Bench-Scale Steam Reforming of Methane for Hydrogen Production
Abstract
1. Introduction
2. Results and Discussion
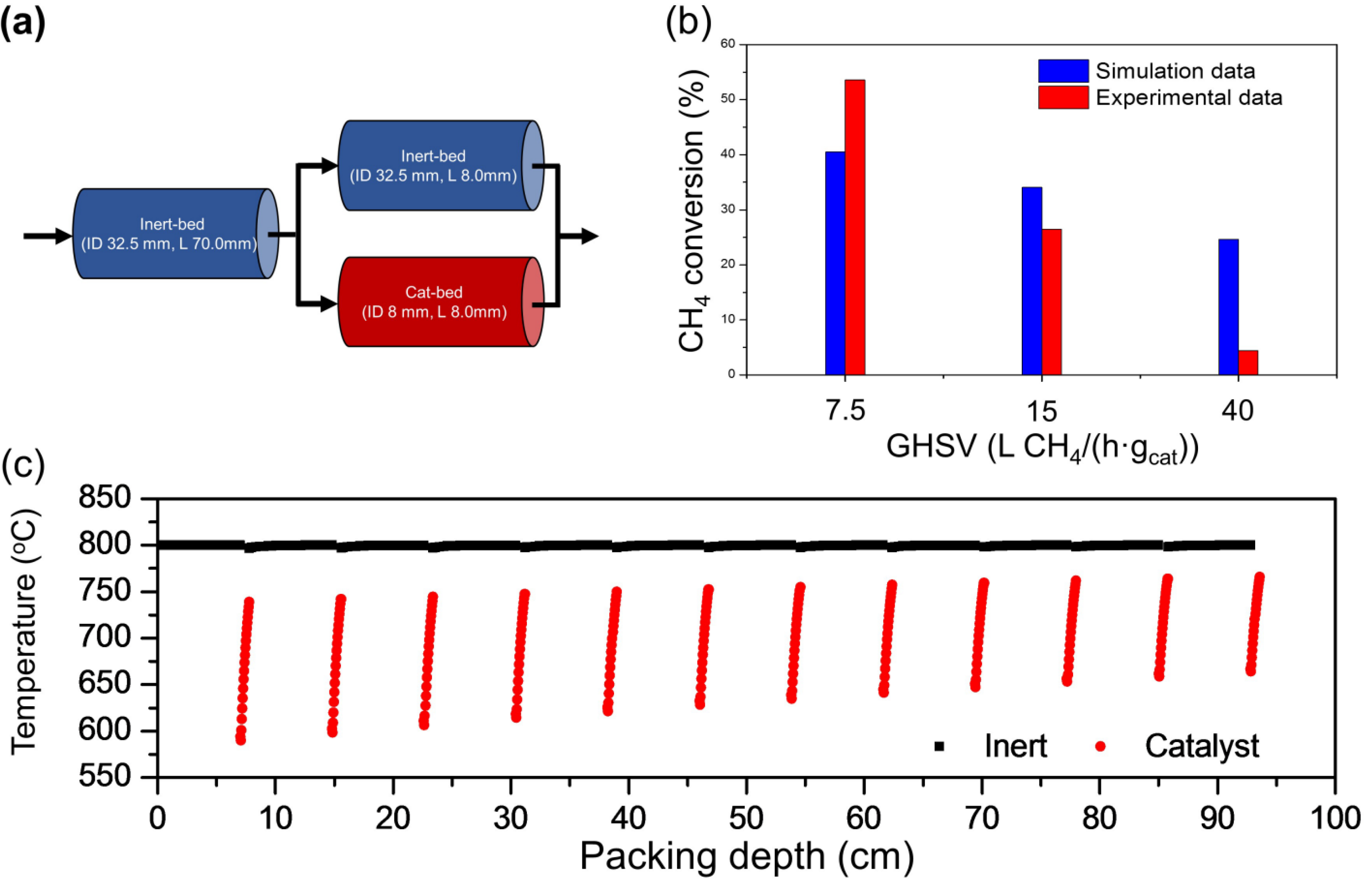
2.1. Methane Steam Reforming Reaction in a Lab-Scale Reactor
2.2. Methane Steam Reforming Reaction in a Bench-Scale Reactor
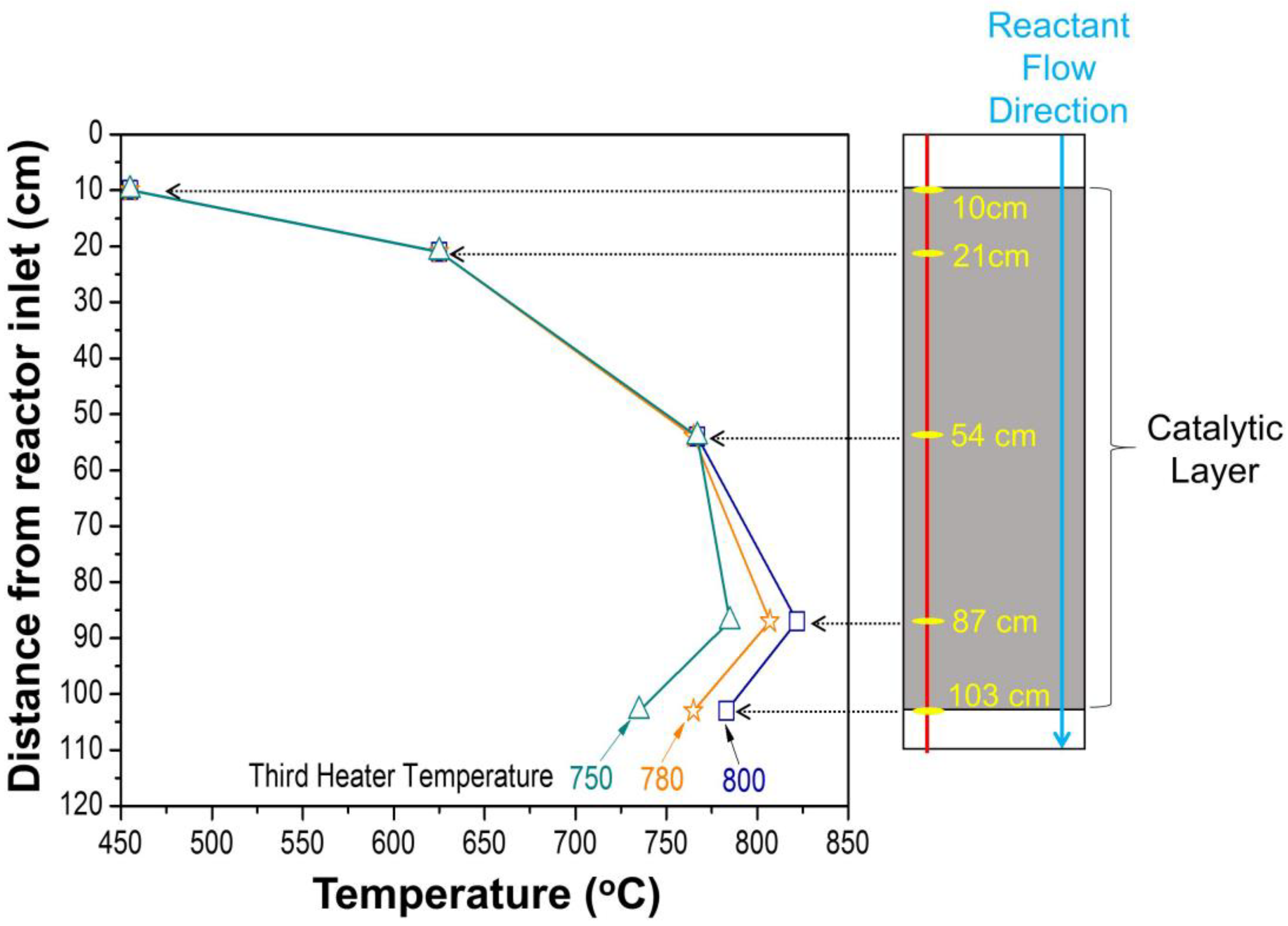
2.2.1. Effect of Reaction Temperature
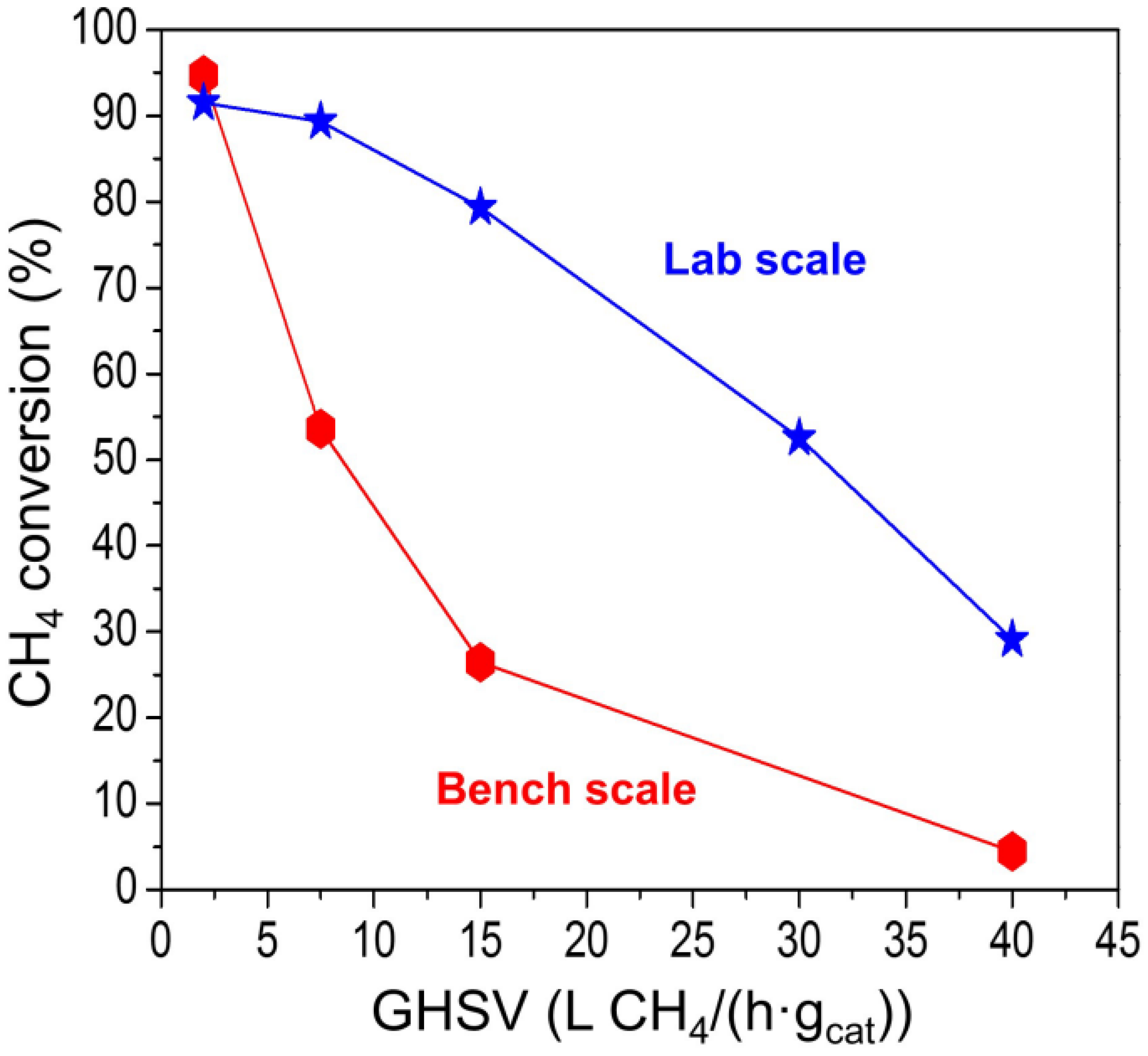
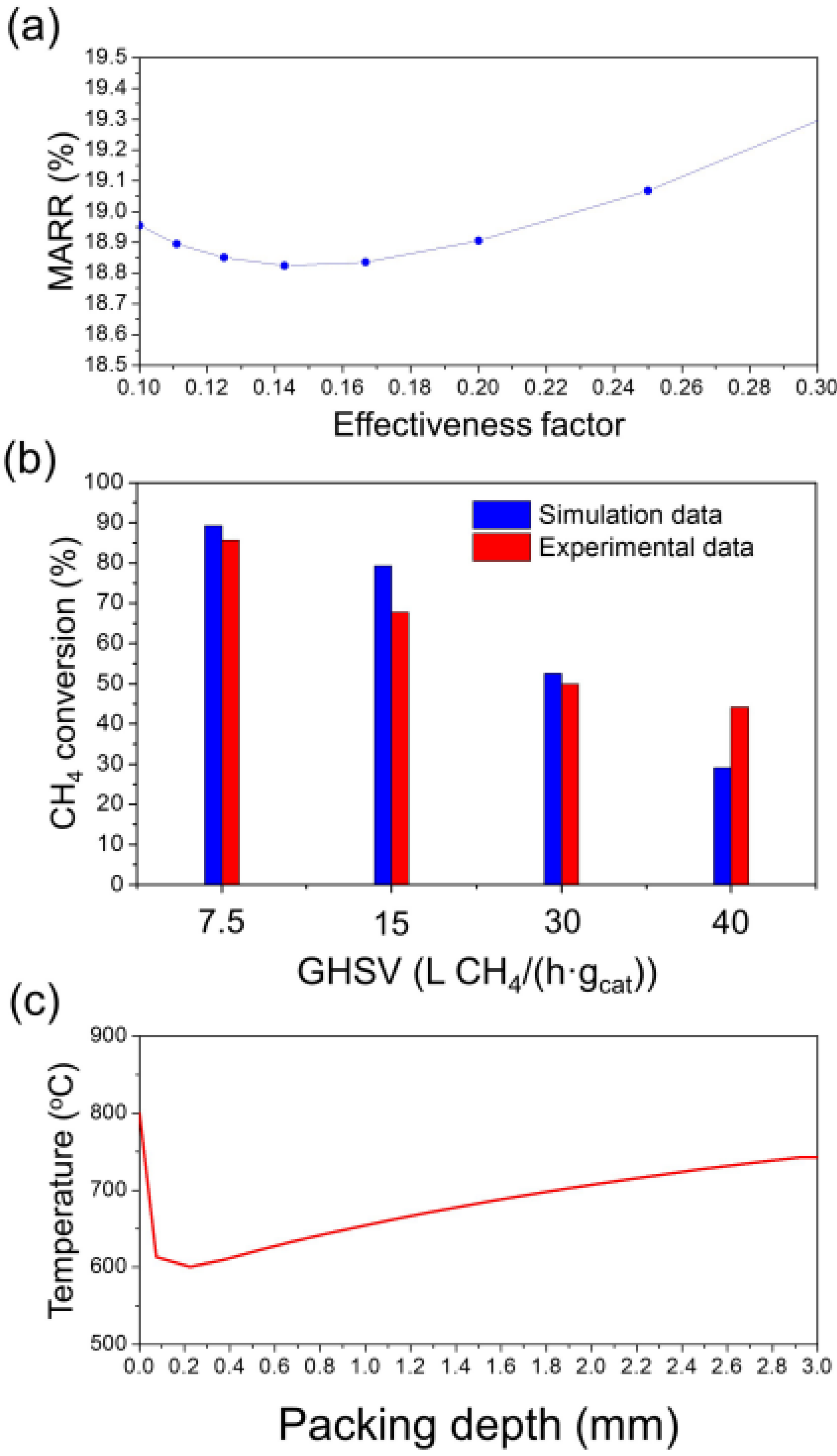
2.2.2. Effect of Space Velocity
2.2.3. Determination of the Effectiveness Factor
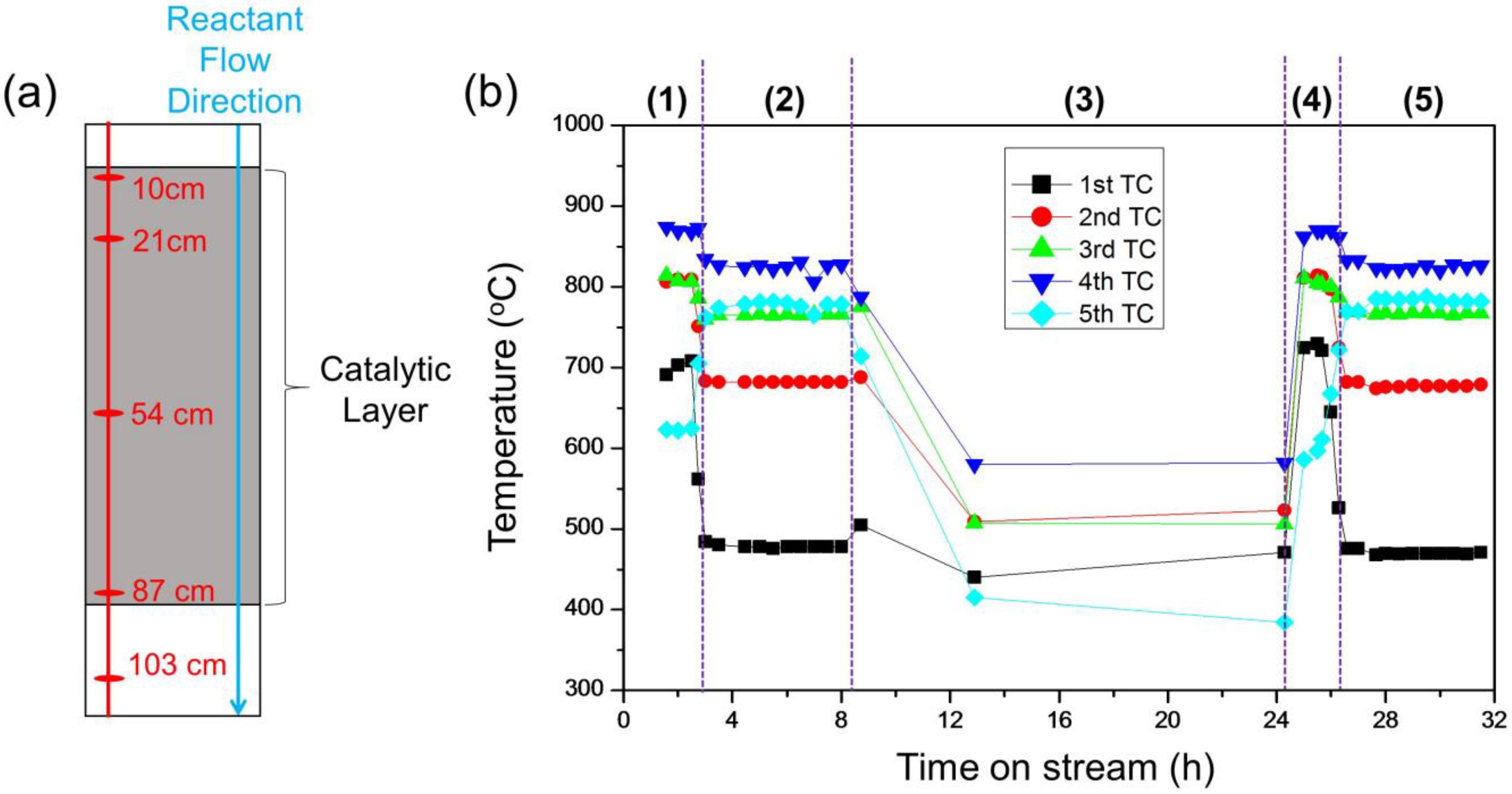
2.3. Idling Conditions
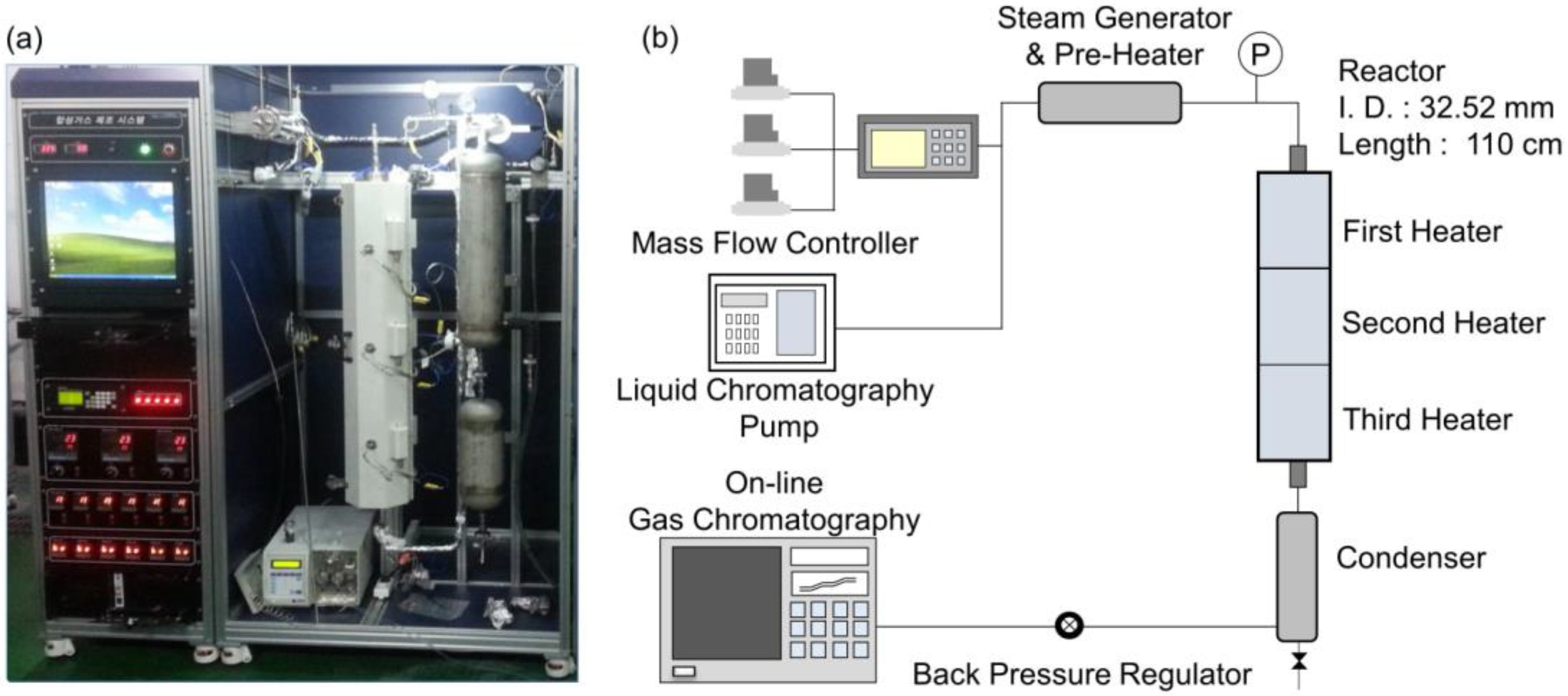
3. Materials and Methods
3.1. Catalyst Characterization
3.2. Steam Reforming Reaction
3.2.1. Methane Steam Reforming Reaction in the Lab-Scale Reactor
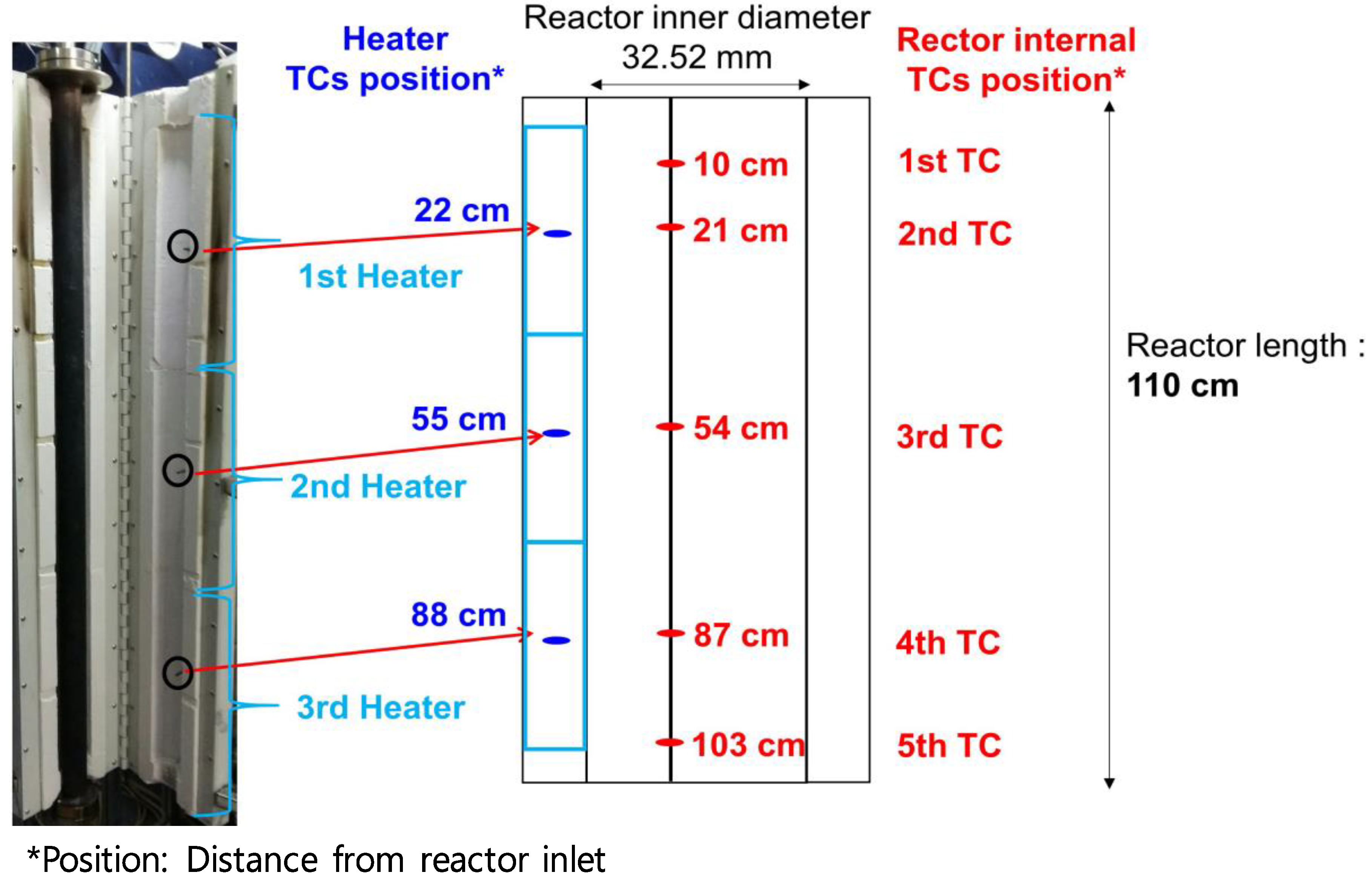
3.2.2. Methane Steam Reforming Reaction in the Bench-Scale Reactor
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iglesia, E. Design, synthesis, and use of cobalt-based Fischer-Tropsch synthesis catalysts. Appl. Catal. A Gen. 1997, 161, 59–78. [Google Scholar] [CrossRef]
- Zhang, C.; Jun, K.-W.; Kwak, G.; Lee, Y.-J.; Park, H.-G. Efficient utilization of carbon dioxide in a gas-to-methanol process composed of CO2/steam mixed reforming and methanol synthesis. J. CO2 Util. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Prater, K.B. Polymer electrolyte fuel cells: A review of recent developments. J. Power Sources 1994, 51, 129–144. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic Fuel Cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Nagaoka, K.; Eboshi, T.; Takeishi, Y.; Tasaki, R.; Honda, K.; Imamura, K.; Sato, K. Carbon-free H2 production from ammonia triggered at room temperature with an acidic RuO2/gamma-Al2O3 catalyst. Sci. Adv. 2017, 3, e1602747. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.K.; Torrente-Murciano, L. Low temperature H2 production from ammonia using ruthenium-based catalysts: Synergetic effect of promoter and support. Appl. Catal. B Environ. 2015, 172, 129–135. [Google Scholar] [CrossRef]
- Cheng, C.; Shi, J.; Hu, Y.; Guo, L. WO3/g-C3N4 composites: One-Pot preparation and enhanced photocatalytic H2 production under visible-light irradiation. Nanotechnology 2017, 28, 164002. [Google Scholar] [CrossRef]
- Ouyang, S.; Tong, H.; Umezawa, N.; Cao, J.; Li, P.; Bi, Y.; Zhang, Y.; Ye, J. Surface-alkalinization-induced enhancement of photocatalytic H2 evolution over SrTiO3-based photocatalysts. J. Am. Chem. Soc. 2012, 134, 1974–1977. [Google Scholar] [CrossRef]
- Qin, J.; Huo, J.; Zhang, P.; Zeng, J.; Wang, T.; Zeng, H. Improving the photocatalytic hydrogen production of Ag/g-C3N4 nanocomposites by dye-sensitization under visible light irradiation. Nanoscale 2016, 8, 2249–2259. [Google Scholar] [CrossRef]
- Lindgren, M.; Panas, I. Confinement dependence of electro-catalysts for hydrogen evolution from water splitting. Beilstein. J. Nanotechnol. 2014, 5, 195–201. [Google Scholar] [CrossRef]
- Yildiz, B.; Kazimi, M.S. Efficiency of hydrogen production systems using alternative nuclear energy technologies. Int. J. Hydrogen Energy 2006, 31, 77–92. [Google Scholar] [CrossRef]
- Nagasawa, K.; Davidson, F.T.; Lloyd, A.C.; Webber, M.E. Impacts of renewable hydrogen production from wind energy in electricity markets on potential hydrogen demand for light-duty vehicles. Appl. Energy 2019, 235, 1001–1016. [Google Scholar] [CrossRef]
- Sarma, S.J.; Brar, S.K.; Sydney, E.B.; Le Bihan, Y.; Buelna, G.; Soccol, C.R. Microbial hydrogen production by bioconversion of crude glycerol: A review. Int. J. Hydrogen Energy 2012, 37, 6473–6490. [Google Scholar] [CrossRef]
- Turner, J.A. Sustainable hydrogen production. Science 2004, 305, 972–974. [Google Scholar] [CrossRef] [PubMed]
- Szima, S.; Nazir, S.M.; Cloete, S.; Amini, S.; Fogarasi, S.; Cormos, A.-M.; Cormos, C.-C. Gas switching reforming for flexible power and hydrogen production to balance variable renewables. Renew. Sustain. Energy Rev. 2019, 110, 207–219. [Google Scholar] [CrossRef]
- Rashid, M.M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen production by water electrolysis: A review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int. J. Eng. Adv. Technol. 2015, 4, 80–93. [Google Scholar]
- Barelli, L.; Bidini, G.; Gallorini, F.; Servili, S. Hydrogen production through sorption-enhanced steam methane reforming and membrane technology: A review. Energy 2008, 33, 554–570. [Google Scholar] [CrossRef]
- Zamaniyan, A.; Ebrahimi, H.; Mohammadzadeh, J.S.S. A unified model for top fired methane steam reformers using three-dimensional zonal analysis. Chem. Eng. Process. Process. Intensif. 2008, 47, 946–956. [Google Scholar] [CrossRef]
- Wu, H.; Parola, V.L.; Pantaleo, G.; Puleo, F.; Venezia, A.M.; Liotta, L.F. Ni-Based Catalysts for low temperature methane steam reforming: Recent results on Ni-Au and comparison with other bi-metallic systems. Catalysts 2013, 3, 563–583. [Google Scholar] [CrossRef]
- Liu, C.-J.; Ye, J.; Jiang, J.; Pan, Y. Progresses in the preparation of coke resistant Ni-based catalyst for steam and CO2 reforming of methane. Chem. Cat Chem. 2011, 3, 529–541. [Google Scholar] [CrossRef]
- Mohammadzadeh, J.S.S.; Zamaniyan, A. Catalyst shape as a design parameter—Optimum shape for methane-steam reforming catalyst. Chem. Eng. Res. Des. 2002, 80, 383–391. [Google Scholar] [CrossRef]
- Saric, M.; Delft, Y.C.; Sumbharaju, R.; Meyer, D.F.; Groot, A. Steam reforming of methane in a bench-scale membrane reactor at realistic working conditions. Catal. Today 2012, 193, 74–80. [Google Scholar] [CrossRef]
- Wang, Y.; Chin, Y.H.; Rozmiarek, R.T.; Johnson, B.R.; Gao, Y.; Watson, J.; Tonkovich, A.Y.L.; Vander Wiel, D.P. Highly active and stable Rh/MgO–Al2O3 catalysts for methane steam reforming. Catal. Today 2004, 98, 575–581. [Google Scholar] [CrossRef]
- Baek, S.M.; Kang, J.H.; Lee, K.-J.; Nam, J.H. A numerical study of the effectiveness factors of nickel catalyst pellets used in steam methane reforming for residential fuel cell applications. Int. J. Hydrogen Energy 2014, 39, 9180–9192. [Google Scholar] [CrossRef]
- Park, N.; Park, M.-J.; Baek, S.-C.; Ha, K.-S.; Lee, Y.-J.; Kwak, G.; Park, H.-G.; Jun, K.-W. Modeling and optimization of the mixed reforming of methane: Maximizing CO2 utilization for non-equilibrated reaction. Fuel 2014, 115, 357–365. [Google Scholar] [CrossRef]
- Smith, J.M.; Van Ness, H.C.; Abbott, M.M. Introduction to Chemical Engineering Thermodynamics, 7th ed.; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
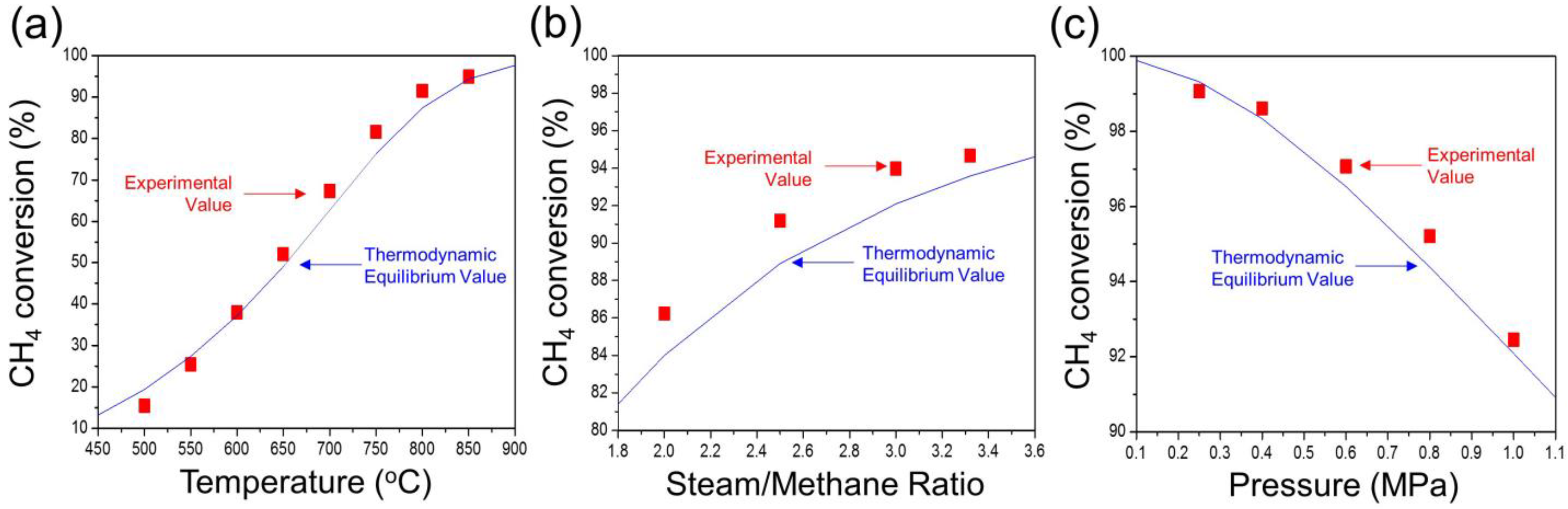

| (a) Experimental Value | |||||
|---|---|---|---|---|---|
| Temperature (°C) | CH4 Conversion (%) | Hydrogen Production Rate (L/min) | |||
| 3rd Heater | 4th TC | 5th TC | Mean Value (between the 4th and 5th TC) | ||
| 800 | 822 | 783 | 802.5 | 94.07 | 10.76 |
| 780 | 807 | 764 | 785.5 | 92.43 | 10.68 |
| 750 | 785 | 738 | 761.5 | 89.63 | 10.53 |
| (b) Thermodynamic Equilibrium Value | |||||
| Temperature (°C) | CH4 Conversion (%) | ||||
| 750 | 87.10 | ||||
| 760 | 89.00 | ||||
| 770 | 90.67 | ||||
| 780 | 92.16 | ||||
| 790 | 93.46 | ||||
| 800 | 94.57 | ||||
| (a) Experimental Value | |||
|---|---|---|---|
| Temperature (°C) | CH4 Conversion (%) | ||
| 1st Heater | 2nd Heater | 3rd Heater | |
| 500 | 650 | 800 | 57.27 |
| 600 | 800 | 600 | 64.13 |
| (b) Thermodynamic Equilibrium Value | |||
| Temperature (°C) | CH4 Conversion (%) | ||
| 620 | 53.40 | ||
| 630 | 56.30 | ||
| 640 | 59.20 | ||
| 650 | 62.20 | ||
| 660 | 65.20 | ||
| 670 | 68.20 | ||
| Temperature (°C) | CH4 Conversion (%) | Rate of Hydrogen Production (L/min) | |||||
|---|---|---|---|---|---|---|---|
| 1st TC | 2nd TC | 3rd TC | 4th TC | 5th TC | |||
| Before idling | 478 | 682 | 766 | 826 | 781 | 92.95 | 6.67 |
| After idling | 469 | 677 | 767 | 825 | 782 | 92.81 | 6.65 |
| Parameters | Data |
|---|---|
| Shape | 1–hole cylinder |
| Size | O.D. 8.17 mm, I.D. 2.85 mm, Height 7.21 mm |
| Composition | Ni 20 wt.%, CaO-Al2O3 80 wt.% |
| Density | 1.80 g/cm3 |
| Packing Density (in bench reactor) | 0.718 g/cm3 |
| Surface Area | 21.26 m2/g |
| Micropore Area | 13.07 m2/g |
| Pore Volume | 0.033 cm3/g |
| Pore Size | 111 Å |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-G.; Han, S.-Y.; Jun, K.-W.; Woo, Y.; Park, M.-J.; Kim, S.K. Bench-Scale Steam Reforming of Methane for Hydrogen Production. Catalysts 2019, 9, 615. https://doi.org/10.3390/catal9070615
Park H-G, Han S-Y, Jun K-W, Woo Y, Park M-J, Kim SK. Bench-Scale Steam Reforming of Methane for Hydrogen Production. Catalysts. 2019; 9(7):615. https://doi.org/10.3390/catal9070615
Chicago/Turabian StylePark, Hae-Gu, Sang-Young Han, Ki-Won Jun, Yesol Woo, Myung-June Park, and Seok Ki Kim. 2019. "Bench-Scale Steam Reforming of Methane for Hydrogen Production" Catalysts 9, no. 7: 615. https://doi.org/10.3390/catal9070615
APA StylePark, H.-G., Han, S.-Y., Jun, K.-W., Woo, Y., Park, M.-J., & Kim, S. K. (2019). Bench-Scale Steam Reforming of Methane for Hydrogen Production. Catalysts, 9(7), 615. https://doi.org/10.3390/catal9070615






