Simultaneous Removal of Estrogens and Antibiotics from Livestock Manure Using Fenton Oxidation Technique
Abstract
:1. Introduction
2. Results and Discussion
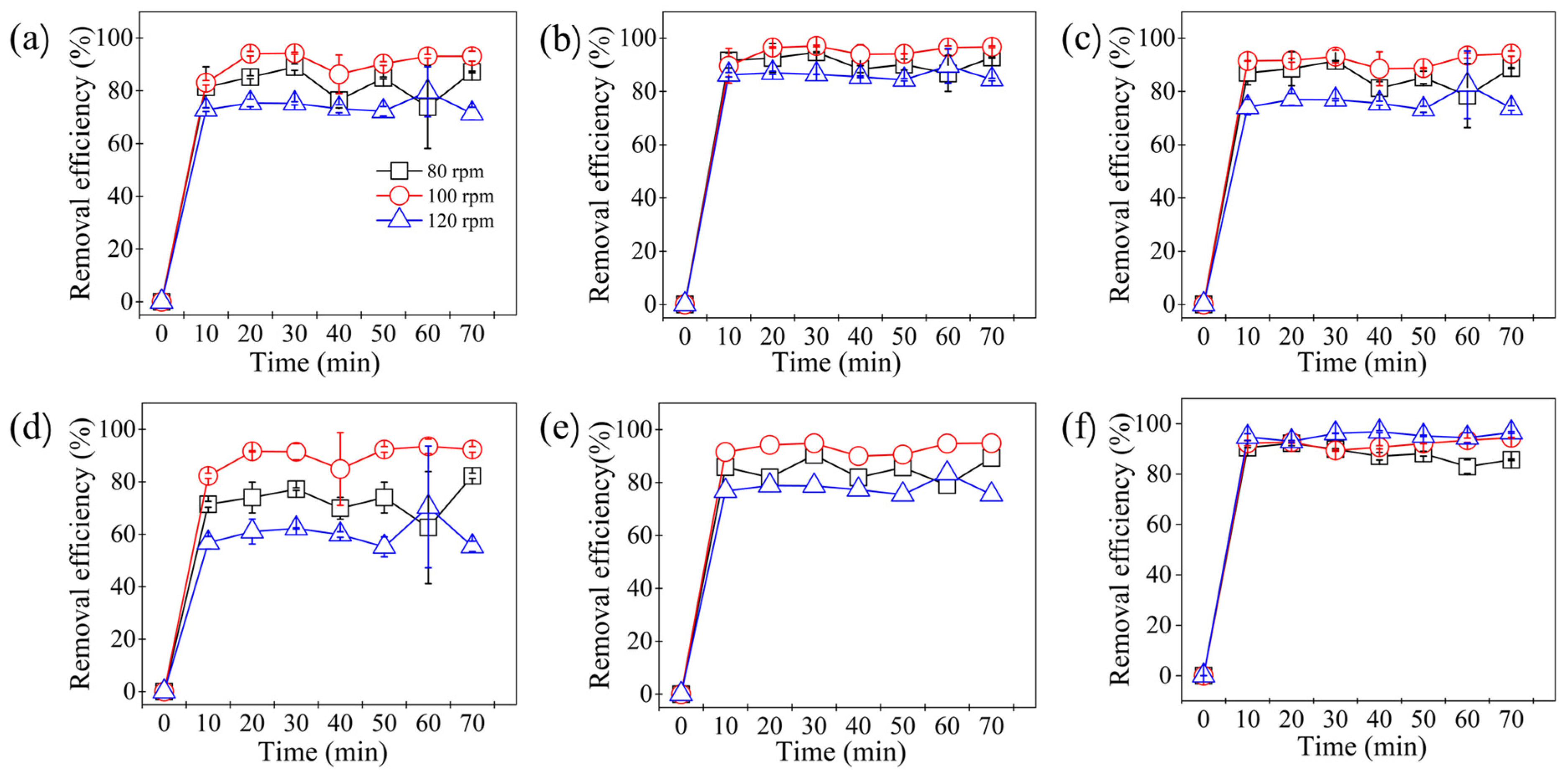
2.1. Effect of Stirring Speed on the Removal of Estrogens and Antibiotic
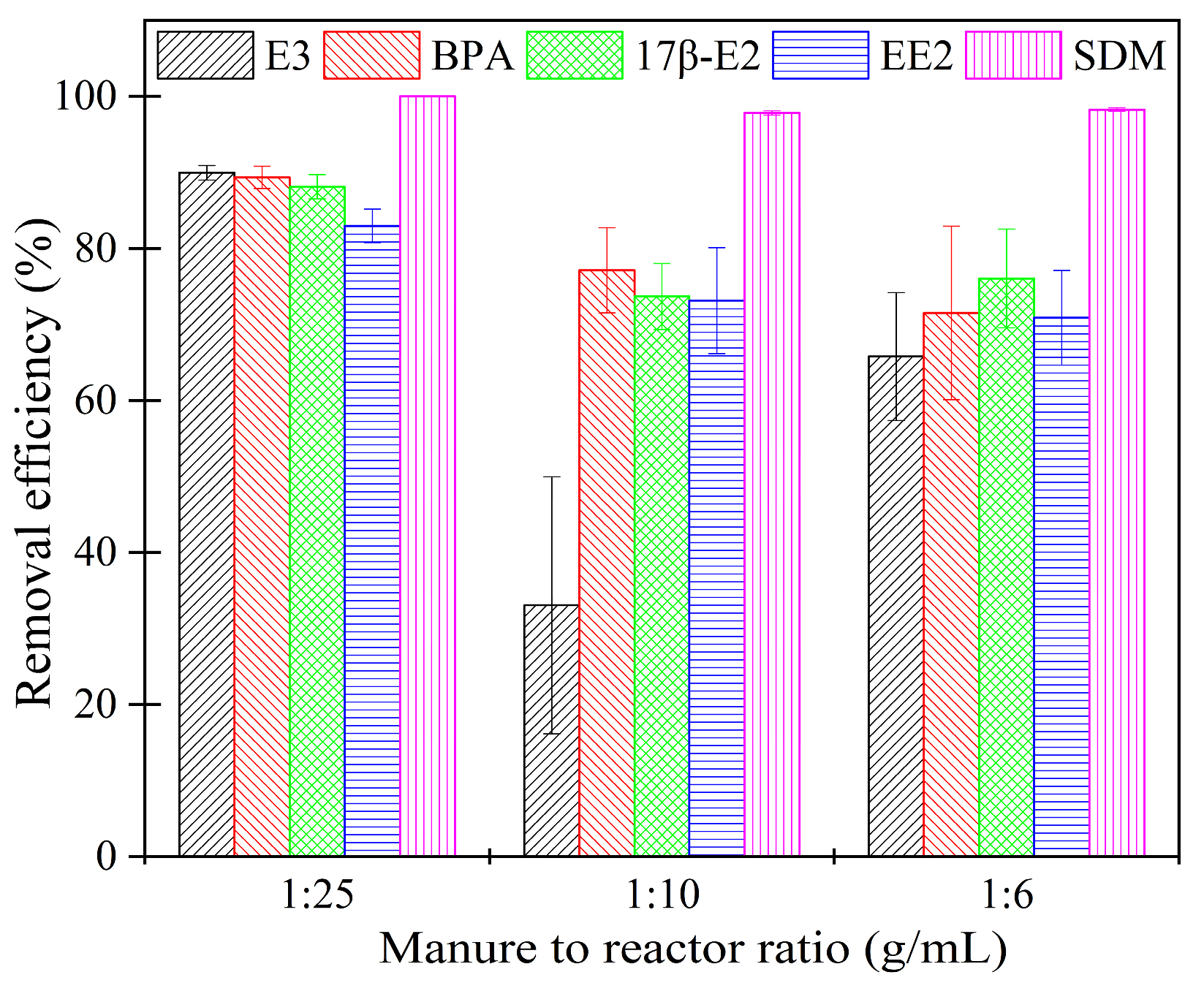
2.2. Effect of the Manure/Reactor Ratio on the Removal of Estrogens and Antibiotic
2.3. Effect of H2O2 Dosification on Estrogens and Antibiotic Removal
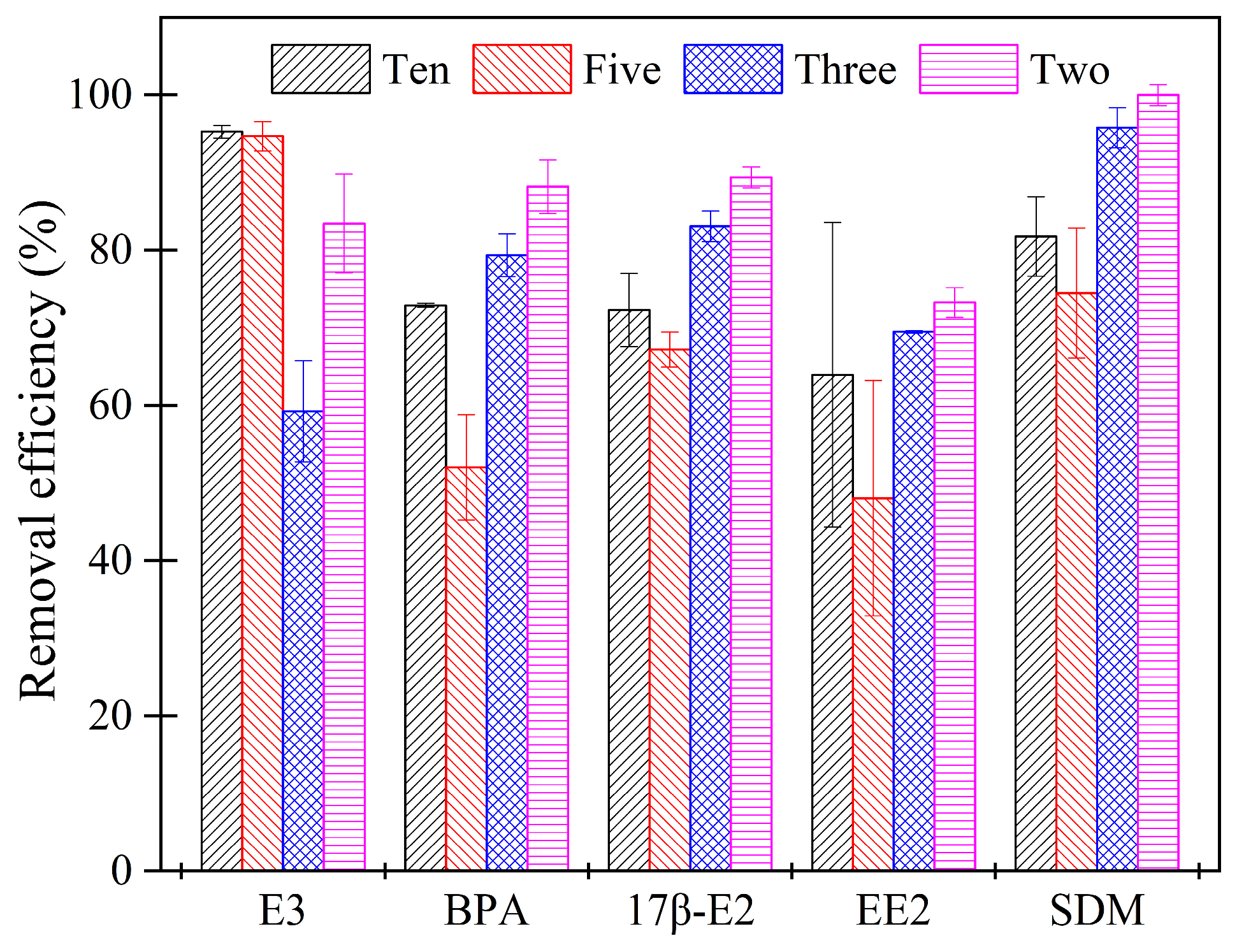
2.4. Effect of Different Types of Manure on the Removal of Tested Estrogens and SDM under Optimum Reaction Conditions
3. Materials and Methods
3.1. Chemicals
3.2. Manure
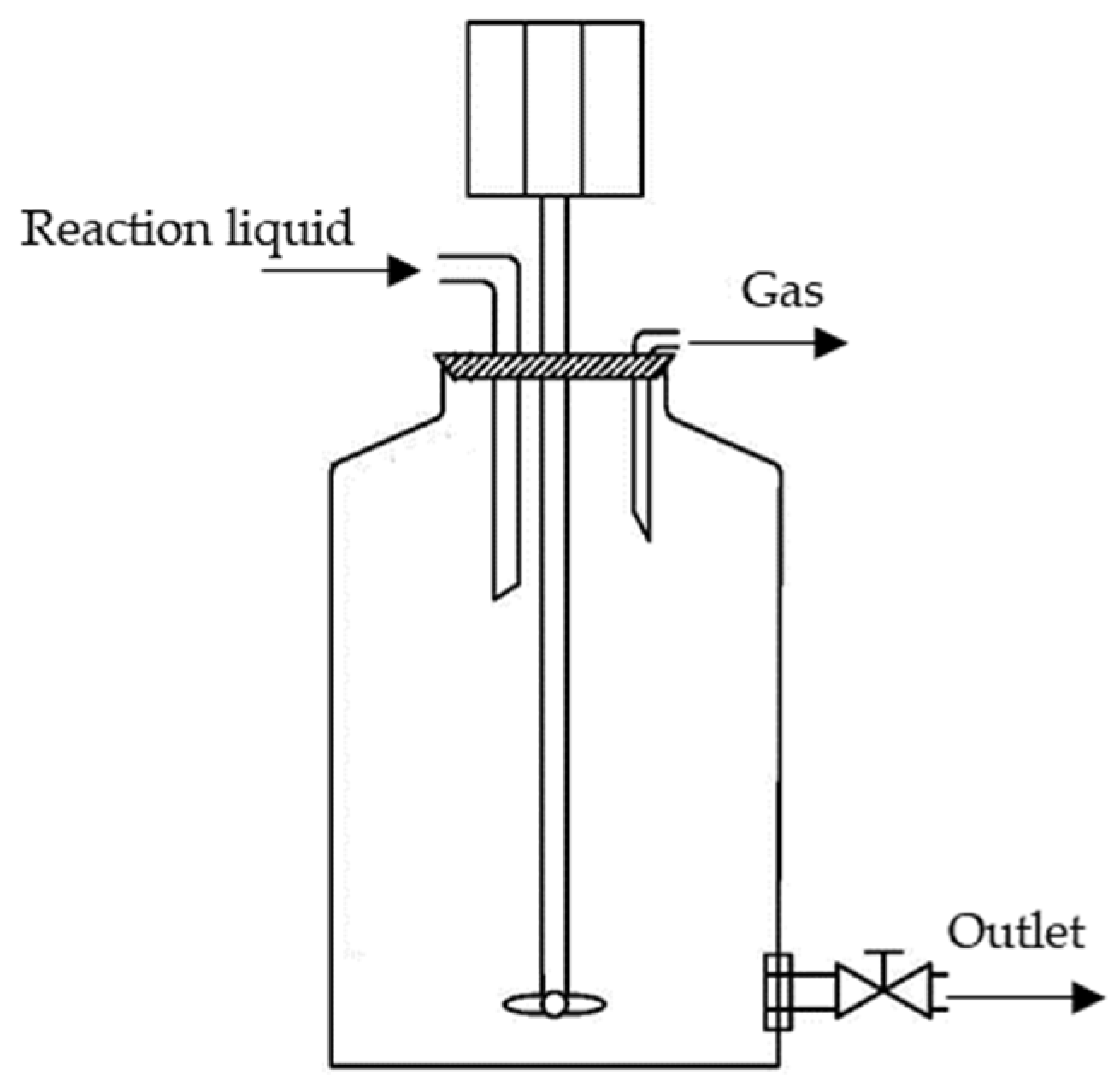
3.3. Fenton Oxidation of Manure
3.4. Estrogens and SDM Analysis
3.4.1. Estrogens Analysis
3.4.2. SDM Analysis
3.5. Other Chemical Analyses
3.6. Data Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramaswamy, J.; Prasher, S.O.; Patel, R.M.; Hussain, S.A.; Barrington, S.F. The effect of composting on the degradation of a veterinary pharmaceutical. Bioresour. Technol. 2009, 101, 2294–2299. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Sengeløv, G.; Tjørnelund, J. Toxicity of tetracyclines and tetracycline degradation products to environmentally relevant bacteria, including selected tetracycline-resistant bacteria. Arch. Environ. Contam. Toxicol. 2002, 42, 263–271. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Literature Review of Contaminants in Livestock and Poultry Manure and Implications for Water Quality; EPA 820-R-13-002; USEPA, Office of Water: Washington, DC, USA, 2013; pp. 1–137.
- Holm-Nielsen, J.B.; Seadi, T.A.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilisation. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Zhang, T.; Fang, H.H.P. Antibiotic resistance genes in water environment. Appl. Microbiol. Biotechnol. 2009, 82, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Zhou, X.; Xu, D.; Xiang, Y.; Ling, W.; Chen, M. Contamination and risk assessment of estrogens in livestock manure: A case study in Jiangsu province, China. Int. J. Environ. Res. Public Health 2018, 15, 125. [Google Scholar] [CrossRef]
- Martinez-Carballo, E.; Gonzalez-Barreiro, C.; Scharf, S.; Gans, O. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria. Environ. Pollut. 2007, 148, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Wu, H.; Wang, J.; Zhang, H.; Zhang, Z.; Zhang, Y.; Lin, H.; Ma, J. Occurrence of trace elements and antibiotics in manure-based fertilizers from the Zhejiang Province of China. Sci. Total Environ. 2016, 559, 174–181. [Google Scholar] [CrossRef]
- Arikan, O.A.; Sikora, L.J.; Mulbry, W.; Khan, S.U.; Rice, C.; Foster, G.D. The fate and effect of oxytetracycline during the anaerobic digestion of manure from therapeutically treated calves. Process Biochem. 2006, 41, 1637–1643. [Google Scholar] [CrossRef]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Degradation of veterinary antibiotics and hormone during broiler manure composting. Bioresour. Technol. 2013, 131, 476–484. [Google Scholar] [CrossRef]
- Derby, N.E.; Hakk, H.; Casey, F.X.M.; DeSutter, T.M. Effects of composting swine manure on nutrients and estrogens. Soil Sci. 2011, 176, 91–98. [Google Scholar] [CrossRef]
- Zhang, M.; He, L.Y.; Liu, Y.S.; Zhao, J.L.; Liu, W.R.; Zhang, J.N.; Chen, J.; He, L.K.; Zhang, Q.Q.; Ying, G.G. Fate of veterinary antibiotics during animal manure composting. Sci. Total Environ. 2019, 650, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shi, J.; Liu, X.; Zhan, X.; Chen, Q. Occurrence and removal of free estrogens, conjugated estrogens, and bisphenol A in manure treatment facilities in East China. Water Res. 2014, 58, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Soares, O.S.G.P.; Rodrigues, C.S.; Madeira, L.M.; Pereira, M.F.R. Heterogeneous Fenton-Like Degradation of p-Nitrophenol over Tailored Carbon-Based Materials. Catalysts 2019, 9, 258. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Highly effective degradation of selected groups of organic compounds by cavitation based AOPs under basic pH conditions. Ultrason. Sonochem. 2018, 45, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Garoma, T.; Umamaheshwar, S.K.; Mumper, A. Removal of sulfadiazine, sulfamethizole, sulfamethoxazole, and sulfathiazole from aqueous solution by ozonation. Chemosphere 2010, 79, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Bhuta, H. Advanced treatment technology and strategy for water and wastewater management. In Industrial Wastewater Treatment, Recycling and Reuse, 1st ed.; Ranade, V.V., Bhandari, V.M., Eds.; Butterworth-Heinemann: Waltham, UK, 2014; Chapter 4; pp. 193–213. [Google Scholar]
- Ramirez, J.H.; Maldonado-Hódar, F.J.; Pérez-Cadenas, A.F.; Moreno-Castilla, C.; Costa, C.A.; Madeira, L.M. Azo-dye Orange II degradation by heterogeneous Fenton-like reaction using carbon-Fe catalysts. Appl. Catal. B Environ. 2007, 75, 312–323. [Google Scholar] [CrossRef]
- Mendez-Arriaga, F.; Esplugas, S.; Gimenez, J. Degradation of the emerging contaminant ibuprofen in water by photo-Fenton. Water Res. 2010, 44, 589–595. [Google Scholar] [CrossRef]
- Sun, M.; Xu, D.; Ji, Y.; Liu, J.; Ling, W.; Li, S.; Chen, M. Using Fenton oxidation to simultaneously remove different estrogens from cow manure. Int. J. Environ. Res. Public Health. 2016, 13, 917. [Google Scholar] [CrossRef]
- Uslu, M.O.; Balcioglu, I.A. Simultaneous removal of oxytetracycline and sulfamethazine antibacterials from animal waste by chemical oxidation processes. J. Agric. Food Chem. 2009, 57, 11284–11291. [Google Scholar] [CrossRef]
- Wang, J.; Xia, K.; Waigi, M.G.; Gao, Y.; Odinga, E.S.; Ling, W.; Liu, J. Application of biochar to soils may result in plant contamination and human cancer risk due to exposure of polycyclic aromatic hydrocarbons. Environ. Int. 2018, 121, 169–177. [Google Scholar] [CrossRef]
- Ben, W.; Qiang, Z.; Pan, X.; Chen, M. Removal of veterinary antibiotics from sequencing batch reactor (SBR) pretreated swine wastewater by Fenton’s reagent. Water Res. 2009, 43, 4392–4402. [Google Scholar] [CrossRef]
- Bouasla, C.; Samar, M.E.H.; Ismail, F. Degradation of methyl violet 6B dye by the Fenton process. Desalination 2010, 254, 35–41. [Google Scholar] [CrossRef]
- Cuiping, B.; Wenqi, G.; Dexin, F.; Mo, X.; Qi, Z.; Shaohua, C.; Zhongxue, G.; Yanshui, Z. Natural graphite tailings as heterogeneous Fenton catalyst for the decolorization of rhodamine B. Chem. Eng. J. 2012, 197, 306–313. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, A. Removal of steroid estrogens from waste activated sludge using Fenton oxidation: Influencing factors and degradation intermediates. Chemosphere 2014, 105, 24–30. [Google Scholar] [CrossRef]
- Duarte, F.; Morais, V.; Maldonado-Hódar, F.J.; Madeira, L.M. Treatment of textile effluents by the heterogeneous Fenton process in a continuous packed-bed reactor using Fe/activated carbon as catalyst. Chem. Eng. J. 2013, 232, 34–41. [Google Scholar] [CrossRef]
- Sun, J.H.; Sun, S.P.; Wang, G.L.; Qiao, L.P. Degradation of azo dye Amido black 10B in aqueous solution by Fenton oxidation process. Dyes Pigments 2007, 74, 647–652. [Google Scholar] [CrossRef]
- Ghiselli, G.; Jardim, W.F.; Litter, M.I.; Mansilla, H.D. Destruction of EDTA using Fenton and photo-Fenton-like reactions under UV-A irradiation. J. Photochem. Photobiol. A Chem. 2004, 167, 59–67. [Google Scholar] [CrossRef]
- Primo, O.; Rivero, M.J.; Ortiz, I. Photo-Fenton process as an efficient alternative to the treatment of landfill leachates. J. Hazard. Mater. 2008, 153, 834–842. [Google Scholar] [CrossRef]
- Zhang, H.; Choi, H.J.; Huang, C.P. Optimization of Fenton process for the treatment of landfill leachate. J. Hazard. Mater. 2005, 125, 166–174. [Google Scholar] [CrossRef]
- Heredia, J.B.D.; Domínguez, J.R.; López, R. Advanced oxidation of cork-processing wastewater using Fenton’s reagent: Kinetics and stoichiometry. J. Chem. Technol. Biotechnol. 2004, 79, 407–412. [Google Scholar] [CrossRef]
- Turan-Ertas, T.; Gurol, M.D. Oxidation of diethylene glycol with ozone and modified Fenton processes. Chemosphere 2002, 47, 293–301. [Google Scholar] [CrossRef]
- Lopez, A.; Pagano, M.; Volpe, A.; Pinto, A.C.D. Fenton’s pre-treatment of mature landfill leachate. Chemosphere 2004, 54, 1005–1010. [Google Scholar] [CrossRef]
- Fei, H.Y.; Chang, Z.Z.; Wang, S.M.; Huang, H.Y.; Chen, X.; Zhu, H. Characterization of moisture in three livestock manures. J. Agro Environ. Sci. 2006, 25, 599–603. (In Chinese) [Google Scholar]
- Nieto, L.M.; Hodaifa, G.; Rodríguez, S.; Giménez, J.A.; Ochando, J. Degradation of organic matter in olive-oil mill wastewater through homogeneous Fenton-like reaction. Chem. Eng. J. 2011, 173, 503–510. [Google Scholar] [CrossRef]
- Sillanpaa, M.; Ncibi, M.C.; Matilainen, A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: A comprehensive review. J. Environ. Manag. 2018, 208, 56–76. [Google Scholar] [CrossRef]
- Chi, G.; Chen, X.; Shi, Y.; Zheng, T. Forms and profile distribution of soil Fe in the Sanjiang Plain of Northeast China as affected by land uses. J. Soils Sediments 2010, 10, 787–795. [Google Scholar] [CrossRef]
- Zhao, L.; Dong, Y.H.; Wang, H. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010, 408, 1069–1075. [Google Scholar] [CrossRef]
- Agriculture Department of People Republic China. Organic Fertilizer; NY 525-2012; China Agriculture Press: Beijing, China, 2012. (In Chinese)
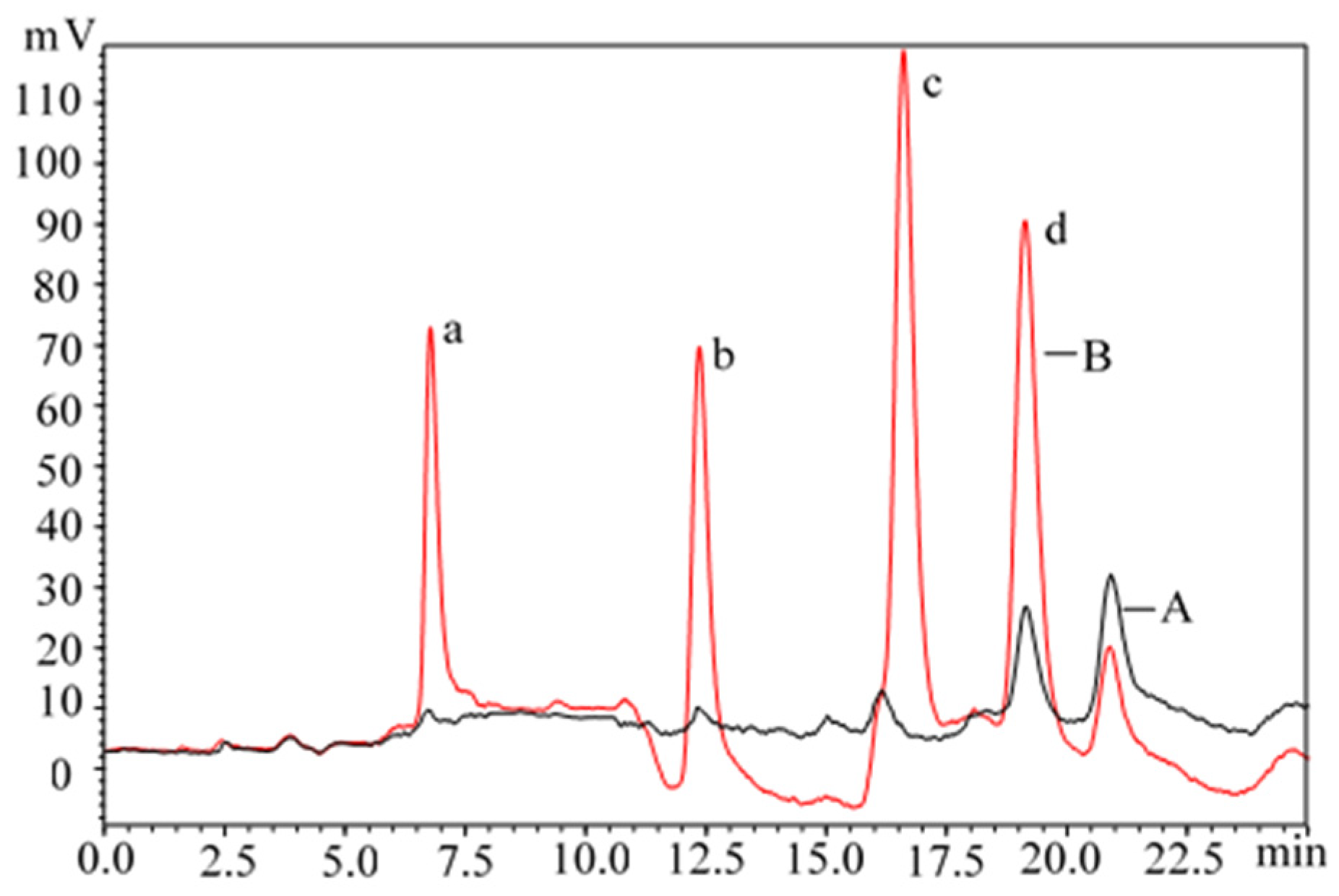
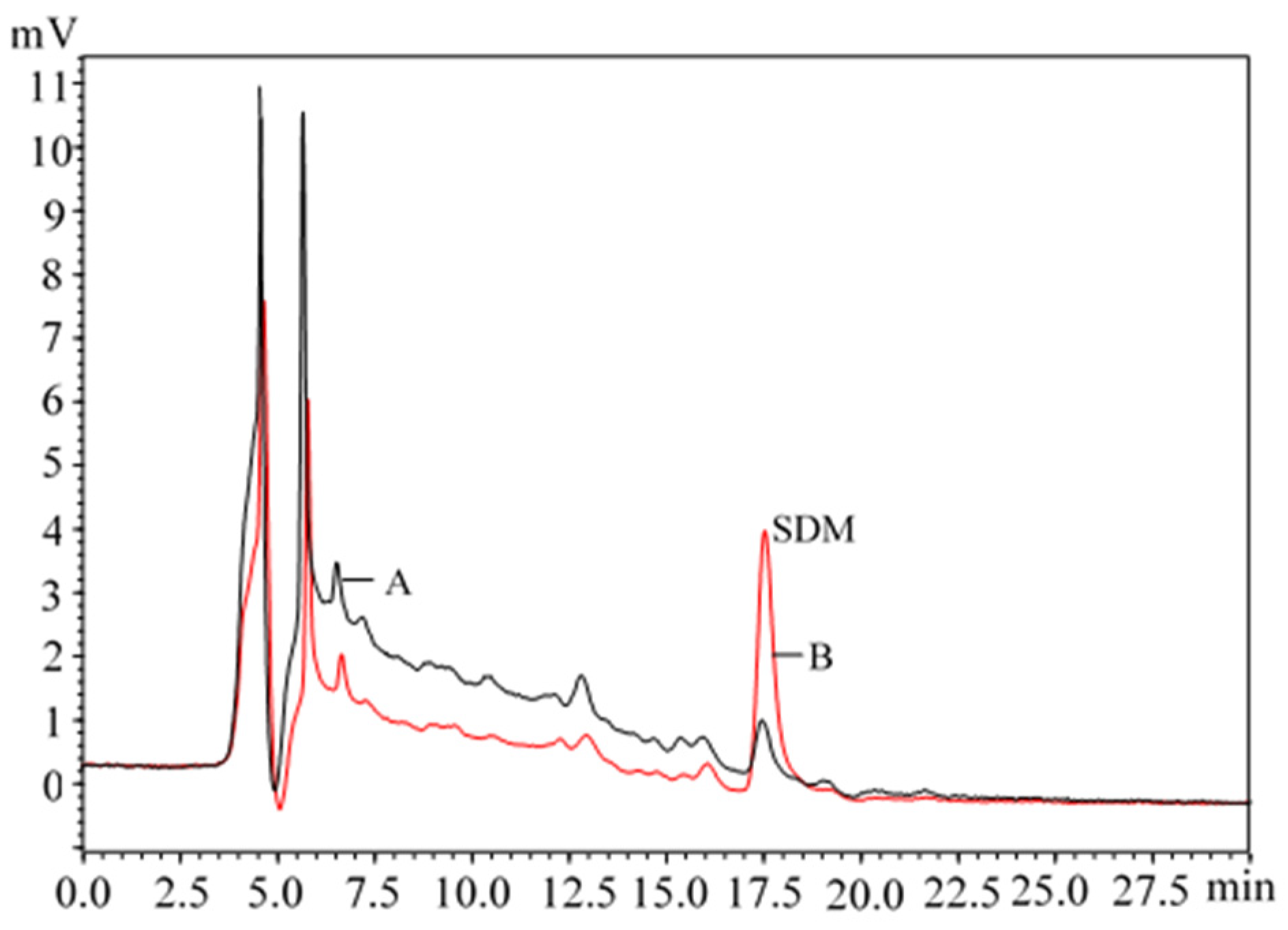
| Sample | The Initial Concentration of Estrogens and SDM in Livestock Feces (μg/kg) | |||||
|---|---|---|---|---|---|---|
| E3 | BPA | 17β-E2 | EE2 | ∑E | SDM | |
| D1 | 6765 ± 848 1 | 17 ± 0 | 23 ± 22 | 188 ± 42 | 6982 ± 897 | 5.6 ± 0.92 |
| D2 | 1115 ± 155 | 0.81 ± 0.77 | ND 2 | 112 ± 0 | 1153 ± 183 | 1.61 ± 0.02 |
| S1 | 592 ± 81 | 137 ± 17 | 106 ± 21 | ND | 568 ± 78 | 7.58 ± 0.86 |
| S2 | 844 ± 165 | ND | 152 ± 76 | ND | 999 ± 151 | 40.89 ± 9.82 |
| C1 | 53 ± 62 | ND | 1.84 ± 2.6 | ND | 55 ± 61 | 2.8 ± 0.22 |
| C2 | 40 ± 22 | 4 ± 0 | 19 ± 14 | ND | 60 ± 26 | 1.22 ± 0.11 |
| Sample | The Residual Concentration of Estrogens and SDM in Livestock Feces (μg/kg) | |||||
|---|---|---|---|---|---|---|
| E3 | BPA | 17β-E2 | EE2 | ∑E | SDM | |
| D1 | 112 ± 121 1 | ND 2 | 0.05 ± 0.07 | 97 ± 0 | 145 ± 98 | 1.48 ± 0.11 |
| D2 | 50 ± 36 | ND | ND | ND | 50 ± 36 | ND |
| S1 | 175 ± 24 | 34 ± 8 | 37 ± 0 | ND | 234 ± 33 | ND |
| S2 | 58 ± 35 | ND | 21 ± 0 | ND | 65 ± 27 | 10.7 ± 1.15 |
| C1 | 36 ± 23 | ND | ND | ND | 36 ± 23 | ND |
| C2 | 4 ± 3 | 13 ± 0 | 2.72 ± 3.57 | ND | 11 ± 8 | ND |
| Sample | The Removal Efficiency of Estrogens and SDM in Livestock Feces (%) | |||||
|---|---|---|---|---|---|---|
| E3 | BPA | 17β-E2 | EE2 | ∑E | SDM | |
| D1 | 97.5 | 100 | 99.8 | 82.7 | 97.9 | 73.5 |
| D2 | 95.5 | - | - | 100 | 95.7 | 100 |
| S1 | 46.2 | 75.4 | 88.5 | - | 60.5 | 100 |
| S2 | 93.1 | - | 95.6 | - | 93.5 | 73.8 |
| C1 | 56.4 | - | 100 | - | 57.9 | 100 |
| C2 | 85.5 | - | 78.0 | - | 81.6 | 100 |
| Sample | TOC | TP | TK | TN | Fe | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | A | B | A | B | A | B | A | B | A | |
| D1 | 556.3 | 464.1 | 24.2 | 19.3 | 2.41 | 2.00 | 24.7 | 19.6 | 0.48 | 1.60 |
| D2 | 528.3 | 378.8 | 21.7 | 17.5 | 3.62 | 4.00 | 18.2 | 16.3 | 0.62 | 1.21 |
| S1 | 573.5 | 492.8 | 83.0 | 62.6 | 0.94 | 0.71 | 26.2 | 23.6 | 0.46 | 1.52 |
| S2 | 534.1 | 350.9 | 51.4 | 48.6 | 3.50 | 3.22 | 28.6 | 25.0 | 0.64 | 1.64 |
| C1 | 481.1 | 368.1 | 24.7 | 23.9 | 3.16 | 2.63 | 28.5 | 23.8 | 0.45 | 1.51 |
| C2 | 564.7 | 388.4 | 39.3 | 34.2 | 3.30 | 2.63 | 22.9 | 17.6 | 0.81 | 1.43 |
| Substances | MS 1 | MW 2 (g/mol) | pKa 3 | logKow 4 |
|---|---|---|---|---|
| Estriol (E3) |  | 288.38 | 10.4 | 2.6 |
| 17beta-Estradiol (17β-E2) | 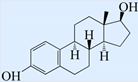 | 272.38 | 10.5 | 3.1 |
| Bisphenol A (BPA) | 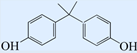 | 228.29 | 10.7 | 3.94 |
| Ethinyloestradiol (EE2) | 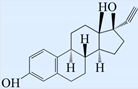 | 296.40 | 11.3 | 3.9 |
| Sulfadimethoxine (SDM) | 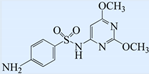 | 310.34 | 6.08 | 1.63 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhou, X.; Gatheru Waigi, M.; Owino Gudda, F.; Cheng, P.; Ling, W. Simultaneous Removal of Estrogens and Antibiotics from Livestock Manure Using Fenton Oxidation Technique. Catalysts 2019, 9, 644. https://doi.org/10.3390/catal9080644
Wang J, Zhou X, Gatheru Waigi M, Owino Gudda F, Cheng P, Ling W. Simultaneous Removal of Estrogens and Antibiotics from Livestock Manure Using Fenton Oxidation Technique. Catalysts. 2019; 9(8):644. https://doi.org/10.3390/catal9080644
Chicago/Turabian StyleWang, Jian, Xian Zhou, Michael Gatheru Waigi, Fredrick Owino Gudda, Pengfei Cheng, and Wanting Ling. 2019. "Simultaneous Removal of Estrogens and Antibiotics from Livestock Manure Using Fenton Oxidation Technique" Catalysts 9, no. 8: 644. https://doi.org/10.3390/catal9080644
APA StyleWang, J., Zhou, X., Gatheru Waigi, M., Owino Gudda, F., Cheng, P., & Ling, W. (2019). Simultaneous Removal of Estrogens and Antibiotics from Livestock Manure Using Fenton Oxidation Technique. Catalysts, 9(8), 644. https://doi.org/10.3390/catal9080644






