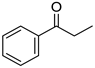
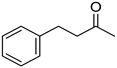
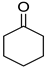
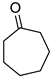
Abstract
A new catalytic system for transfer hydrogenation of carbonyl compounds using glucose as a hydrogen donor was developed. Various ketones and aldehydes were efficiently converted to corresponding alcohols with two equivalents of glucose in the presence of a small amount (0.1 to 1.0 mol%) of iridium catalyst that had a functional ligand. In this catalytic system, transfer hydrogenation reactions proceeded based on the cooperativity of iridium and a functional ligand. It should be noted that environmentally benign water could have been used as a solvent in the present catalytic system for the reduction of various carbonyl substrates. Furthermore, the reaction scope could be extended by using N,N-dimethylacetamide as a reaction solvent.
Keywords:
transfer hydrogenation; iridium catalyst; functional ligand; glucose; ketone; aldehyde; alcohol; water solvent 1. Introduction
Reductive conversion of carbonyl compounds to alcohols is one of the most important and fundamental reactions in the field of synthetic organic chemistry. This method has been predominantly used to prepare various alcohols. On an industrial scale and as a conventional technology, such reductive conversion has been performed via catalytic reactions using hydrogen as a reductant [1]. For small-scale laboratory experiments, reduction of carbonyl compounds to alcohols is often performed with stoichiometric amounts of metal hydride reductant, such as lithium aluminum hydride or sodium borohydride. Both the aforementioned methods are well-established; however, hydrogen poses safety issues owing to its explosive nature. Additionally, using a metal hydride reductant not only adversely affects the chemoselectivity of the reaction but also produces a stoichiometric amount of waste.
On the other hand, catalytic transfer hydrogenation reactions using a less toxic hydrogen donor are also important and widely employed in methods for converting carbonyl compounds to alcohols [2,3,4]. The most well-known of these would be the Meerwein–Ponndorf–Verley (MPV)-type reduction using 2-propanol as a hydrogen donor. Although an aluminum catalyst is typically used in MPV-type reductions [5,6], many highly efficient systems using transition metal catalysts have been reported [7,8,9,10,11,12]. However, MPV-type reduction depends on the equilibrium between alcohols and carbonyl compounds; therefore, a large excess of 2-propanol as a hydrogen donor must be used to obtain an alcohol in high yield. For example, Li et al. recently reported highly efficient MPV-type reduction of aldehydes using an iridium catalyst [12]; however, 65 equivalents of 2-propanol relative to the aldehyde substrates had to be used to obtain the product primary alcohols in satisfactory yields. Currently, 2-propanol is produced from propylene, which is obtained from fossil resources. Hence, it is essential to search for a low-cost hydrogen donor that is sustainably available from natural resources.
In these situations, we focused on carbohydrate as an alternative hydrogen donor for catalytic transfer hydrogenation. Thus, we studied the catalytic transfer hydrogenation of carbonyl compounds to form alcohols using glucose as a hydrogen donor. Glucose is inexpensive, easily obtained from natural renewable resources, and safe to handle [13]. Furthermore, as it is extremely soluble in water, it is expected to be an ideal hydrogen donor if the reduction can be performed in aqueous media [14]. However, few catalytic transfer hydrogenation reactions that use glucose as a hydrogen donor have been reported. Manna and Antonchick recently reported a new system for transfer hydrogenation of unsaturated organic compounds using glucose as a hydrogen donor [15]. Their system involved relatively large amounts of rhodium complex, [Cp*RhCl2]2 (6 to 12 mol% Rh), as catalyst. The main target of this catalytic system was the reductive transformation of alkynes to alkenes, and alkenes to alkanes; therefore, only five examples of the transfer hydrogenation of carbonyl substrates have been demonstrated.
Our group developed various iridium catalysts exhibiting high catalytic performances in dehydrogenation and hydrogen transfer reactions based on the cooperativity of iridium and functional ligands [16,17,18,19,20]. As an expansion of our study, here, we reported the transfer hydrogenation of various ketones and aldehydes using glucose as a hydrogen donor, catalyzed by a small amount of iridium complex (0.1 to 1 mol% Ir) (Scheme 1). It should be noted that water could be used as a solvent in the present catalytic system for various carbonyl substrates, although N,N-dimethylacetamide (DMAc), was indispensable as an organic solvent for improving the reduction efficiency for some substrates.
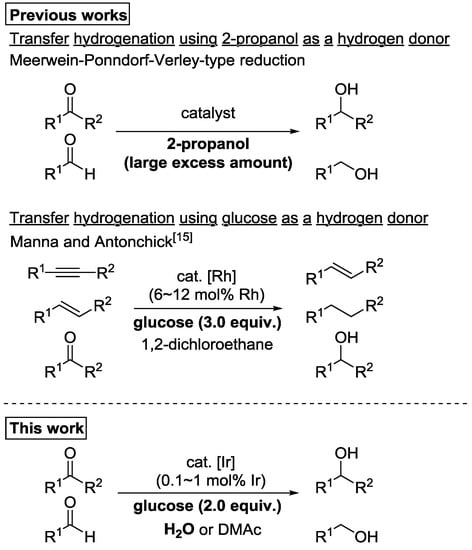
Scheme 1.
Catalytic transfer hydrogenation using easily available hydrogen donors.
2. Results
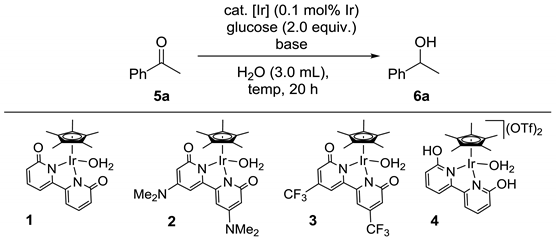
We initially optimized the reaction conditions for the transfer hydrogenation of acetophenone (5a) to 1-phenylethanol (6a) using glucose as a hydrogen donor. The results are shown in Table 1. Reactions were conducted in a sealed stainless-steel reactor using 5a (2.0 mmol) and glucose (4.0 mmol) in water (3.0 mL) in the presence of catalytic amounts of iridium complex and base for 20 h at 80 °C to 120 °C. As indicated in entry 1, a simple iridium complex, [Cp*IrCl2]2, exhibited no catalytic activity, which resulted in no formation of 6a. When the reaction of 5a was performed in the presence of 0.1 mol% Ir of aqua(2,2′-bipyridine-6,6′-dionato)(pentamethylcyclopentadienyl)iridium (1) and 5.0 mol% of Na2CO3 at 100 °C, transfer hydrogenation proceeded selectively to give 6a in 85% yield (entry 2). Other iridium catalysts 2 and 3 having substituents of the functional bipyridonate ligand exhibited lower activity than 1 (entries 3 and 4). Related dicationic iridium catalyst 4 was also inferior to 1 (entry 5). Reactions at lower and higher temperatures (80 °C and 120 °C) both resulted in lower yields of 6a (entries 6 and 7). Employing other bases such as K2CO3, NaOtBu, and KOtBu also decreased the yield of 6a (entries 8–10). Two equivalents of glucose were indispensable in obtaining 6a in high yield, as the reaction using just one equivalent of glucose resulted in a moderate yield of 6a (entry 11). Finally, the highest yield of 6a (89%) was achieved by employing 0.2 mol% Ir of the catalyst 1 (entry 12). Additionally, DMAc could be used as a solvent in place of water, maintaining a high yield of 6a (entry 13).

Table 1.
Optimization of reaction conditions for the transfer hydrogenation of acetophenone (5a) to 1-phenylethanol (6a) using glucose as a hydrogen donor.
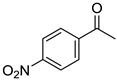
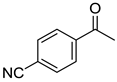
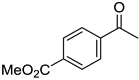
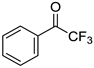
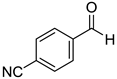
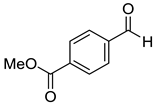
To explore the substrate scope for the transfer hydrogenation catalyzed by 1 using glucose as a hydrogen donor, reactions were conducted with various ketones. The results are shown in Table 2. Reactions of acetophenone derivatives bearing various substituents on the phenyl ring were initially examined in water (Conditions A). In most of these reactions, 1.0 mol% Ir of catalyst 1 was required to achieve efficient conversion of the ketone substrates. The meta-methyl-substituted acetophenone 5b was converted to the corresponding secondary alcohol 6b in 61% yield. Acetophenone derivatives 5c–f bearing electron-withdrawing substituents such as trifluoromethyl, nitro, cyano, and methoxycarbonyl groups at the para-position on the phenyl ring were selectively converted to the corresponding secondary alcohols 6c–f in moderate to good yields. The 2,2,2-trifluoroacetophenone (5g) was also converted to the corresponding 2,2,2-trifluoro-1-phenylethanol (6g) in 89% yield. Propiophenone (5h) and other ketones 5i–k were also applicable to this catalytic system. Conversely, reactions of acetophenone derivatives bearing methoxy and halogen substituents in water gave lower yields of secondary alcohols. Here, intermolecular dehydration predominantly proceeded to afford ethers [bis-(α-methylbenzyl)ether derivatives]. For these substrates, the reaction in DMAc as a solvent (Conditions B) significantly improved the yields of secondary alcohols. Starting from acetophenone derivatives 5l–q, the desired alcohol products 6l–q were obtained in good to excellent yields under catalytic conditions B.

Table 2.
Transfer hydrogenation of various ketones catalyzed by 1 using glucose as a hydrogen donor.
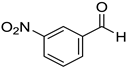
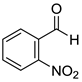
The present catalytic system was also suitable for reducing various aromatic aldehydes to primary benzyl alcohols. The results are summarized in Table 3. Reaction conditions A, which were applied for reducing ketones, as mentioned in Table 2, were also effective for reducing benzaldehyde (7a) and p-methylbenzaldehyde (7b) to benzyl alcohol (8a) and p-methylbenzyl alcohol (8b), respectively. Benzaldehyde derivatives 7c–f bearing electron-withdrawing substituents such as cyano, nitro, and methoxycarbonyl groups were also converted to corresponding benzylic alcohols 8c–f in moderate to good yields under conditions A. Benzaldehyde derivatives 7g–k bearing methoxy, tert-butyl, and halogen substituents were efficiently converted to benzylic alcohols 8g–k under conditions B using DMAc as a solvent. Other aldehydes 7l and 7m with naphthyl rings were also converted to corresponding primary alcohols 8l and 8m in excellent yields under conditions B.

Table 3.
Transfer hydrogenation of various aldehydes catalyzed by 1 using glucose as a hydrogen donor.
To determine which part of glucose functioned as a hydrogen donor, two additional experiments were conducted (Figure 1). The reaction of 5a using methyl α-glucopyranoside (9), in which one of the hydroxy groups of glucose at the C1 position was protected, did not proceed (Equation 1). Conversely, 2,3,4,6-tetra-O-methyl α-glucopyranose (10), in which all four hydroxy groups except for that at the C1 position were protected, was an effective hydrogen donor for the transfer hydrogenation of 5a to give 6a in 97% yield (Equation 2). These results verified that hydrogen transfer occurred from the hydroxy group at the C1 position in glucose to the carbonyl substrates during the catalytic processes.
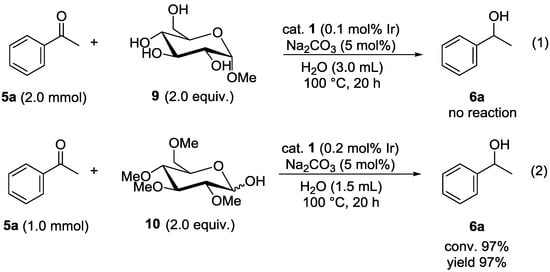
Figure 1.
Additional experiments to determine which part of glucose functioned as a hydrogen donor.
A possible mechanism for the transfer hydrogenation of carbonyl compounds to the corresponding alcohol products catalyzed by iridium complex 1 is shown in Scheme 2. First, elimination of the aquo ligand in 1 occurred to generate coordinatively unsaturated species I. Then, dehydrogenation at the hydroxy moiety at the C1 position of glucose, based on the cooperativity of iridium and the functional ligand, proceeded through transition state II, affording gluconolactone and iridium-hydride species III. (NMR analysis of the crude mixture obtained under optimal conditions (Table 1, entry 12) was performed. Signals due to gluconolactone were observed, indicating that catalytic hydrogen transfer from glucose to acetophenone surely occurred.) Transfer hydrogenation from species III to carbonyl substrates occurred through transition state IV to give the alcohol products along with regeneration of catalytically active species I.
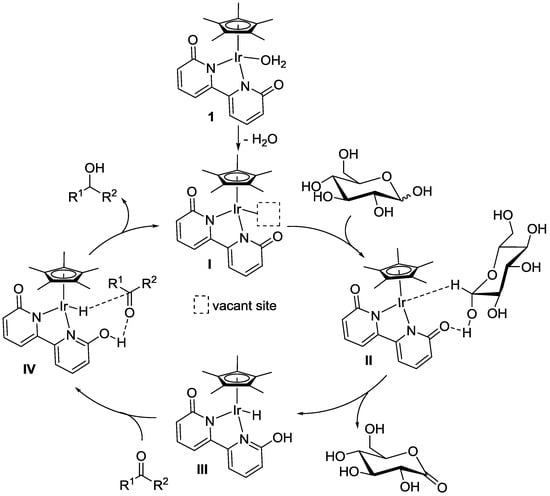
Scheme 2.
A possible mechanism for the transfer hydrogenation of carbonyl compounds catalyzed by 1 using glucose as a hydrogen donor.
3. Materials and Methods
3.1. General
1H and 13C{1H} NMR spectra were recorded on JEOL ECX-500 and ECS-400 spectrometers (JEOL Ltd., Tokyo, Japan). Gas chromatography (GC) analyses were performed on a GL-Sciences GC353B gas chromatograph (GL Sciences Inc., Tokyo, Japan) with a capillary column (GL-Sciences and InertCap Pure WAX (GL Sciences Inc., Tokyo, Japan)). Silica-gel column chromatography was carried out by using Wako-gel C-200 (FUJIFILM Wako Pure Chemical Corp., Osaka, Japan). Ketones and aldehydes were purchased from FUJIFILM Wako Pure Chemical Corp. (Osaka, Japan), Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) or nacalai tesque (Kyoto, Japan). Distilled water and N,N-dimethylacetamide(super dehydrated) were purchased from FUJIFILM Wako Pure Chemical Corp. (Osaka, Japan). The compounds, [Cp*IrCl2]2 (Cp* = η5-pentamethylcyclopentadienyl) [21] and iridium complexes 1–4 were prepared according to the literature methods [18,19,22,23].
3.2. General Procedure for Transfer Hydrogenation of Acetophenone to 1-phenylethanol Using Glucose (Table 1)
In a 5 mL stainless-steel reactor under argon atmosphere, catalyst (0.1 mol% Ir), acetophenone (2.0 mmol), glucose (4.0 mmol), base (5.0 or 10.0 mol%) and degassed distilled water (3.0 mL) were placed. Then, the reactor was sealed with a stainless-steel stopper, and the mixture was stirred at 100 °C for 20 h. After cooling to room temperature, the mixture was diluted with toluene (50 mL). The conversion of acetophenone and the yield of 1-phenylethanol were determined by GC analysis using biphenyl as an internal standard.
3.3. General Procedure for Transfer Hydrogenation of Ketones to the Corresponding Secondary Alcohols Using Glucose (Table 2)
3.3.1. Conditions A
In a 5 mL stainless-steel reactor under argon atmosphere, catalyst 1 (10.6 mg, 0.020 mmol, 1.0 mol%), ketone (2.0 mmol), glucose (720.6 mg, 4.0 mmol, 2.0 equiv.), Na2CO3 (10.6 mg, 0.10 mmol, 5.0 mol%) and degassed distilled water (3.0 mL) were placed. Then, the reactor was sealed with a stainless-steel stopper, and the mixture was stirred at 100 °C for 20 h. After cooling to room temperature, the products were extracted with dichloromethane (20 mL × 3). After evaporation of the solvent, the yield was determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard. The products were isolated by column chromatography (eluent = ethyl acetate/hexane). 1H and 13C{1H} NMR spectra of each isolated products are shown in Supplementary Materials.
1-Phenylethanol (6a) [24]: 1H NMR (500 MHz, CDCl3) δ 7.40–7.33 (m, 4H, aromatic), 7.31–7.25 (m, 1H, aromatic), 4.91 (qd, J = 6.5, 3.5 Hz, 1H, CHOH), 1.85 (d, 3.5 Hz, 1H, CHOH), 1.50 (d, J = 6.0 Hz, 3H, CH3). 13C{1H} NMR (125 MHz, CDCl3) δ 145.9, 128.6, 127.5, 125.5, 70.4, 25.2.
1-(3’-Methylphenyl)ethanol (6b) [25]: 1H NMR (400 MHz, CDCl3) δ 7.25 (t, J = 8.0 Hz, 1H, aromatic), 7.23–7.15 (m, 2H, aromatic), 7.09 (d, J = 7.2 Hz, 1H, aromatic), 4.87 (qd, J = 6.4, 2.8 Hz, 1H, CH(OH)CH3), 2.36 (s, 3H, ArCH3), 1.80 (br, 1H, OH), 1.49 (d, J = 6.4 Hz, 3H, CH(OH)CH3 ). 13C{1H} NMR (100 MHz, CDCl3) δ 145.9, 138.3, 128.6, 128.4, 126.2, 122.6, 70.6, 25.3, 21.6.
1-(4’-Trifluoromethylphenyl)ethanol (6c) [25]: 1H NMR (400 MHz, CDCl3) δ 7.57 (d, J = 8.0 Hz, 2H, aromatic), 7.42 (d, J = 8.4 Hz, 2H, aromatic), 4.88 (q, J = 2.4 Hz, 1H, -CH(OH)CH3), 2.93 (br, 1H, OH), 1.44 (d, J = 6.4 Hz, 3H, CH3). 13C{1H} NMR (100 MHz, CDCl3) δ 149.8, 129.6 (q, JCF3 = 31.7 Hz), 125.7, 125.4 (q, JCF3 = 3.9 Hz), 124.3 (q, JCF3 = 271.2 Hz), 69.8, 25.3.
1-(4’-Nitrophenyl)ethanol (6d) [24]: 1H NMR (500 MHz, CDCl3) δ 8.20 (dt, J = 9.0, 2.0 Hz, 2H, aromatic), 7.55 (ddt, J = 8.5, 2.0, 0.5 Hz, 2H, aromatic), 5.03 (q, J = 6.5 Hz, 1H, CH(OH)CH3), 2.16 (br, 1H, OH), 1.52 (d, J = 6.5 Hz, CH3). 13C{1H} NMR (125 MHz, CDCl3) δ 153.2, 147.3, 126.2, 123.9, 69.6, 25.6.
1-(4’-Cyanophenyl)ethanol (6e) [26]: 1H NMR (500 MHz, CDCl3) δ 7.65 (dd, J = 8.5, 1.0 Hz, 2H, aromatic), 7.49 (d, J = 8.0 Hz, 2H, aromatic), 4.97 (ddd, J = 13.0, 4.0, 2.5 Hz, CH(OH)CH3), 1.92 (d, J = 4.0 Hz, OH), 1.50 (d, J = 6.0 Hz, 3H, CH3). 13C{1H} NMR (100 MHz, CDCl3) δ 151.4, 132.2, 126.1, 118.9, 110.5, 69.3, 25.2.
Methyl-4-(1-hydroxyethyl)benzoate (6f) [27]: 1H NMR (500 MHz, CDCl3) δ. 8.00 (dt, J = 8.0, 2.0 Hz, 2H, aromatic), 7.43 (d, J = 8.0 Hz, 2H, aromatic), 4.95 (q, J = 5.5 Hz, 1H, CH(OH)CH3), 3.90 (s, 3H, C(O)OCH3), 2.19 (br, 1H, OH), 1.49 (d, J = 6.5 Hz, 3H, CH(OH)CH3). 13C{1H} NMR (125 MHz, CDCl3) δ 167.1, 151.1, 130.0, 129.3, 125.4, 70.1, 52.2, 25.4.
2,2,2-Trifluoro-1-phenylethanol (6g) [28]: 1H NMR (400 MHz, CDCl3) δ 7.52–7.45 (m, 2H, aromatic), 7.45-7.37 (m, 3H, aromatic), 5.03 (m, 1H, CHOHCF3), 2.60–2.58 (br, 1H, OH). 13C{1H} NMR (100 MHz, CDCl3) δ .134.0, 129.7, 128.8, 127.6, 124.3 (q, JCF3 = 280.1 Hz), 72.9 (q, JCF3 = 32.1 Hz).
1-Phenyl-1-propanol (6h) [29]: 1H NMR (500 MHz, CDCl3) δ 7.40–7.31 (m, 4H, aromatic), 7.29–7.23 (m, 1H, aromatic), 4.58 (t, J = 6.5 Hz, 1H, CH(OH)CH2CH3), 1.96–1.98 (br, 1H, OH), 1.86-1.70 (m, 2H, CH(OH)CH2CH3), 0.91 (t, J = 7.5 Hz, CH(OH)CH2CH3). 13C{1H} NMR (125 MHz, CDCl3) δ. 144.7, 128.5, 127.6, 126.1, 76.1, 32.0, 10.3.
4-Phenylbutan-2-ol (6i) [29]: 1H NMR (500 MHz, CDCl3) δ 7.31-7.26 (m, 2H, aromatic), 7.23–7.17 (m, 3H, aromatic), 3.84 (sep, J = 6.0 Hz, 1H, CH(OH)), 2.80-2.64 (m, 2H, CH2CH3), 1.85–1.72 (m, 2H, CH2), 1.34 (br, 1H, OH), 1.23 (d, J = 6.5 Hz, 3H, CH3). 13C{1H} NMR (125 MHz, CDCl3) δ 142.2, 128.5, 125.9, 67.5, 40.9, 32.2, 23.7.
Cyclohexanol (6j) [29]: 1H NMR (500 MHz, CDCl3) δ 3.61 (m, 1H, CH2CHOHCH2), 1.92–1.88 (m. 2H, CH2), 1.78–1.68 (m, 2H, CH2), 1.59–1.51 (m, 1H, CH2), 1.37 (s, 1H, CH2), 1.35–1.24 (m, 4H, CH2), 1.22–1.12 (m, 1H, CH2). 13C{1H} NMR (125 MHz, CDCl3) δ 70.4, 35.6, 25.5, 24.3.
Cycloheptanol (6k) [30]: 1H NMR (500 MHz, CDCl3) δ 3.85 (m, 1H, CH2CHOHCH2), 1.92 (m. 2H, CH2), 1.65 (m, 2H, CH2), 1.61–1.50 (m, 6H, CH2), 1.40 (m, 2H, CH2), 1.30 (br, 1H, OH). 13C{1H} NMR (100 MHz, CDCl3) δ 72.9, 37.7, 28.2, 22.7.
3.3.2. Conditions B
In a 5 mL stainless-steel reactor under argon atmosphere, catalyst 1 (10.6 mg, 0.020 mmol, 1.0 mol%), ketone (2.0 mmol), glucose (720.6 mg, 4.0 mmol, 2.0 equiv.), Na2CO3 (10.6 mg, 0.10 mmol, 5.0 mol%) and N,N-dimethylacetamide (3.0 mL) were placed. Then, the reactor was sealed with a stainless-steel stopper, and the mixture was stirred at 100 °C for 20 h. After cooling to room temperature, the reaction mixture was poured into water (50 mL) and the products were extracted with a mixed solvent having a volume ratio of hexane: AcOEt of 1: 1 (20 mL × 3). After evaporation of the solvent, the yield was determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard. The products were isolated by column chromatography (eluent = ethyl acetate/hexane). 1H and 13C{1H} NMR spectra of each isolated products are shown in Supplementary Materials.
1-(4’-Methoxyphenyl)ethanol (6l) [24]: 1H NMR (500 MHz, CDCl3) δ 7.31 (dt, J = 8.5, 2.0 Hz, 2H, aromatic), 6.89 (dt, J = 9.0, 2.0 Hz, 2H, aromatic), 4.86 (q, J = 6.0 Hz, 1H, CHOH), 3.81 (s, 3H, OMe), 1.78-1.75 (br, 1H, OH), 1.48 (d, J = 6.5 Hz, 3H, CH3). 13C{1H} NMR (125 MHz, CDCl3) δ 159.1, 138.1, 126.8, 113.9, 70.1, 55.4, 25.2.
1-(2’-Methoxyphenyl)ethanol (6m) [30]: 1H NMR (500 MHz, CDCl3) δ 7.34 (dd, J = 7.5, 1.5 Hz, 1H, aromatic), 7.28-7.22 (m, 1H, aromatic), 6.97 (td, J = 7.5, 1.0 Hz, 1H, aromatic), 6.88 (d, J = 8.0 Hz, 1H, aromatic), 5.09 (quint, J = 6.5 Hz, CH(OH)CH3), 3.87 (s, 3H, OMe), 2.69 (d, J = 5.0 Hz, OH), 1.51 (d, J = 7.0 Hz, CH(OH)CH3). 13C{1H} NMR (125 MHz, CDCl3) δ 156.7, 133.5, 128.4, 126.2, 120.9, 110.5, 66.7, 55.4, 22.9.
1-(4’-Chlorophenyl)ethanol (6n) [24]: 1H NMR (500 MHz, CDCl3) δ 7.35–7.27 (m, 4H, aromatic), 4.89 (m, 1H, CHOH), 1.91-1.84 (br, 1H, CHOH), 1.47 (d, J = 6.5 Hz, 3H, CH3). 13C{1H} NMR (125 MHz, CDCl3) δ 144.3, 133.2, 128.7, 126.9, 69.9, 25.4.
1-(3’-Chlorophenyl)ethanol (6o) [25]: 1H NMR (500 MHz, CDCl3) δ 7.37 (t, J = 2.0 Hz, 1H, aromatic), 7.30–7.22 (m, 3H, aromatic), 4.88 (qd, J = 6.5, 3.5 Hz, CH(OH)CH3), 1.92 (d, J = 3.5 Hz, 1H, OH), 1.48 (d, J = 6.5 Hz, 3H, CH3). 13C{1H} NMR (125 MHz, CDCl3) δ 148.0, 134.5, 129.9, 127.7, 125.8, 123.7, 70.0, 25.4.
1-(2’-Chlorophenyl)ethanol (6p) [31]: 1H NMR (500 MHz, CDCl3) δ 7.58 (dd, J = 8.0, 2.0 Hz, 1H, aromatic), 7.33–7.27 (m, 2H, aromatic), 7.20 (td, J = 8.0, 2.0 Hz, 1H, aromatic), 5.28 (qd, 6.5, 3.5 Hz, 1H, CH(OH)CH3), 2.13 (d, J = 4.0 Hz, 1H, OH), 1.48 (d, J = 6.5 Hz, CH3). 13C{1H} NMR (125 MHz, CDCl3) δ 143.2, 131.7, 129.5, 128.5, 127.3, 126.4, 67.1, 23.6.
1-(4’-Bromophenyl)ethanol (6q) [24]: 1H NMR (500 MHz, CDCl3) δ 7.46 (dt, J = 8.5, 2.5, 1.5 Hz, 2H, aromatic), 7.24 (d, J = 8.5 Hz, 2H, aromatic), 4.85 (q, J = 6.5 Hz, 1H, CH(OH)CH3), 1.98 (br, 1H, OH), 1.46 (d, J = 6.5 Hz, 3H, CH(OH)CH3). 13C{1H} NMR (125 MHz, CDCl3) δ 144.9, 131.7, 127.3, 121.3, 69.9, 25.4.
3.4. General Procedure for Transfer Hydrogenation of Aldehydes to the Corresponding Alcohols Using Glucose (Table 3)
3.4.1. Conditions A
In a 5 mL stainless-steel reactor under argon atmosphere, catalyst 1 (10.6 mg, 0.020 mmol, 1.0 mol%), aldehyde (2.0 mmol), glucose (720.6 mg, 4.0 mmol, 2.0 equiv.), Na2CO3 (10.6 mg, 0.10 mmol, 5.0 mol%) and degassed distilled water (3.0 mL) were placed. Then, the reactor was sealed with a stainless-steel stopper, and the mixture was stirred at 100 °C for 20 h. After cooling to room temperature, the products were extracted with dichloromethane (20 mL × 3). After evaporation of the solvent, the yields were determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard. The products were isolated by column chromatography (eluent = ethyl acetate/hexane). 1H and 13C{1H} NMR spectra of each isolated products are shown in Supplementary Materials.
Benzyl alcohol (8a) [32]: 1H NMR (500 MHz, CDCl3) δ 7.41–7.35 (m, 4H, aromatic), 7.33–7.28 (m, 1H, aromatic), 4.70 (d, J = 6.0 Hz, 2H, CH2(OH)), 1.75 (t, J = 6.0 Hz, 1H, OH).13C{1H} NMR (125 MHz, CDCl3) δ 141.0, 128.6, 127.7, 127.1, 65.2.
p-Methylbenzyl alcohol (8b) [32]: 1H NMR (500 MHz, CDCl3) δ 7.21 (d, J = 8.0 Hz, 2H, aromatic), 7.14 (d, J = 8.0 Hz, 2H, aromatic), 4.57 (d, J = 3.5 Hz, 2H, ArCH2OH), 2.33 (s, 3H, Me), 2.23–2.12 (br, 1H, OH).13C{1H} NMR (125 MHz, CDCl3) δ138.0, 137.4, 129.3, 127.2, 65.2, 21.2.
p-Cyanobenzyl alcohol (8c) [30]: 1H NMR (500 MHz, CDCl3) δ 7.62–7.57 (m, 2H, aromatic), 7.46–7.41 (m, 2H, aromatic), 4.73 (s, 2H, CH2), 2.61 (br, 1H, OH). 13C{1H} NMR (125 MHz, CDCl3) δ 146.5, 132.3, 127.1, 119.0, 110.9, 64.1.
m-Nitrobenzyl alcohol (8d) [33]: 1H NMR (500 MHz, CDCl3) δ 8.26 (s, 1H, aromatic), 8.16 (dd, J = 8.5, 1.0 Hz, 1H, aromatic), 7.71 (dd, J = 8.0, 1.0 Hz, 1H, aromatic), 7.54 (t, J = 8.0 Hz, 1H, aromatic), 4.84 (s, 2H, CH2), 1.95 (br, 1H, OH). 13C{1H} NMR (125 MHz, CDCl3) δ 148.4, 143.0, 132.8, 129.6, 122.6, 121.6, 64.0.
o-Nitrobenzyl alcohol (8e) [33]: 1H NMR (500 MHz, CDCl3) δ 8.12 (dd, J = 8.0, 1.0 Hz, 1H, aromatic), 7.75 (d, J = 7.0 Hz, 1H, aromatic), 7.69 (td, J = 7.5, 1.0 Hz, 1H, aromatic), 7.49 (td, J = 8.0, 1.0 Hz, 1H, aromatic), 4.99 (d, J = 6.0 Hz, 2H, –CH(OH)–), 2.53 (t, J = 7.0 Hz, 1H, OH).13C{1H} NMR (125 MHz, CDCl3) δ 147.7, 136.9, 134.3, 130.2, 128.7, 125.2, 62.7.
Methyl-4-(hydroxymethyl)benzoate (8f) [34]: 1H NMR (500 MHz, CDCl3) δ 7.95 (d, J = 8.0 Hz, 2H, aromatic), 7.37 (d, J = 8.0 Hz, 2H, aromatic), 4.69 (s, 2H, CH2(OH)), 3.88 (s, 3H, OCH3), 3.21 (br, 1H, OH). 13C{1H} NMR (125 MHz, CDCl3) δ 167.2, 146.3, 129.8, 129.0, 126.4, 64.4, 52.2.
3.4.2. Conditions B
In a 5 mL stainless-steel reactor under argon atmosphere, catalyst 1 (10.6 mg, 0.020 mmol, 1.0 mol%), aldehyde (2.0 mmol), glucose (720.6 mg, 4.0 mmol, 2.0 equiv.), Na2CO3 (10.6 mg, 0.10 mmol, 5.0 mol%) and N,N-dimethylacetamide (3.0 mL) were placed. Then, the reactor was sealed with a stainless-steel stopper, and the mixture was stirred at 100 °C for 20 h. After cooling to room temperature, the reaction mixture was poured into water (50 mL) and the products were extracted with a mixed solvent having a volume ratio of hexane: AcOEt of 1:1 (20 mL × 3). After evaporation of the solvent, the yield was determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard. The products were isolated by column chromatography (eluent = ethyl acetate/hexane). 1H and 13C{1H} NMR spectra of each isolated products are shown in Supplementary Materials.
p-Methoxybenzyl alcohol (8g) [32]: 1H NMR (500 MHz, CDCl3) δ 7.28 (dt, J = 9.0, 3.0, 2.0 Hz, 2H, aromatic), 6.89 (dt, J = 8.5, 3.0, 2.0 Hz, 2H, aromatic), 4.60 (s, 2H, CH2), 3.80 (s, 3H, OMe), 1.87 (br, 1H, OH). 13C{1H} NMR (125 MHz, CDCl3) δ 159.2, 133.2, 128.8, 114.0, 65.1, 55.4.
p-Chlorobenzyl alcohol (8h) [32]: 1H NMR (500 MHz, CDCl3) δ 7.34–7.23 (m, 4H, aromatic), 4.62 (s, 2H, ArCH2OH), 2.21 (br, 1H, ArCH2OH).13C{1H} NMR (125 MHz, CDCl3) δ 139.3, 133.4, 128.8, 128.4, 64.6.
p-Bromobenzyl alcohol (8i) [30]: 1H NMR (500 MHz, CDCl3) δ 7.48 (dt, J = 8.5, 2.0 Hz, 2H, aromatic), 7.23 (d, J = 8.5 Hz, 2H, aromatic), 4.65 (s, 2H, CH2), 1.87 (br, 1H, OH). 13C{1H} NMR (125 MHz, CDCl3) δ 139.8, 131.7, 128.7, 121.6, 64.7.
p-tert-Butylbenzyl alcohol (8j) [35]: 1H NMR (500 MHz, CDCl3) δ 7.39 (d, J = 8.0 Hz, 2H, aromatic), 7.30 (d, J = 8.5 Hz, 2H, aromatic), 4.64 (d, J = 1.5 Hz, 2H, CH2), 1.32 (d, J = 2.0 Hz, 9H, C(CH3)3). 13C{1H} NMR (125 MHz, CDCl3) δ 150.8, 138.0, 127.0, 125.6, 65.2, 34.6, 31.5.
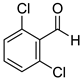
2,6-Dichlorobenzyl alcohol (8k) [36]: 1H NMR (500 MHz, CDCl3) δ 7.34–7.28 (m, 2H, aromatic), 7.18 (m, 1H, aromatic), 4.95 (d, J = 3.5 Hz, CH2OH), 2.31 (br, 1H, OH). 13C{1H} NMR (125 MHz, CDCl3) δ 136.0, 135.7, 129.9, 128.5, 60.2.
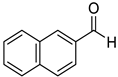
2-Naphthalenemethanol (8l) [37]: 1H NMR (500 MHz, CDCl3) δ 7.83–7.75 (m, 3H, aromatic), 7.72 (s, 1H, aromatic), 7.49–7.38 (m, 3H, aromatic), 4.76 (s, 2H, CH2), 2.33 (br, 1H, OH). 13C{1H} NMR (125 MHz, CDCl3) δ 138.3, 133.4, 133.0, 128.3, 128.0, 127.8, 126.2, 126.0, 125.5, 125.3, 65.4.
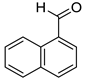
1-Naphthalenemethanol (8m) [33]: 1H NMR (500 MHz, CDCl3) δ 8.04 (d J = 8.0 Hz, 1H, aromatic), 7.88–7.74 (m, 2H, aromatic), 7.54–7.36 (m, 4H, aromatic), 5.05 (s, 2H, CH2), 2.30–2.10 (br, 1H, OH). 13C{1H} NMR (125 MHz, CDCl3) δ 136.3, 133.8, 131.3, 128.7, 128.6, 126.4, 126.0, 125.5, 125.4, 123.7, 63.6.
3.5. Preparation of 2,3,4,6-tetra-O-methyl-D-glucopyranose (10). (Equation 2)
In a two-necked round-bottomed flask, aqueous NaOH (50 wt%, 4.0 mL), methyl α-D-glucopyranoside (9) (1.94 g, 10.0 mmol) and DMSO (35 mL) were placed. After stirring the mixture at room temperature for 5 min, iodomethane (3.3 mL, 50 mmol) was added. The mixture was stirred at room temperature for 4 h. The reaction mixture was poured into water (100 mL) and extracted with Et2O. An intermediate product was obtained after evaporation of the organic layer. (colorless oil, 1.64 g, 6.5 mmol, 65% yield).
In a round-bottomed flask, above intermediate product (1.64 g, 6.5 mmol) and aqueous HCl (9.6 M, 25 mL) were placed. The mixture was stirred at 60 °C for 16 h. After cooling to room temperature, the crude product was obtained by evaporation of the reaction mixture. After purifying by column chromatography (eluent = EtOH/CH2Cl2), the product 10 was obtained (653.5 mg, 2.8 mmol, 43% yield).
2,3,4,6-tetra-O-methyl-D-glucopyranose (10) [38]:1H NMR (500 MHz, CDCl3) δ 5.31 (d, J = 3.5 Hz, 1H), 4.56 (d, J = 7.5 Hz, 0.5H), 3.91 (dt, J = 10.5 Hz, 2.5 Hz, 1H), 3.70 (q, J = 7.0 Hz, 1H), 3.66–3.60 (m, 6H), 3.59–3.5 (m, 6H), 3.53–3.50 (m, 4H), 3.42–3.30 (m, 5H,), 3.22–3.05 (m, 3H), 2.97 (dd, J = 9.0, 8.0 Hz, 0.5 H). 13C NMR (125 MHz, CDCl3) δ 96.9, 90.5, 86.4, 84.7, 83.1, 81.9, 79.7, 79.7, 74.1, 71.6, 71.4, 69.6, 60.9, 60.8, 60.5, 60.4, 59.1, 58.7.
3.6. Reaction of Acetophenone Using α-D-glucopyranoside (9) (Equation 1)
In a 5 mL stainless-steel reactor under argon atmosphere, catalyst 1 (1.0 mg, 0.002 mmol, 0.1 mol% Ir), acetophenone (240.5 mg, 2.0 mmol), α-D-glucopyranoside (777.3 mg, 4.0 mmol), Na2CO3 (10.5 mg, 0.1 mmol, 5.0 mol%) and degassed distilled water (3.0 mL) were placed. Then, the reactor was sealed with a stainless-steel stopper, and the mixture was stirred at 100 °C for 20 h. After cooling to room temperature, the mixture was diluted with toluene (50 mL). The conversion of acetophenone and the yield of 1-phenylethanol were determined by GC analysis using biphenyl as an internal standard. No reaction occurred.
3.7. Reaction of Acetophenone Using 2,3,4,6-tetra-O-methyl-D-glucopyranose (10) (Equation 2)
In a 5 mL stainless-steel reactor under argon atmosphere, catalyst 1 (1.1 mg, 0.002 mmol, 0.2 mol%Ir), acetophenone (120.6 mg, 1.0 mmol), 2,3,4,6-tetra-O-methyl-D-glucopyranose (472.4 mg, 2.0 mmol, 2.0 equiv.), Na2CO3 (5.4 mg, 0.05 mmol, 5 mol%) and degassed distilled water (1.5 mL) were placed. Then, the reactor was sealed with a stainless-steel stopper, and the mixture was stirred at 100 °C for 20 h. After cooling to room temperature, the mixture was diluted with toluene (25 mL). The conversion of acetophenone and the yield of 1-phenylethanol were determined by GC analysis using biphenyl as an internal standard. The conversion and the yield were 97% and 97%, respectively.
4. Conclusions
In conclusion, we developed a new system for transfer hydrogenation of various ketones and aldehydes using glucose as a hydrogen donor catalyzed by a small amount of iridium complex (0.1 to 1 mol% Ir). It should be noted that environmentally benign water could be used as a solvent in the present catalytic system for various carbonyl substrates. To the best of our knowledge, the results disclosed in this paper were the first example of transfer hydrogenation in water using glucose as a hydrogen donor. Furthermore, the reaction scope could be extended by using DMAc as a reaction solvent instead of water.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/6/503/s1, detailed description of experimental procedures, 1H and 13C{1H} NMR data of the isolated products with spectral charts.
Author Contributions
M.Y. performed the experiments, analyzed the results, and wrote the draft of the manuscript. R.Y. and T.I. performed the experiments. T.S. supported the analysis of the experimental results and the writing of the manuscript. K.F. guided the research, designed the experiments, and wrote the manuscript.
Funding
This work was supported by The Research Grant against Global Warming of the Ichimura Foundation for New Technology. This work was also financially supported by JSPS KAKENHI Grant Number JP18H04255, JP19H02715, and JP19H05053.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dupau, P. Ruthenium-Catalyzed Selective Hydrogenation for Flavor and Fragrance Applications. In Organometallics as Catalysts in the Fine Chemical Industry; Beller, M., Blaser, H.-U., Eds.; Topics in Organometallic Chemistry; Springer: Berlin/Heidelberg, Germany, 2012; pp. 47–63. ISBN 978-3-642-32833-6. [Google Scholar]
- Štefane, B.; Požgan, F. Metal-Catalysed Transfer Hydrogenation of Ketones. Top. Curr. Chem. 2016, 374, 1–67. [Google Scholar]
- Brieger, G.; Nestrick, T.J. Catalytic transfer hydrogenation. Chem. Rev. 1974, 74, 567–580. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef] [PubMed]
- Meerwein, H.; Schmidt, R. Ein neues Verfahren zur Reduktion von Aldehyden und Ketonen. Liebigs Ann. 1925, 444, 221–238. [Google Scholar] [CrossRef]
- Li, J.J. Meerwein–Ponndorf–Verley reduction. In Name Reactions: A Collection of Detailed Mechanisms and Synthetic Applications; Li, J.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 345–346. ISBN 978-3-642-01053-8. [Google Scholar]
- Saidi, O.; Williams, J.M.J. Iridium-Catalyzed Hydrogen Transfer Reactions. In Iridium Catalysis; Andersson, P.G., Ed.; Topics in Organometallic Chemistry; Springer: Berlin/Heidelberg, Germany, 2011; pp. 77–106. ISBN 978-3-642-15334-1. [Google Scholar]
- Wang, C.; Wu, X.; Xiao, J. Broader, Greener, and More Efficient: Recent Advances in Asymmetric Transfer Hydrogenation. Chem. Asian J. 2008, 3, 1750–1770. [Google Scholar] [CrossRef] [PubMed]
- Noyori, R.; Hashiguchi, S. Asymmetric Transfer Hydrogenation Catalyzed by Chiral Ruthenium Complexes. Acc. Chem. Res. 1997, 30, 97–102. [Google Scholar] [CrossRef]
- Ikariya, T.; Blacker, A.J. Asymmetric Transfer Hydrogenation of Ketones with Bifunctional Transition Metal-Based Molecular Catalysts. Acc. Chem. Res. 2007, 40, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Miecznikowski, J.R.; Crabtree, R.H. Hydrogen Transfer Reduction of Aldehydes with Alkali-Metal Carbonates and Iridium NHC Complexes. Organometallics 2004, 23, 629–631. [Google Scholar] [CrossRef]
- Wang, R.; Tang, Y.; Xu, M.; Meng, C.; Li, F. Transfer Hydrogenation of Aldehydes and Ketones with Isopropanol under Neutral Conditions Catalyzed by a Metal–Ligand Bifunctional Catalyst [Cp*Ir(2,2′-bpyO)(H2O)]. J. Org. Chem. 2018, 83, 2274–2281. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, L. Industrial Catalysts. In Handbook of Industrial Catalysts; Lloyd, L., Ed.; Fundamental and Applied Catalysis; Springer: Boston, MA, USA, 2011; pp. 1–22. ISBN 978-0-387-49962-8. [Google Scholar]
- Simon, M.-O.; Li, C.-J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef]
- Manna, S.; Antonchick, A.P. Catalytic Transfer Hydrogenation Using Biomass as Hydrogen Source. ChemSusChem. in press. [CrossRef] [PubMed]
- Fujita, K.; Tanino, N.; Yamaguchi, R. Ligand-Promoted Dehydrogenation of Alcohols Catalyzed by Cp*Ir Complexes. A New Catalytic System for Oxidant-Free Oxidation of Alcohols. Org. Lett. 2007, 9, 109–111. [Google Scholar] [CrossRef]
- Fujita, K.; Yoshida, T.; Imori, Y.; Yamaguchi, R. Dehydrogenative Oxidation of Primary and Secondary Alcohols Catalyzed by a Cp*Ir Complex Having a Functional C,N-Chelate Ligand. Org. Lett. 2011, 13, 2278–2281. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, R.; Fujita, K.; Yamaguchi, R. Dehydrogenative Oxidation of Alcohols in Aqueous Media Using Water-Soluble and Reusable Cp*Ir Catalysts Bearing a Functional Bipyridine Ligand. J. Am. Chem. Soc. 2012, 134, 3643–3646. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, R.; Fujita, K.I.; Yamaguchi, R. Cooperative Catalysis by Iridium Complexes with a Bipyridonate Ligand: Versatile Dehydrogenative Oxidation of Alcohols and Reversible Dehydrogenation–Hydrogenation between 2-Propanol and Acetone. Angew. Chem. Int. Ed. 2012, 51, 12790–12794. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Uejima, T.; Yamaguchi, R. Hydrogen-transfer Oxidation of Primary Alcohols Catalyzed by Iridium Complexes Bearing a Functional Pyridonate Ligand Using Isopropenyl Acetate as a Hydrogen Acceptor. Chem. Lett. 2013, 42, 1496–1498. [Google Scholar] [CrossRef]
- Ball, R.G.; Graham, W.A.G.; Heinekey, D.M.; Hoyano, J.K.; McMaster, A.D.; Mattson, B.M.; Michel, S.T. Synthesis and structure of dicarbonylbis(η-pentamethylcyclopentadienyl)diiridium. Inorg. Chem. 1990, 29, 2023–2025. [Google Scholar] [CrossRef]
- Kuwahara, M.; Nishioka, M.; Yoshida, M.; Fujita, K. A Sustainable Method for the Synthesis of Acetic Acid Based on Dehydrogenation of an Ethanol–Water Solution Catalyzed by an Iridium Complex Bearing a Functional Bipyridonate Ligand. ChemCatChem 2018, 10, 3636–3640. [Google Scholar] [CrossRef]
- Fujita, K.; Wada, T.; Shiraishi, T. Reversible Interconversion between 2,5-Dimethylpyrazine and 2,5-Dimethylpiperazine by Iridium-Catalyzed Hydrogenation/Dehydrogenation for Efficient Hydrogen Storage. Angew. Chem. Int. Ed. 2017, 56, 10886–10889. [Google Scholar] [CrossRef]
- Nogueira Fernandes, J.L.; de Souza, M.C.; Brenelli, E.C.S.; Brenelli, J.A. Reduction of Acetophenones Using Borohydride Exchange Resins (BER) and a BER-Lithium Salt System. Synthesis 2009, 2009, 4058–4062. [Google Scholar]
- Yamamoto, Y.; Hasegawa, H.; Yamataka, H. Dynamic Path Bifurcation in the Beckmann Reaction: Support from Kinetic Analyses. J. Org. Chem. 2011, 76, 4652–4660. [Google Scholar] [CrossRef] [PubMed]
- Bastin, S.; Eaves, R.J.; Edwards, C.W.; Ichihara, O.; Whittaker, M.; Wills, M. A Soluble-Polymer System for the Asymmetric Transfer Hydrogenation of Ketones. J. Org. Chem. 2004, 69, 5405–5412. [Google Scholar] [CrossRef]
- Rahaim, R.J.; Maleczka, R.E. C−O Hydrogenolysis Catalyzed by Pd-PMHS Nanoparticles in the Company of Chloroarenes. Org. Lett. 2011, 13, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Hevia, E.; Kennedy, A.R.; Klett, J.; Livingstone, Z.; McCall, M.D. New insights into addition reactions of dialkylzinc reagents to trifluoromethyl ketones: Structural authentication of a β-hydride elimination product containing a tetranuclear zinc chain. Dalton Trans. 2009, 39, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Maytum, H.C.; Francos, J.; Whatrup, D.J.; Williams, J.M.J. 1,4-Butanediol as a Reducing Agent in Transfer Hydrogenation Reactions. Chem. Asian J. 2010, 5, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.C.M.; Bézier, D.; Sortais, J.-B.; Darcel, C. Iron Dihydride Complex as the Pre-catalyst for Efficient Hydrosilylation of Aldehydes and Ketones Under Visible Light Activation. Adv. Synth. Catal. 2011, 353, 1279–1284. [Google Scholar] [CrossRef]
- Azerraf, C.; Gelman, D. New Shapes of PC(sp3)P Pincer Complexes. Organometallics 2009, 28, 6578–6584. [Google Scholar] [CrossRef]
- Koren-Selfridge, L.; Londino, H.N.; Vellucci, J.K.; Simmons, B.J.; Casey, C.P.; Clark, T.B. A Boron-Substituted Analogue of the Shvo Hydrogenation Catalyst: Catalytic Hydroboration of Aldehydes, Imines, and Ketones. Organometallics 2009, 28, 2085–2090. [Google Scholar] [CrossRef]
- Basu, B.; Mandal, B.; Das, S.; Das, P.; Nanda, A.K. Chemoselective reduction of aldehydes by ruthenium trichloride and resin-bound formates. Beilstein J. Org. Chem. 2008, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, N.S.; Junge, K.; Beller, M. A Convenient and General Iron-Catalyzed Hydrosilylation of Aldehydes. Org. Lett. 2007, 9, 5429–5432. [Google Scholar] [CrossRef]
- Dieskau, A.P.; Begouin, J.-M.; Plietker, B. Bu4N[Fe(CO)3(NO)]-Catalyzed Hydrosilylation of Aldehydes and Ketones. Eur. J. Org. Chem. 2011, 2011, 5291–5296. [Google Scholar] [CrossRef]
- Pouchert, C.J.; Behnke, J. The Aldrich Library of 13C and 1H FT NMR Spectra, 1st ed.; Aldrich Chemical Company Inc.: St. Louis, MI, USA, 1993; Volume 2, p. 357B. [Google Scholar]
- Bhattacharya, P.; Krause, J.A.; Guan, H. Iron Hydride Complexes Bearing Phosphinite-Based Pincer Ligands: Synthesis, Reactivity, and Catalytic Application in Hydrosilylation Reactions. Organometallics 2011, 30, 4720–4729. [Google Scholar] [CrossRef]
- Xu, G.; Moeller, K.D. Anodic Coupling Reactions and the Synthesis of C-Glycosides. Org. Lett. 2010, 12, 2590–2593. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).