the corresponding batch was withdrawn from the series and adjusting pH to stop the reaction. As the above, analyzing the concentration of bound PAH residues.
Experiment on the effect of different factors on Fenton oxidation, the oxidation processes were conducted with different factors (H2O2: Fe2+ (m/m), H2O2 concentration, pH, solid: liquid (g/mL) and acid kind). Test conditions are shown in Table 4 (Sets 2–Sets 6). 3 g of soil was poured into a glass reaction bottle. The volume of ferric sulfate and hydrogen peroxide are listed in Tables S2 and S3, respectively. The test operation was carried out as described above, and the bottles were incubated with shaker incubator under 160 rpm, 25 °C for 24 h. The reaction ended by adjusting the pH. Triplicate samples from each were taken at t = 0, 24 h for analysis of concentrations of bound PAH residues in contaminated soils as described in Section 4.2.
4.4. H2O2 Concentration Assay
The H2O2 concentration was determined by potassium titanium oxalate spectrophotometry [58]. Briefly, H2O2 in the sample was reacted with titanium (IV) ions in acidic conditions to produce stable orange complex-pertitanic acid, and its absorbance was measured at 400 nm.
4.5. Data Analysis
All data were processed using Microsoft Office Excel (2016) and SPSS (v.19.0) software. Figures were generated using Origin (2018 version) software. Each data point represented an average value of three replicate samples in the figure. The standard deviation (SD) of each data point was obtained from three replicates samples and was shown as an error bar.
5. Conclusions
In this manuscript, an initial investigation on exploiting the Fenton oxidation technique to degrade the bound PAH residues in contaminated soil was conducted. The present study demonstrated that citric acid enhanced the Fenton treatment in bound PAH residues (NAP, FLU, and ANT) degradation, which followed pseudo-first-order kinetics (4.22 × 10−2, 1.25 × 10−1 and 2.72 × 10−1 h−1 for NAP, FLU and ANT, respectively). The study showed that adding citric acid can effectively degrade bound PAH residues (NAP, FLU, and ANT) in contaminated soil (up to 89.5%). The degradation rate of the Fenton oxidation process of individually bound PAH residues depends on the physicochemical properties of specific PAHs, including ionization potential. The lower ionization potentials of the tested PAHs were susceptible to Fenton oxidation. Oxalic acid could degrade bound PAH residues more efficiently than citric acid an chelating agent. The results of this investigation may provide valuable insight into the removal of less bioavailable PAHs in contaminated soils and reduce PAH-related risks in ecological niches. Furthermore, future studies should be conducted to establish the nature, toxicity, and migration transformation of the formation of organic by-products. Finally, the development of low-cost, environmentally friendly technologies is required to advance PAH chemical remediation in soils.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/7/619/s1. Figure S1: Pseudo-first-order kinetics fitting figure of bound PAH residues degradation. Figure S2: Effects of soil as a catalyst for the degradation of bound PAH residues without Fe2+. Figure S3: Effect pH on bound PAH residues oxidation without Fenton reagent. Table S1: The concentration of bound PAH residues in soil with different reaction time. Table S2: The amount of reagent adding at different H2O2 and Fe2+ ratios. Table S3: The amount of reagent adding at different H2O2 concentration. Table S4: The basic physicochemical properties of the main chemical reagents.
Author Contributions
The experimental work was designed and supported by L.Q. and W.L.; X.Z. and L.Q. analyzed the data; L.Q. and W.L. contributed the reagents/materials/analysis tools, and all supporting materials; The manuscript was written by all authors. All authors have given approval of the final version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (41771523), and the National Key R & D Program of China (2016YFD0800203).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rubio-Clemente, A.; Torres-Palma, R.A.; Peñuela, G.A. Removal of polycyclic aromatic hydrocarbons in aqueous environment by chemical treatments: A review. Sci. Total. Environ. 2014, 478, 201–225. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.O.; Choi, S.D. Polycyclic aromatic hydrocarbons (PAHs) in soils from a multi-industrial city, South Korea. Sci. Total Environ. 2014, 470, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, K.; Waigi, M.G.; Gao, Y.; Odinga, E.S.; Ling, W.; Liu, J. Application of biochar to soils may result in plant contamination and human cancer risk due to exposure of polycyclic aromatic hydrocarbons. Environ. Int. 2018, 121, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liao, X.; Huling, S.G.; Xue, T.; Liu, Q.; Cao, H.; Lin, Q. The combined effects of surfactant solubilization and chemical oxidation on the removal of polycyclic aromatic hydrocarbon from soil. Sci. Total. Environ. 2019, 647, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Hong, Y.; Liu, J.; Gao, Y.; Ma, Z.; Yang, B.; Ling, W.; Waigi, M.G. A PAH-degrading bacterial community enriched with contaminated agricultural soil and its utility for microbial bioremediation. Environ. Pollut. 2019, 251, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Thavamani, P.; Megharaj, M.; Krishnamurti, G.; McFarland, R.; Naidu, R. Finger printing of mixed contaminants from former manufactured gas plant (MGP) site soils: Implications to bioremediation. Environ. Int. 2011, 37, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.B.; Kang, Y.; Wang, H.S.; Lau, W.; Li, H.; Sun, X.L.; Giesy, J.P.; Chow, K.L.; Wong, M.H. Cancer risk assessments of Hong Kong soils contaminated by polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2013, 261, 770–776. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Zeng, Y.; Zhu, X. Phytoavailability and Rhizospheric Gradient Distribution of Bound-Polycyclic Aromatic Hydrocarbon Residues in Soils. Soil Sci. Soc. Am. J. 2013, 77, 1572–1583. [Google Scholar] [CrossRef]
- Führ, F.; Ophoff, H.; Burauel, P.; Wanner, U.; Haider, K. Modification of the Definition of Bound Residues. Pesticide Bound Residues in Soil; Wiley-VCH: Weinheim, Germany, 1998; pp. 175–176. [Google Scholar]
- Gevao, B.; Mordaunt, C.; Semple, K.T.; Piearce, T.G.; Jones, K.C. Bioavailability of Nonextractable (Bound) Pesticide Residues to Earthworms. Environ. Sci. Technol. 2001, 35, 501–507. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, X.; Zhou, Z.; Zhang, W.; Wang, Y.; Sun, B. Phytoavailability and mechanism of bound PAH residues in filed contaminated soils. Environ. Pollut. 2017, 222, 465–476. [Google Scholar] [CrossRef]
- Poirier, V.; Angers, D.A.; Whalen, J.K. Formation of millimetric-scale aggregates and associated retention of 13C–15N-labelled residues are greater in subsoil than topsoil. Soil Boil. Biochem. 2014, 75, 45–53. [Google Scholar] [CrossRef]
- Boesten, J. Proposal for field-based definition of soil bound pesticide residues. Sci. Total. Environ. 2016, 544, 114–117. [Google Scholar] [CrossRef]
- Richnow, H.H.; Eschenbach, A.; Mahro, B.; Kästner, M.; Annweiler, E.; Seifert, R.; Michaelis, W. Formation of nonextractable soil residues: A stable isotope approach. Environ. Sci. Technol. 1999, 33, 3761–3767. [Google Scholar] [CrossRef]
- Northcott, G.L.; Jones, K.C. Experimental approaches and analytical techniques for determining organic compound bound residues in soil and sediment. Environ. Pollut. 2000, 108, 19–43. [Google Scholar] [CrossRef]
- Barraclough, D.; Kearney, T.; Croxford, A. Bound residues: Environmental solution or future problem? Environ. Pollut. 2005, 133, 85–90. [Google Scholar] [CrossRef]
- Gao, Y.; Yuan, X.; Lin, X.; Sun, B.; Zhao, Z. Low-molecular-weight organic acids enhance the release of bound PAH residues in soils. Soil Tillage Res. 2015, 145, 103–110. [Google Scholar] [CrossRef]
- Yap, C.L.; Gan, S.; Ng, H.K. Ethyl lactate-Fenton treatment of soil highly contaminated with polycyclic aromatic hydrocarbons (PAHs). Chem. Eng. J. 2012, 200, 247–256. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Gitipour, S.; Sorial, G.A.; Ghasemi, S.; Bazyari, M. Treatment technologies for PAH-contaminated sites: A critical review. Environ. Monit. Assess. 2018, 190, 546. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, J.; Song, Y.; Zheng, X.; Qu, L.L.; Wu, Z.; Wu, X. Remediation of Phenanthrene Contaminated Soil by a Solid-State Photo-Fenton Reagent Based on Mesoporous Magnetite/Carboxylate-Rich Carbon Composites and Its Phytotoxicity Evaluation. ACS Sustain. Chem. Eng. 2018, 6, 13262–13275. [Google Scholar] [CrossRef]
- Ke, Y.; Ning, X.A.; Liang, J.; Zou, H.; Sun, J.; Cai, H.; Lin, M.; Li, R.; Zhang, Y. Sludge treatment by integrated ultrasound-Fenton process: Characterization of sludge organic matter and its impact on PAHs removal. J. Hazard. Mater. 2018, 343, 191–199. [Google Scholar] [CrossRef]
- Bocos, E.; Fernández-Costas, C.; Pazos, M.; Sanromán, M. Ángeles Removal of PAHs and pesticides from polluted soils by enhanced electrokinetic-Fenton treatment. Chemosphere 2015, 125, 168–174. [Google Scholar] [CrossRef]
- Lemaire, J.; Laurent, F.; Leyval, C.; Schwartz, C.; Buès, M.; Simonnot, M.-O. PAH oxidation in aged and spiked soils investigated by column experiments. Chemosphere 2013, 91, 406–414. [Google Scholar] [CrossRef]
- Venny; Gan, S.; Ng, H.K. Inorganic chelated modified-Fenton treatment of polycyclic aromatic hydrocarbon (PAH)-contaminated soils. Chem. Eng. J. 2012, 180, 1–8. [Google Scholar] [CrossRef]
- Jorfi, S.; Rezaee, A.; Moheb-Ali, G.A.; Jaafarzadeh, N.A. Pyrene removal from contaminated soils by modified Fenton oxidation using iron nano particles. J. Environ. Heal. Sci. Eng. 2013, 11, 17. [Google Scholar] [CrossRef]
- Gryzenia, J.; Cassidy, D.; Hampton, D. Production and accumulation of surfactants during the chemical oxidation of PAH in soil. Chemosphere 2009, 77, 540–545. [Google Scholar] [CrossRef]
- Venny; Gan, S.; Ng, H.K. Modified Fenton oxidation of polycyclic aromatic hydrocarbon (PAH)-contaminated soils and the potential of bioremediation as post-treatment. Sci. Total. Environ. 2012, 419, 240–249. [Google Scholar] [CrossRef]
- Li, Y.C.; Bachas, L.G.; Bhattacharyya, D. Selected chloro-organic detoxifications by polychelate (poly (acrylic acid)) and citrate-based Fenton reaction at neutral pH environment. Ind. Eng. Chem. Res. 2007, 46, 7984–7992. [Google Scholar] [CrossRef]
- Usman, M.; Hanna, K.; Haderlein, S. Fenton oxidation to remediate PAHs in contaminated soils: A critical review of major limitations and counter-strategies. Sci. Total. Environ. 2016, 569, 179–190. [Google Scholar] [CrossRef]
- Jönsson, S.; Persson, Y.; Frankki, S.; Van Bavel, B.; Lundstedt, S.; Haglund, P.; Tysklind, M. Degradation of polycyclic aromatic hydrocarbons (PAHs) in contaminated soils by Fenton’s reagent: A multivariate evaluation of the importance of soil characteristics and PAH properties. J. Hazard. Mater. 2007, 149, 86–96. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. Advanced oxidation of phenol: A comparison between Fenton, electro-Fenton, sono-electro-Fenton and photo-electro-Fenton processes. Chem. Eng. J. 2012, 183, 1–9. [Google Scholar] [CrossRef]
- Gan, S.; Yap, C.L.; Ng, H.K.; Venny. Investigation of the impacts of ethyl lactate-based Fenton treatment on soil quality for polycyclic aromatic hydrocarbons (PAHs)-contaminated soils. J. Hazard. Mater. 2013, 262, 691–700. [Google Scholar] [CrossRef]
- Usman, M.; Chaudhary, A.; Biache, C.; Faure, P.; Hanna, K. Effect of thermal pre-treatment on the availability of PAHs for successive chemical oxidation in contaminated soils. Environ. Sci. Pollut. Res. 2016, 23, 1371–1380. [Google Scholar] [CrossRef]
- Sun, H.W.; Yan, Q.S. Influence of pyrene combination state in soils on its treatment efficiency by Fenton oxidation. J. Environ. Manag. 2008, 88, 556–563. [Google Scholar] [CrossRef]
- Usman, M.; Faure, P.; Ruby, C.; Hanna, K. Remediation of PAH-contaminated soils by magnetite catalyzed Fenton-like oxidation. Appl. Catal. B Environ. 2012, 117, 10–17. [Google Scholar] [CrossRef]
- Souza e Silva, P.T.D.; Da Silva, V.L.; de Barros Neto, B.; Simonnot, M.O. Phenanthrene and pyrene oxidation in contaminated soils using Fenton’s reagent. J. Hazard. Mater. 2009, 161, 967–973. [Google Scholar] [CrossRef]
- Lin, M.; Ning, X.A.; An, T.; Zhang, J.; Chen, C.; Ke, Y.; Wang, Y.; Zhang, Y.; Sun, J.; Liu, J. Degradation of polycyclic aromatic hydrocarbons (PAHs) in textile dyeing sludge with ultrasound and Fenton processes: Effect of system parameters and synergistic effect study. J. Hazard. Mater. 2016, 307, 7–16. [Google Scholar] [CrossRef]
- Virkutyte, J.; Vičkačkaite, V.; Padarauskas, A. Sono-oxidation of soils: Degradation of naphthalene by sono-Fenton-like process. J. Soils Sediments 2010, 10, 526–536. [Google Scholar] [CrossRef]
- Kanel, S.R.; Neppolian, B.; Jung, H.; Choi, H. Comparative removal of polycyclic aromatic hydrocarbons using iron oxide and hydrogen peroxide in soil slurries. Environ. Eng. Sci. 2004, 21, 741–751. [Google Scholar] [CrossRef]
- Valderrama, C.; Alessandri, R.; Aunola, T.; Cortina, J.L.; Gamisans, X.; Tuhkanen, T. Oxidation by Fenton’s reagent combined with biological treatment applied to a creosote-comtaminated soil. J. Hazard. Mater. 2009, 166, 594–602. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, J.; Li, L.; Li, X.; Wang, C. Transformation of polycyclic aromatic hydrocarbons (PAHs) on Fe(III)-modified clay minerals: Role of molecular chemistry and clay surface properties. Appl. Catal. B Environ. 2014, 154, 238–245. [Google Scholar] [CrossRef]
- Forsey, S.P.; Thomson, N.R.; Barker, J.F. Oxidation kinetics of polycyclic aromatic hydrocarbons by permanganate. Chemosphere 2010, 79, 628–636. [Google Scholar] [CrossRef]
- Laurent, F.; Cébron, A.; Schwartz, C.; Leyval, C. Oxidation of a PAH polluted soil using modified Fenton reaction in unsaturated condition affects biological and physico-chemical properties. Chemosphere 2012, 86, 659–664. [Google Scholar] [CrossRef]
- Shih, Y.J.; Binh, N.T.; Chen, C.W.; Chen, C.F.; Dong, C.D. Treatability assessment of polycyclic aromatic hydrocarbons contaminated marine sediments using permanganate, persulfate and Fenton oxidation processes. Chemosphere 2016, 150, 294–303. [Google Scholar] [CrossRef]
- Ferrarese, E.; Andreottola, G.; Oprea, I.A. Remediation of PAH-contaminated sediments by chemical oxidation. J. Hazard. Mater. 2008, 152, 128–139. [Google Scholar] [CrossRef]
- Abo-Farha, S.A. Comparative study of oxidation of some azo dyes by different advanced oxidation processes: Fenton, Fenton-like, photo-Fenton and photo-Fenton-like. J. Am. Sci. 2010, 6, 128–142. [Google Scholar] [CrossRef]
- Ling, W.; Ren, L.; Gao, Y.; Zhu, X.; Sun, B. Impact of low-molecular-weight organic acids on the availability of phenanthrene and pyrene in soil. Soil Boil. Biochem. 2009, 41, 2187–2195. [Google Scholar] [CrossRef]
- Baba, Y.; Yatagai, T.; Harada, T.; Kawase, Y. Hydroxyl radical generation in the photo-Fenton process: Effects of carboxylic acids on iron redox cycling. Chem. Eng. J. 2015, 277, 229–241. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, X.; Mao, J.; Zhang, Y.; Lim, T.T. Rapid degradation of sulfonamides in a novel heterogeneous sonophotochemical magnetite-catalyzed Fenton-like (US/UV/Fe3O4/oxalate) system. Appl. Catal. B Environ. 2014, 160, 325–334. [Google Scholar] [CrossRef]
- Chen, C.W.; Binh, N.T.; Hung, C.M.; Chen, C.F.; Dong, C.D. Removal of polycyclic aromatic hydrocarbons from sediments using chemical oxidation processes. J. Adv. Oxid. Technol. 2015, 18, 15–22. [Google Scholar] [CrossRef]
- Lee, B.D.; Iso, M.; Hosomi, M. Prediction of Fenton oxidation positions in polycyclic aromatic hydrocarbons by Frontier electron density. Chemosphere 2001, 42, 431–435. [Google Scholar] [CrossRef]
- Lundstedt, S.; Persson, Y.; Öberg, L. Transformation of PAHs during ethanol-Fenton treatment of an aged gasworks’ soil. Chemosphere 2006, 65, 1288–1294. [Google Scholar] [CrossRef]
- Yu, B.; Jin, X.; Kuang, Y.; Megharaj, M.; Naidu, R.; Chen, Z. An integrated biodegradation and nano-oxidation used for the remediation of naphthalene from aqueous solution. Chemosphere 2015, 141, 205–211. [Google Scholar] [CrossRef]
- Dabestani, R.; Ivanov, I.N. A Compilation of Physical, Spectroscopic and Photophysical Properties of Polycyclic Aromatic Hydrocarbons. Photochem. Photobiol. 1999, 70, 10–34. [Google Scholar] [CrossRef]
- De Lima Ribeiro, F.A.; Ferreira, M.M.C. QSPR models of boiling point, octanol–water partition coefficient and retention time index of polycyclic aromatic hydrocarbons. J. Mol. Struct. 2003, 663, 109–126. [Google Scholar] [CrossRef]
- Crunkilton, R.L.; DeVita, W.M. Determination of aqueous concentrations of polycyclic aromatic hydrocarbons (PAHs) in an urban stream. Chemosphere 1997, 35, 1447–1463. [Google Scholar] [CrossRef]
- Sellers, R.M. Spectrophotometric determination of hydrogen peroxide using potassium titanium (IV) oxalate. Analyst 1980, 105, 950–954. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).