1. Introduction
Biogenic amines are small molecular weight amines which are derived from amino acids by the action of pyridoxal 5-phosphate-dependent amino acid-specific decarboxylases [
1]. The biogenic amines are often found in fermented foods, such as wines, cheeses, sausages, soybean paste seasoning, fish sauces, and so on [
2,
3,
4]. Of the biogenic amines, histamine is considered as one of natural toxins [
5,
6], because the excess intake of histamine from diets may cause skin irritation, headaches, edemas, and rashes typical of allergic reactions [
6]. Of other biogenic amines, tyramine, tryptamine and β-phenylethylamine are suspected of causing blood pressure to increase and triggering hypertensive crises [
7]. In addition, toxicological risk associated with diamines, such as putrescine and cadaverine arise, which are involved in the formation of nitrosamines and cytotoxicity [
8,
9]. Therefore, it is worth noting the importance of monitoring of biogenic amines in foods for food safety issues.
Although the contents of biogenic amines in foods are traditionally determined by using chromatographic methods [
10], it has drawn much attention to develop rapid detection methods for simple and routine monitoring, such as immuno-enzymatic assay [
11], immuno-polymerase chain reaction method [
12], chromogenic sensing [
13], amperometric biosensing [
14], and enzymatic assay [
15,
16]. Of these methods, enzymatic determination using amine oxidases is very useful due to its simple detection of hydrogen peroxide as the by-product of amine oxidase-catalyzed oxidative degradation for biogenic amines. In addition, the broad substrate spectrum of bacterial monoamine oxidases for aromatic biogenic amines including tyramine, tryptamine, 2-phenylehtylamine, and histamine is helpful to determine total contents of biogenic amines in foods [
17,
18]. Recently, our group has developed a bifunctional fusion enzyme protein using a copper-containing monoamine oxidase (AMAO2) and a flavin-containing putrescine oxidases (APUO) from
Arthrobacter aurescens to expand the list of detectable biogenic amines to one of aliphatic biogenic amines, putrescine [
19]. Despite the potential of the fused enzyme, termed Monoamine Putrescine Oxidase (MAPO) as a biocatalyst to determine total contents of biogenic amines in foods, the enzyme productivity using a recombinant
Escherichia coli has been markedly reduced.
Cross-linked enzyme aggregate (CLEAs) technology is an attractive carrier-free enzyme immobilization due to the potential to improve enzyme stability as well as the opportunity to co-immobilize different enzymes [
20,
21,
22]. In this work, a combined CLEAs using AMAO2 and APUO, termed combi-CLEAs hereafter, was prepared, and its properties were compared to those of free AMAO2 and APUO mixture, MAPO, and CLEAs of the fusion enzyme (CLEAs-MAPO).
2. Results and Discussion
2.1. Preparation of CLEAs-MAPO and Combi-CLEAs
AMAO2, APUO, and MAPO were prepared from 2-liter culture in Luria-Bertani (LB) medium. As all amine oxidases have a 6xHis tag at their N-terminus, they were purified using Ni−nitrilotriacetic acid (NTA) affinity chromatography. The productivities of the pure AMAO2 and APUO were 142 ± 24 mg and 156 ± 19 mg per liter of cell culture, respectively, whereas MAPO was 57 ± 9 mg per liter culture. As AMAO2 requests Cu
2+ ion as a cofactor [
14,
16], AMAO2 and MAPO were incubated in the buffer containing CuSO
4. After the Cu
2+ ion charge, the specific activity of AMAO2 (315 U/mg) and the monoamine oxidase of MAPO (134 U/mg) increased by 4.9-fold and 2.5-fold compared to the corresponding purified enzymes.
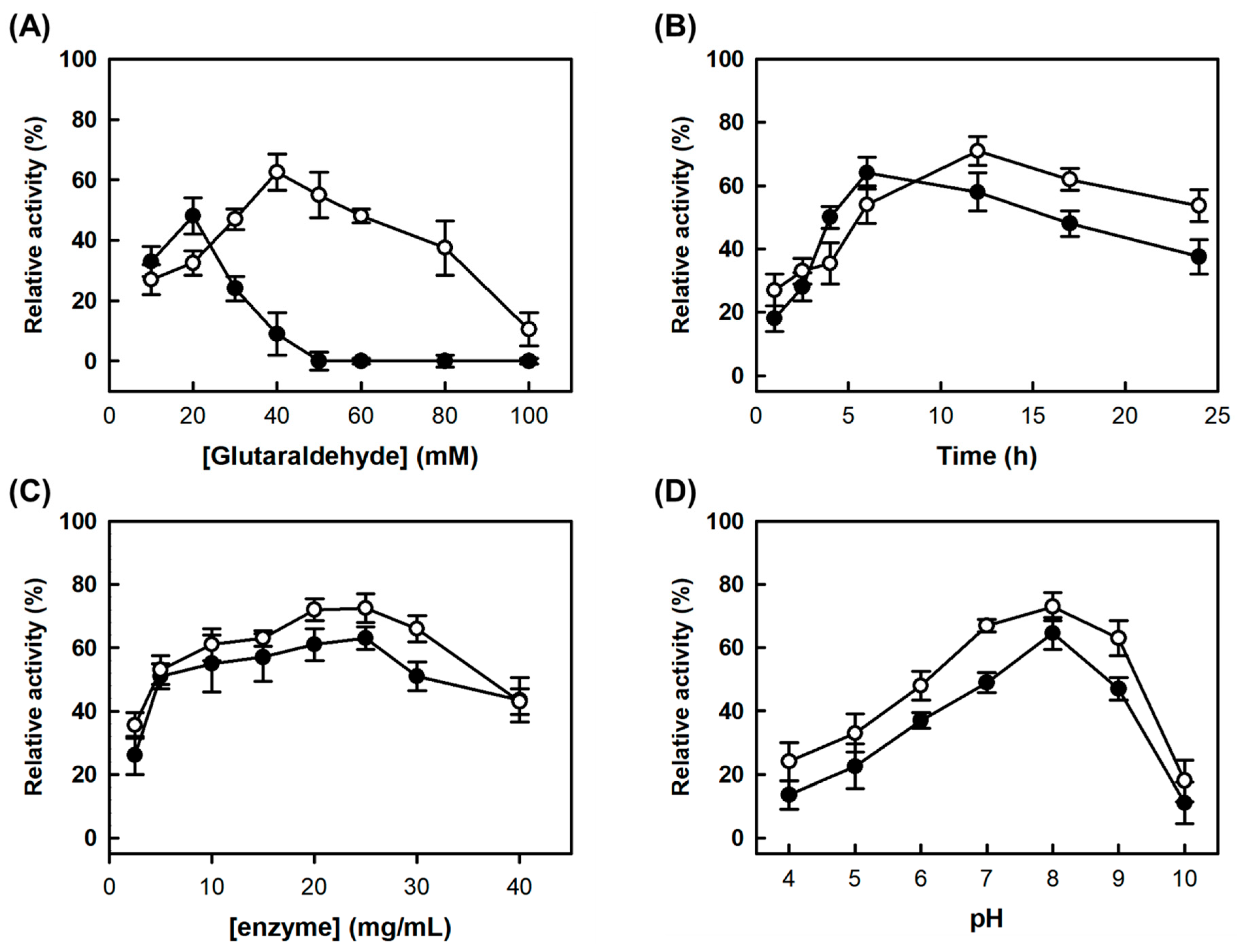
As ammonium sulfate (AS) has been previously selected as the precipitant for AMAO2 [
23], AS was selected as the precipitant for CLEAs-MAPO and combi-CLEAs formation. As in the previous work, AMAO2 was completely precipitated at the concentrations of AS higher than 1.6 M, and fully recovered by the dilution of AS (
Figure 1A). Due to the request of 2 M AS for full aggregation of APUO (
Figure 1B), 2 M AS concentration was determined for simultaneous precipitation of AMAO2 and APUO. Through the optimization based on AMAO2 activity, the final condition for combi-CLEAs was determined as 1.25 mg/mL of each enzyme in 100 mM phosphate buffer (pH 8.0) containing 2 M AS and 40 mM glutaraldehyde (GA) for 12 h at 25 °C was determined. The relative residual tyramine-activity of the CLEAs based on the activities of the subjected enzymes (described as the yield of the CLEAs hereafter) was 71% (open circles in
Figure 2), which was similar to the condition for CLEAs-AMAO2 (50 mM of GA and cross-linking for 17 h at pH 8.0 with 1 mg/mL of AMAO2) [
23]. The optimum condition for APUO activity was same as that of AMAO2 (data not shown).
Upon varying the concentration of AS, MAPO was fully precipitated at the concentrations higher than 2.4 M of AS (
Figure 1C,D). After aggregation at 2.4 M AS, followed by cross-linking at the same condition for combi-CLEAs, there was no activities for neither tyramine nor putrescine. The main reasons were GA concentration and cross-linking time, which were lower (20 mM) and shorter (6 h), respectively, for CLEAs-MAPO than those for combi-CLEAs (
Figure 1A,B). The optimum condition of 2.5 mg/mL MAPO in 100 mM phosphate buffer (pH 8.0) containing 2.4 M AS and 20 mM GA for 6 h at 25 °C was determined, yielding 64% of a yield (
Figure 2).
In a preparative scale production, the reaction volume for cross-linking increased by 100-fold (10 mL) compared to the optimization procedure. Upon using a 15 mL conical tube, the yields of combi-CLEAs and CLEAs-MAPO were 64% and 55%, respectively, based on the initial activity of the enzymes subjected to the CLEAs formation. In order to improve the yields, the container for cross-linking was changed to a 50 mL conical tube, resulting in 82% and 84% of the yields for combi-CLEAs based on the tyramine and putrescine activities, respectively. In the case of CLEAs-MAPO, the yields were also improved (78% and 75%, respectively) compared to those using 15 mL conical tube. These results suggest that enough mixing in a sufficiently large container is an important factor for preparation of CLEAs enzymes.
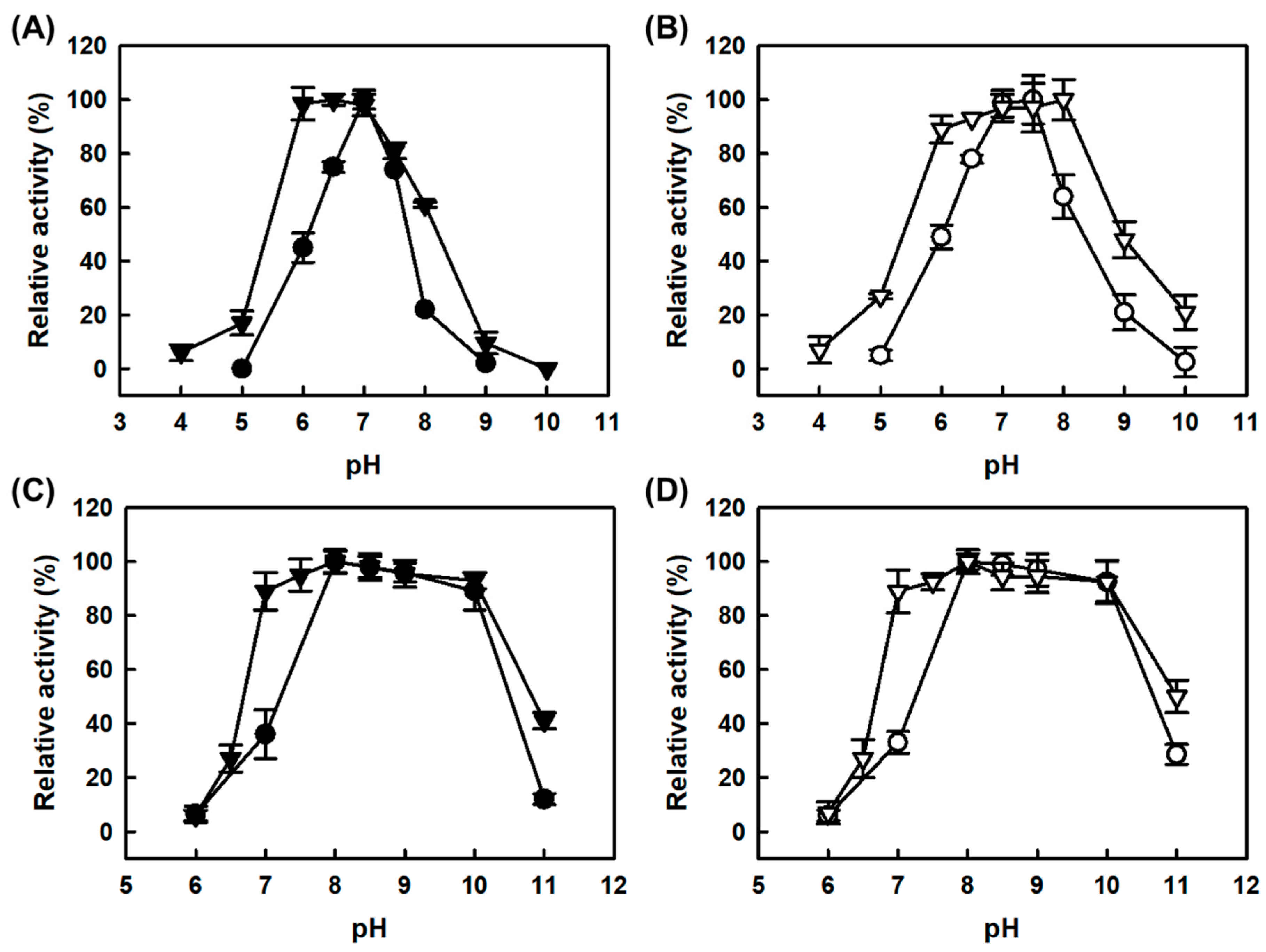
2.2. Effect of pH on the Activities of Free and CLEAs Amine Oxidases
One of the drawbacks for determination of total biogenic amines using the free MAPO is the mismatch of optimal pHs for AMAO2 and APUO (pH 7.5 and pH 8.0, respectively) [
16]; the relative specific activities for tyramine and putrescine of free MAPO were 100:15.7 and 100:273 at the optimal pHs for tyramine and putrescine-activities, respectively [
19]. The pH profile for tyramine activity of free MAPO was much narrower than free AMAO2 due to the more sensitive effect of pH on the AMAO2 domain in MAPO than free AMAO2 (
Figure 3A). Although there was no positive effect on the tyramine-activities for both CLEAs-MAPO and combi-CLEAs at the acidic pH range, those activities were improved at basic pH range, leading to the shift of optimal pHs of the tyramine-activities to pH 7.5 and pH 8.0, respectively (
Figure 3B). Notably, such optimum pH shift for the tyramine-activities of combi-CLEAs resulted in the synchronization of the optimal performances of combi-CLEAs over pH for tyramine and putrescine as the substrates; the relative specific activities for tyramine and putrescine of combi-CLEAs were 100:74 at pH 8.0. Such pH shifts for the tyramine-activities of both CLEAs enzymes might not be caused from the increase of stability at alkaline pHs because there was no significant difference in the enzyme stability over pH (data not shown). The fusion enzyme (MAPO) with a high molecular weight showed markedly low putrescine-activity over pH 7.0 to 8.0 compared to the APUO [
16]. The pH profiles for the putrescine-activities of CLEAs-MAPO and combi-CLEAs were almost same as those of free enzymes, respectively (
Figure 3C,D). Consequently, combi-CLEAs exhibited much better performance over pH than to any other catalysts in this work, displaying more than 90% of the maximal activities for both tyramine and histamine at a pH ranging from pH 7.0 to 8.0.
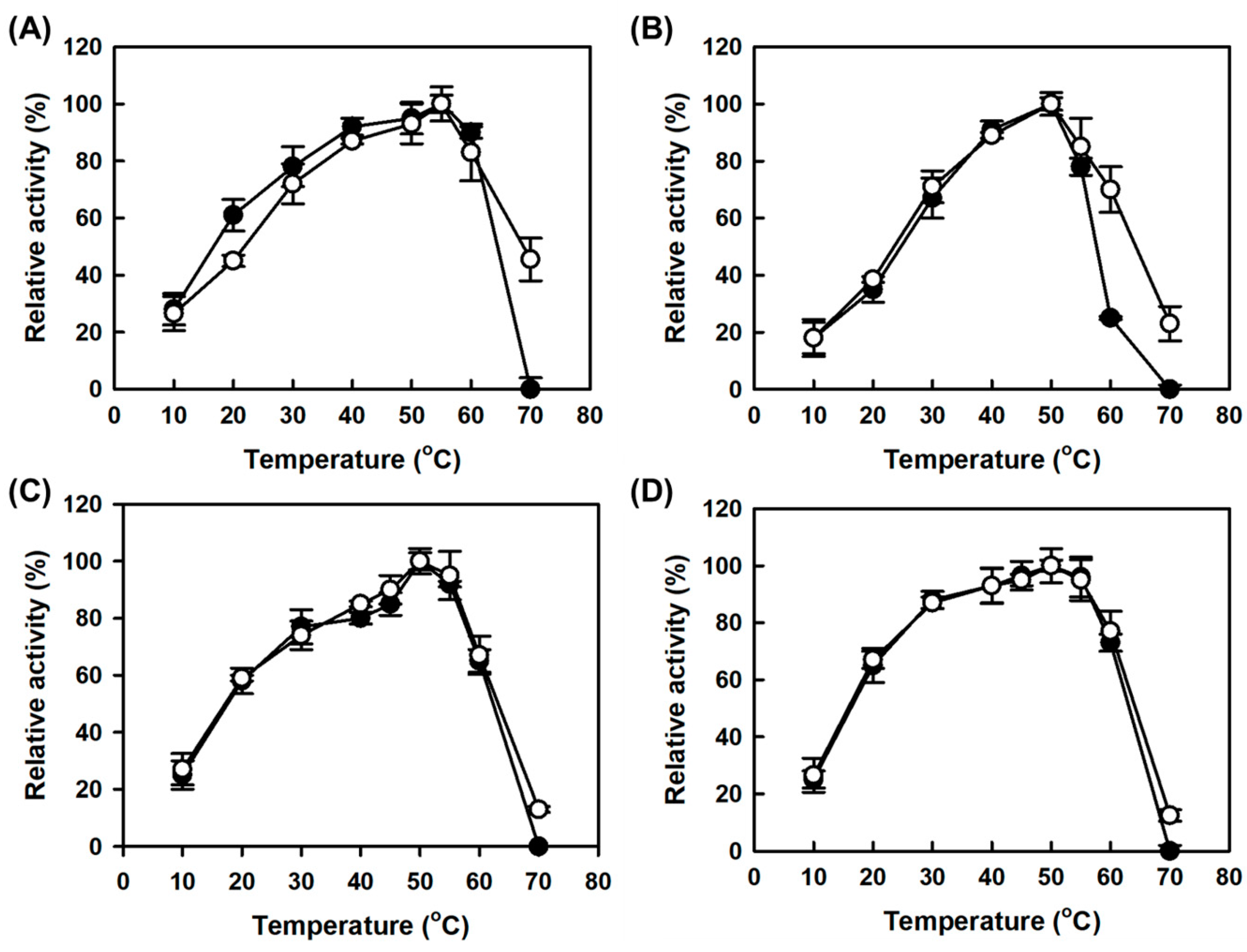
2.3. Effect of Temperature on Activity and Stability of Free and CLEAs Amine Oxidases
MAPO showed a lower optimum temperature for monoamine oxidase activity than AMAO2 by 5 °C (closed circles in
Figure 4A,B for AMAO2 and MAPO, respectively), which was consistent with the previous work [
19]. By contrast, the monoamine oxidase activities for both CLEAs enzymes were maximal at the same optimal temperature of the corresponding free enzymes (
Figure 4). In addition, both CLEAs enzymes showed improved monoamine oxidase activities at a temperature higher than their optimal temperatures, suggesting that the CLEAs enzymes achieved thermostability without the decrease in optimal temperature. As seen in the pH profile, there was no significant difference in the temperature profiles for putrescine activity between the CLEAs enzyme and the corresponding free enzymes (
Figure 4C,D).
The half-lives at 65 °C for combi-CLEAs for tyramine and putrescine activities were 48.1 and 46.8 min, respectively, which can be translated as a 2.4 and 2.1-fold improvement compared to those of the corresponding free enzymes (20.1 min for AMAO2 and 22.3 min for APUO) (
Table 1). The thermostability of MAPO was also improved through the formation of the CLEAs; 18.3 and 17.3 min of half-lives at 65 °C for tyramine and putrescine activity of MAPO, respectively, whereas 30.4 and 27.4 min of those of CLEAs-MAPO, respectively. Such thermostabilization of CLEAs of other enzymes has been observed in many previous studies [
24,
25], and the stabilization of the enzyme conformation by rigidification of the enzyme structure through multipoint covalent attachment has been considered as the main cause for the stabilization of CLEAs [
26,
27]. Upon preparing CLEAs-MAPO, the residual activity seriously decreased at the conditions for the level of cross-linking for the mixed enzymes (
Figure 1), suggesting that the higher rigidity of combi-CLEAs than that of CLEAs-MAPO would yield better thermostability.
2.4. Kinetic Analysis of CLEAs-MAPO and Combi-CLEAs
Of the two CLEAs enzymes, combi-CLEAs exhibited proper performance compared with CLEAs-MAPO. Therefore, combi-CLEAs were chosen, and their kinetic parameters were determined using tyramine, histamine, and putrescine as substrates, followed by comparison with those of free enzymes; the mixture of two individual enzymes (AMAO2 and APUO), the fusion enzyme, MAPO.
After aggregation of the enzymes, the remaining proteins in the supernatants were negligible (less than 0.1% of the initial protein concentration), suggesting that all enzymes subjected to the CLEAs formation existed in the combi-CLEAs. Given the aggregation results, the enzyme concentration of each enzyme (AMAO2 and APUO) in the assay system was calculated.
The substrate specificities based the relative catalytic efficiencies (
kcat/
KM) for tyramine, histamine, and putrescine of combi-CLEAs were 100:36:125, respectively, which were almost same as those of the mixture of AMAO2 and APUO (100:26:167) (
Table 2). The catalytic efficiencies of combi-CLEAs for tyramine, histamine and putrescine were reduced by 41%, 56%, and 31% of the corresponding activities of the mixed free enzymes, respectively, due to the reduction of
kcat values, and that for putrescine of combi-CLEAs were severely reduced. Such reduction in the catalytic efficiency of combi-CLEAs would request either more enzymes or a longer time to determine the biogenic amine contents in foods than that use the free enzymes. However, combi-CLEAs exhibited a beneficial performance in terms of substrate inhibition. As shown in the previous study [
19], the activities of all enzymes for tyramine were inhibited. Interestingly, however,
Ki for combi-CLEAs (
Ki = 1.32 mM) were higher than the double of those for free enzymes (
Ki = 0.61 mM for both) (
Table 2). The substrate inhibition by histamine was also found, albeit much lower (
Ki > 10 mM), and the
Ki for histamine for combi-CLEAs also increased.
2.5. Determination of Biogenic Amines Using Combi-CLEAs
In order to demonstrate the effectiveness of combi-CLEAs to determine biogenic amines, doenjangs, Korean traditional fermented soy seasonings were chosen as a model food system. Individual biogenic amine contents were determined by high performance liquid chromatography (HPLC). Except Sample 3 which had total biogenic amine contents higher than 1300 μM in the extract solution, which can be translated into 1780 ppm of tyramine-equivalent biogenic amines in the sample, the enzyme assay using combi-CLEAs detected 101–103% of the detectable biogenic amines, such as mono biogenic amines and putrescine (
Table 3). Given the detection range of biogenic amines using AMAO2 was up to 120 μM of tyramine equivalent biogenic amines [
17], the high concentration of the substrates in the 10-fold diluted extract for Sample 3 might reduce the reaction rate by substrate inhibition (
Ki for tyramine for combi-CLEAs = 1.32 mM,
Table 2), resulting in underestimation of biogenic amines by combi-CLEAs compared to the HPLC method. Therefore, combi-CLEAs seemed to perform little bit less than free enzymes for biogenic amine detection. In the case of Samples 4 and 5, the enzymatic method detected only 85% of total biogenic amines due to the relatively high ratio of cadaverine and spermidine.
3. Materials and Methods
3.1. Production of Amine Oxidases
Production of AMAO2, APUO, and MAPO using recombinant
Escherichia coli BL21(DE3) containing pET28-6×HAMAO2, pET28-6×HAPUO, and pET29-MAPO6×H, respectively. The recombinant cells were cultured in LB medium at 37 °C, and after low temperature induction (0.2 mM Isopropyl β-D-1-thiogalactopyranoside, 20 °C, 24 h) these amine oxidases were purified using Ni-NTA affinity chromatography as described in previous work [
17,
19]. The eluted fractions containing the target protein were combined and dialyzed against 100 mM potassium phosphate buffer (pH 7.0). In the case of AMAO2 and MAPO, the purified enzymes were incubated in 50 mM sodium phosphate buffer (pH 7.0) containing 0.1 mM CuSO
4 at 4 °C overnight, followed by dialysis against 50 mM Na phosphate buffer (pH 7.0) to remove the excess amount of CuSO
4.
3.2. Assay of Amine Oxidases
The activities of the free and CLEAs amine oxidases were measured using the method described in the previous work [
23]. The diluted enzyme solution was incubated in 100 mM potassium phosphate buffer (pH 7.0) containing tyramine or putrescine as the substrates for 10 min at 37 °C. After boiling the reaction mixture to terminate the reaction, the reaction mixture (75 μL) was mixed with 225 μL chromogen solution, consisting of 1 mM 4-aminoantipyrine, 10 mM hydroxyl benzoate, and 1 U/mL horseradish peroxidase (Sigma, St. Louis, MO, USA) in 100 mM potassium phosphate buffer (pH 7.0), followed by incubation at 37 °C for 30 min. Then, the absorbance of the mixture was measured with a microplate reader (VERSAmax
TM, Molecular devices, Molecular devices, San Jose, CA, USA) at a wavelength of 540 nm.
3.3. Selection of Concentration of Ammonium Sulfate for Enzyme Aggregation
In order to determine the minimum concentration of AS as the precipitant, the enzyme solution (10 μL, 20 mg/mL of MAPO or a mixture of AMAO2 and APUO with the concentrations of 10 mg/mL of each) and 80 μL of AS solutions with different concentrations were mixed. After aggregation (1 h at 25 °C), the aggregated enzyme pellets were separated by centrifugation (10,000× g, 4 °C for 20 min), followed by dissolving the pellets in 100 μL of 50 mM phosphate buffer (pH 7.0). Based on the activities of the supernatants and the pellets for tyramine and putrescine as substrates, the best concentrations for the enzyme mixture and MAPO were selected.
3.4. Optimization of Conditions for Formation of CLEAs
The starting condition for the formation of CLEAs was conducted for 4 h at 25 °C using a mixture of 10 μL of GA and 90 μL of precipitated enzyme solution containing 1 mg/mL of each AMAO2 and APUO in 100 mM phosphate buffer (pH 8.0) with 2 M. After cross-linking, the collected CLEAs by centrifugation (12,000× g for 10 min) were washed twice and resuspended in 1 mL of phosphate buffer (100 mM, pH 7.0). First, through varying GA concentration ranging from 10 to 100 mM at a fixed condition for the other factors, the optimum GA concentration for each CLEAs was determined based on the yield. Next, one of the other factors was optimized stepwise at a fixed condition for the factors previously optimized by varying individually cross-linking time (1–24 h), enzyme concentrations (final 0.25 to 2.5 mg/mL), and pH for cross-linking (pH 3.0−9.0).
3.5. Preparation of CLEAs on Preparative Scale
CLEAs-MAPO and combi-CLEAs were prepared in a 25 mg protein scale at the respective optimal conditions. Each reaction mixture was agitated at 100 rpm during cross-linking. After centrifugation at 10,000× g for 10 min, the pellet was washed three times with 3 mL of 50 mM potassium phosphate buffer/pH 7.0, followed by resuspension with 10 mL of the same buffer, and store at 4 °C for the further experiments.
3.6. Biochemical Characterization of CLEAs-MAPO and Combi-CLEAs
The activities of the free and the CLEAs was determined using tyramine for monoamine oxidase and putrescine for putrescine oxidase as the substrates as described previously [
19]. The optimal pHs and temperatures were determined in the pH range of 4.0−10.0 at 50 °C and 10−70 °C in phosphate buffer (50 mM, pH 7.0), respectively. The effect of pH on the stabilities of the enzymes was investigated by measuring the residual activity after incubating at 4 °C for 24 h in a buffer. The thermostabilities of the enzymes were evaluated as previously described [
23]. The first-order rate constant of irreversible thermal denaturation (
kd) was obtained from the slope of the plots of ln (residual activity/initial activity) versus time, and the half-lives were calculated as ln2/
kd.
3.7. Kinetic Analysis of CLEAs-MAPO and Combi-CLEAs
The kinetic analysis for tyramine, histamine, and putrescine was conducted in 50 mM phosphate buffer (pH 7.0) at 35 °C in the microplate reader (VERSA
maxTM) as previously described [
19]. To determine the initial rate of the reaction, the absorbance was continuously measured using a microplate reader at a wavelength of 540 nm. The absorbance was converted into the concentration biogenic amines using a standard curve prepared using the standard biogenic amines. The kinetic parameters were calculated by fitting the data to the Michaelis−Menten equation. When substrate inhibition was observed, the kinetic parameters were calculated using the data in the following equation, using SigmaPlot software (version 11; Systa Software Inc., San Jose. CA, USA):
3.8. Determination of BA Contents in Doenjangs Using Combi-CLEAs
Doenjangs were purchased from domestic markets. Five grams of the sample was extracted with 25 mL of water, twice. The extracts were combined and filtrated with 90-mm filter paper (Advantech, Tokyo, Japan). The BA contents of the extracts was measured using combi-CLEAs as follows: 40 μL of 10-fold diluted sample, 80 μL of 50 mM potassium phosphate buffer (pH 7.0), 20 μL of combi-CLEAs (1.2 unit), and 60 μL of the chromogen solution was mixed and incubated at 37 °C for 30 min. The absorbance of the mixture was monitored at 540 nm with a microplate reader. The final absorbance was converted into the amount of tyramine-equivalent biogenic amines using a standard curve prepared using tyramine as a standard substance.
3.9. HPLC Analysis of BAs in Doenjang and Gochujang Samples
All BAs were purchased from Sigma-Aldrich. In order to detect the biogenic amines in the samples using HPLC, the amine group of BAs was modified by dansyl chloride as previously described [
17]. The dansylated samples were analyzed by HPLC (YL9100; Younglin, Anyang, Korea) equipped with a SunFire
TM-C18 column (150 × 4.6 mm; Waters, Milford, MA, USA) and a UV/Vis detector at 254 nm. The samples were eluted with a step gradient of 1 M ammonium acetate (eluent A) and acetonitrile (eluent B) at a flow rate of 1 mL/min: 45% of eluent A and 55% of eluent B for 1 min, 35% of eluent A and 65% of eluent B for the next 5 min, 10% of eluent A and 90% of eluent B for the next 15 min. The concentration of each biogenic amine was calculated using a standard curve prepared using the corresponding biogenic amine as a standard substance.
4. Conclusions
Combi-CLEAs of the monoamine oxidase (AMAO2) and the putrescine oxidase (APUO) and CLEAs of the fusion enzyme of the enzymes were prepared by optimizing the factors for the CLEAs preparation. The sufficient mixing in a large volume-container during cross-linking was one of the most important factors for improvement of the residual activity after the CLEAs formation. Combi-CLEAs showed several benefits compared with CLEAs-MAPO in terms of yield, thermostability, and optimal pHs for two activities. The thermostability of combi-CLEAs was improved at high temperatures, suggesting that the half-life time at room temperature or 4 °C would markedly increase compared to the free enzyme. Such stabilization effect would provide an extended shelf-life time of a commercialized kit to detect biogenic amines using combi-CLEAs. Finally, although combi-CLEAs exhibited slightly lower performance to determine biogenic amine contents in foods with high contents of biogenic amines compared to the free enzymes due to the decrease in the catalytic efficiencies of both enzymes, the synchronization of optimal pHs of AMAO2 and APUO in combi-CLEAs would give benefits to determine biogenic monoamines and putrescine together. The findings in this study suggest that combi-CLEAs with AMAO2 and APUO are a promising catalyst for cumulative assay for main biogenic amines in foods, such as tyramine, histamine, and putrescine.