Modeling and Simulations of NOx and SO2 Seawater Scrubbing in Packed-Bed Columns for Marine Applications
Abstract
:1. Introduction
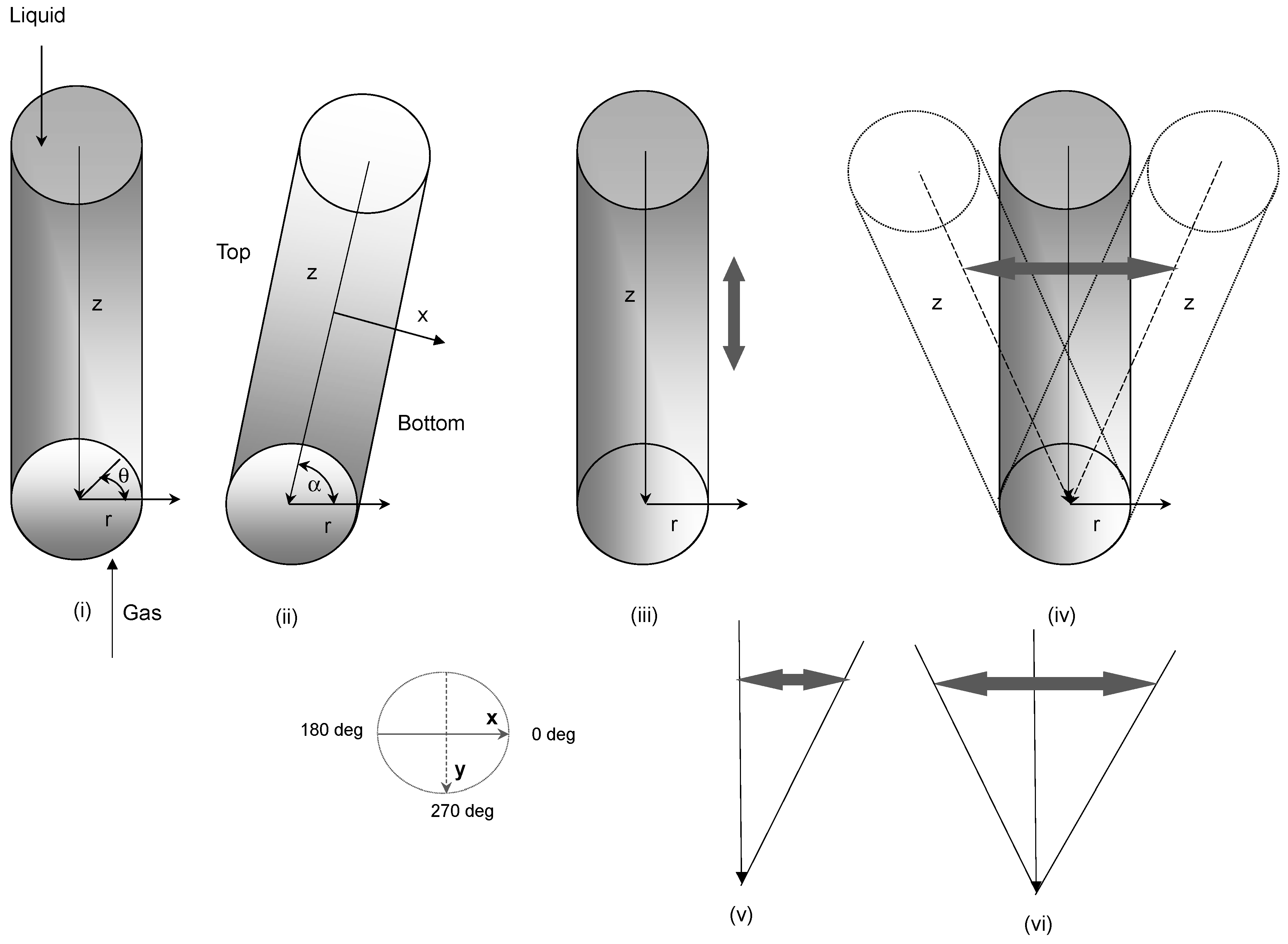
2. Mathematical Model
2.1. Hydrodynamic Model
2.2. Mass and Energy Transport Model
2.2.1. Nitrogen Oxides Absorption Mechanism
2.2.2. SO2 Absorption Mechanism
2.2.3. CO2 Absorption Mechanism
2.2.4. Mass Conservative Equations
2.2.5. Energy Balance Equations
2.3. Model Parameters
2.4. Numerical Implementation
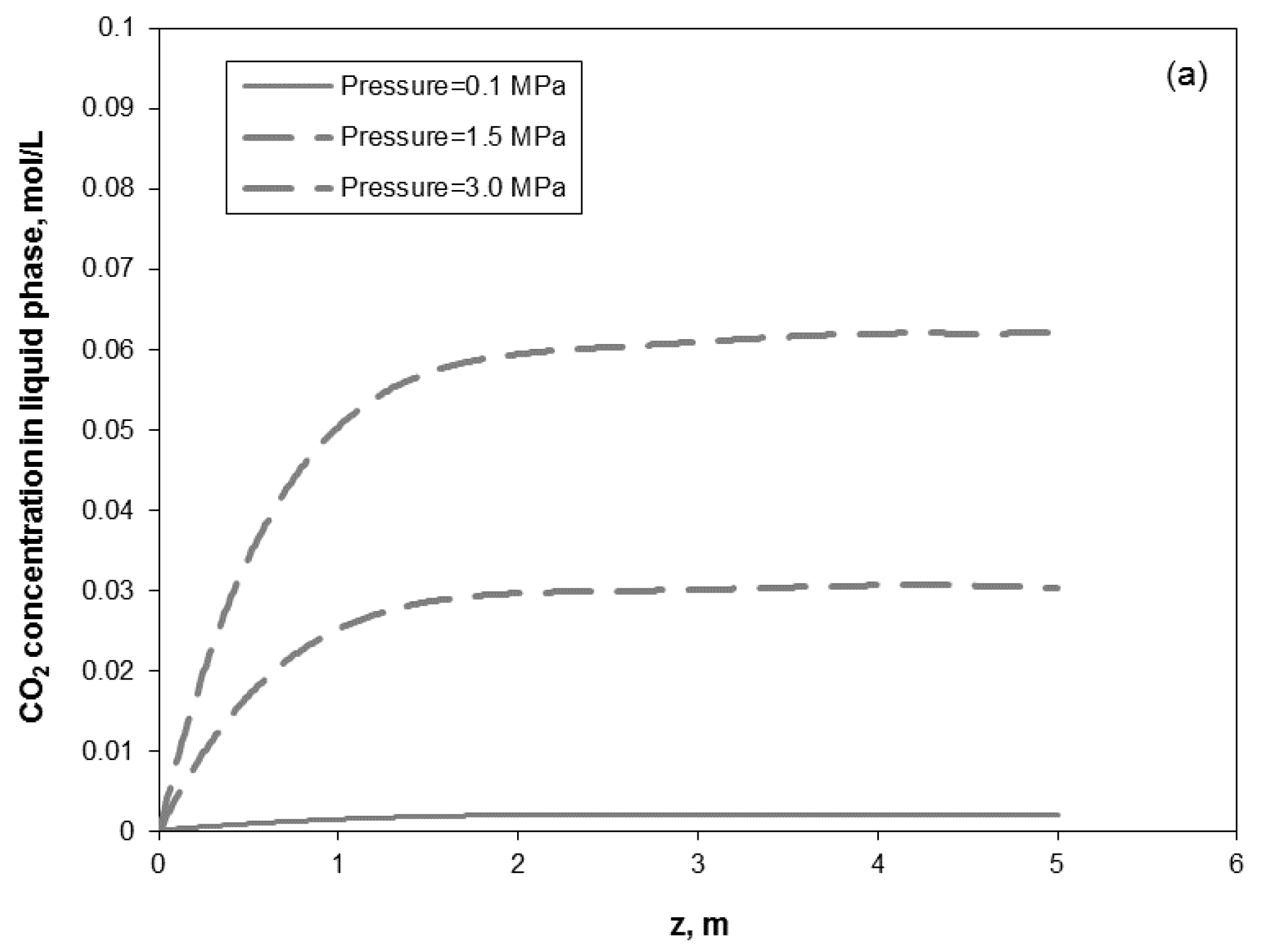
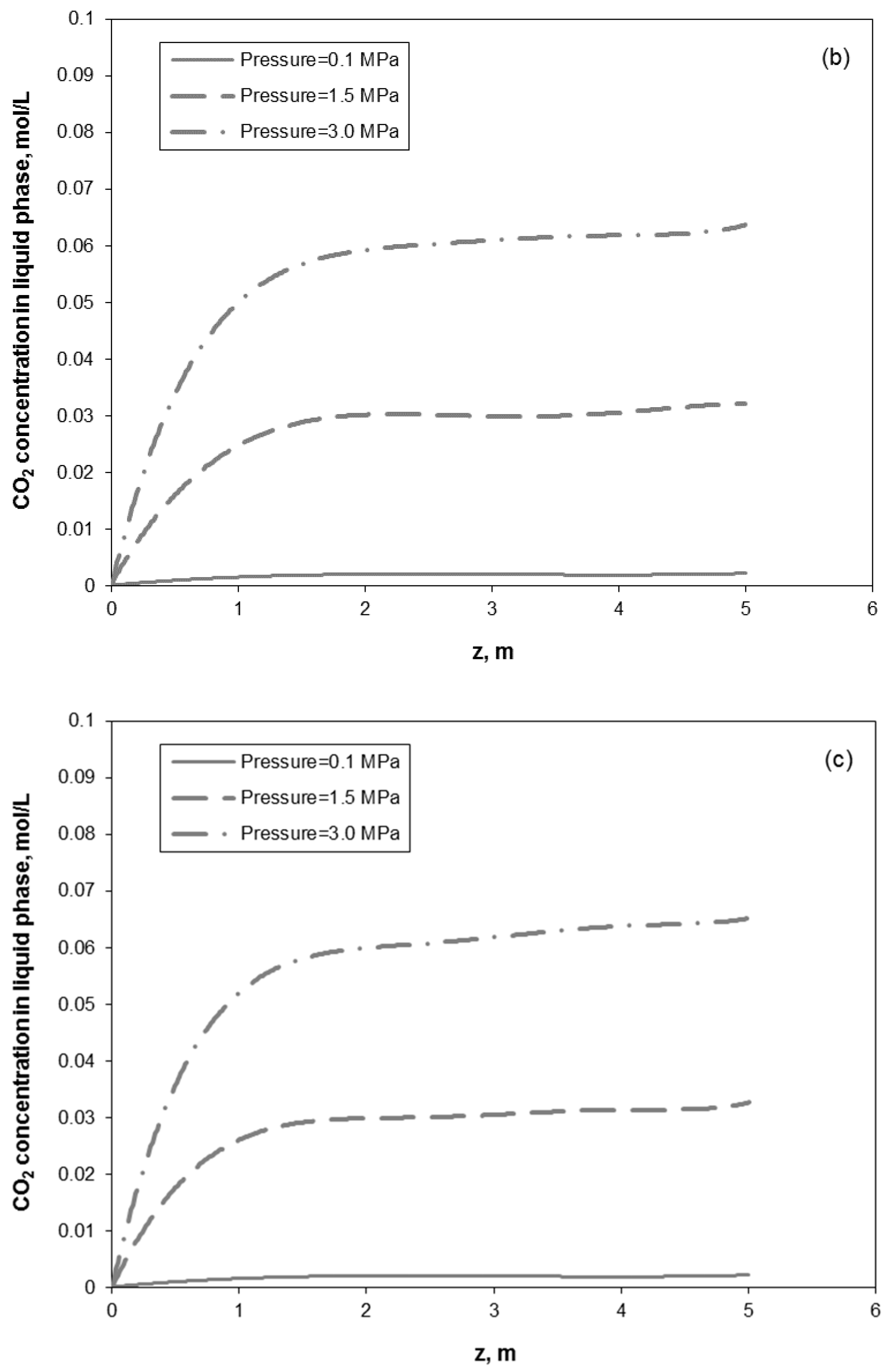
3. Results and Discussion
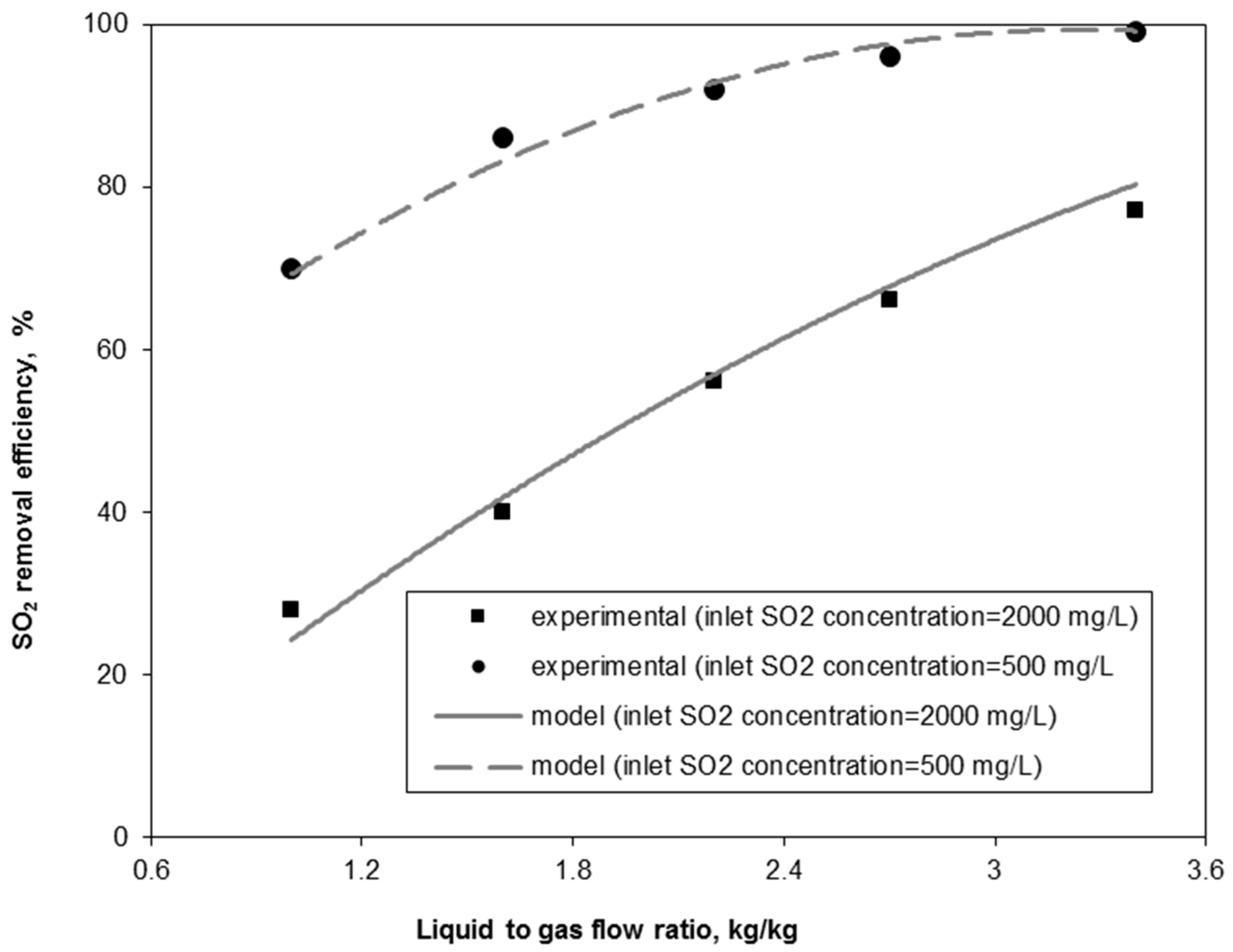
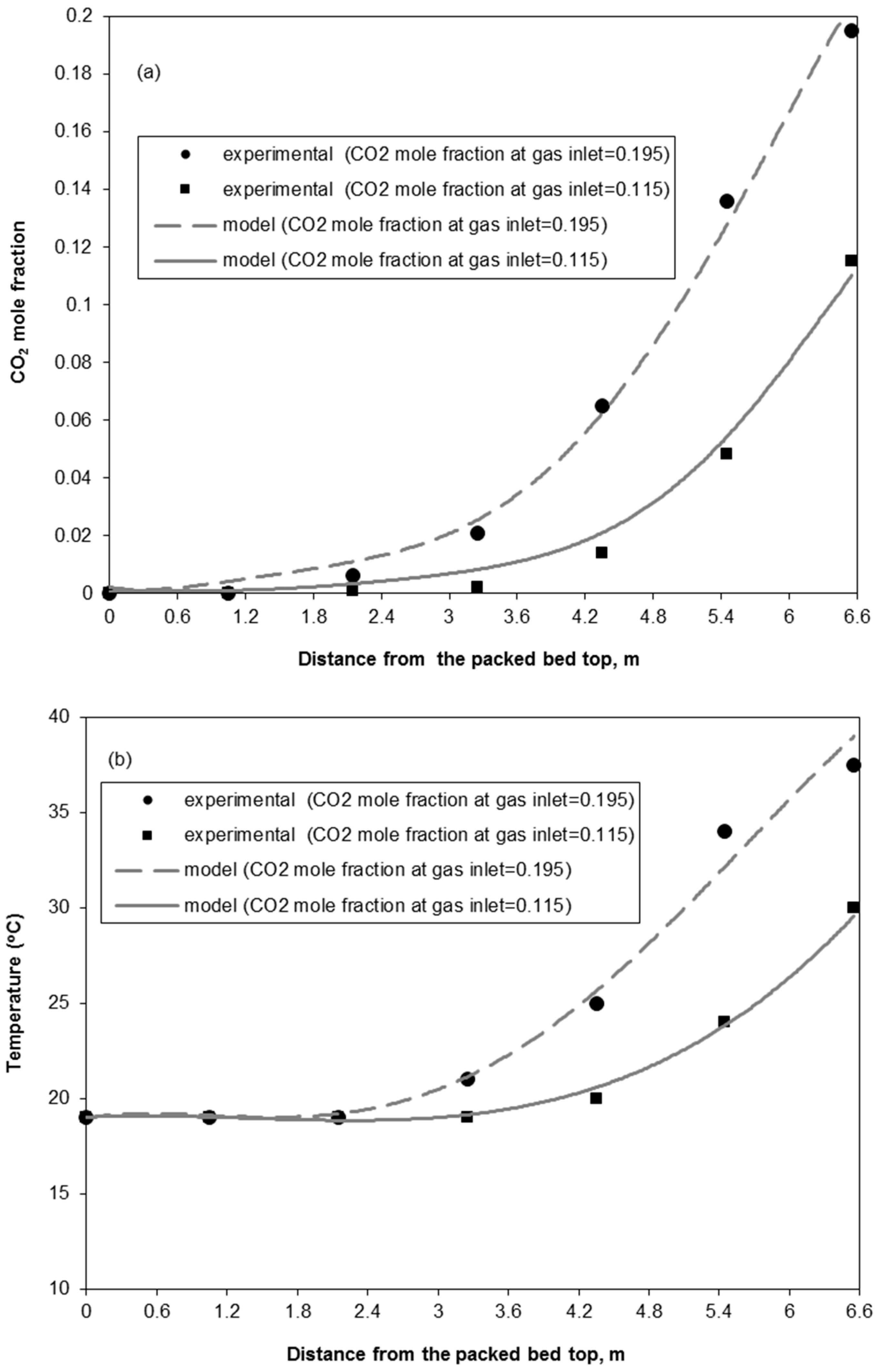
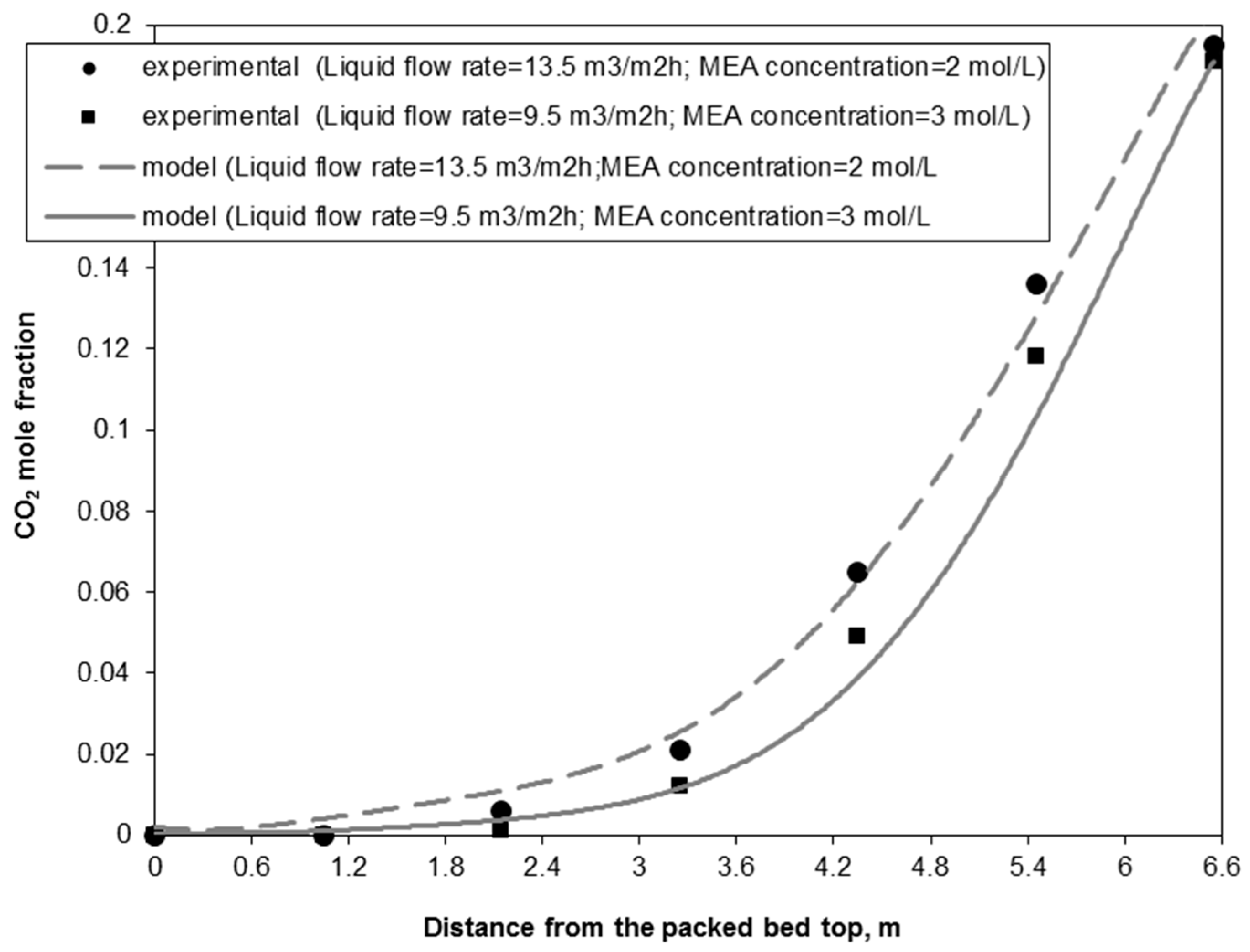
3.1. Model Experimental Validation
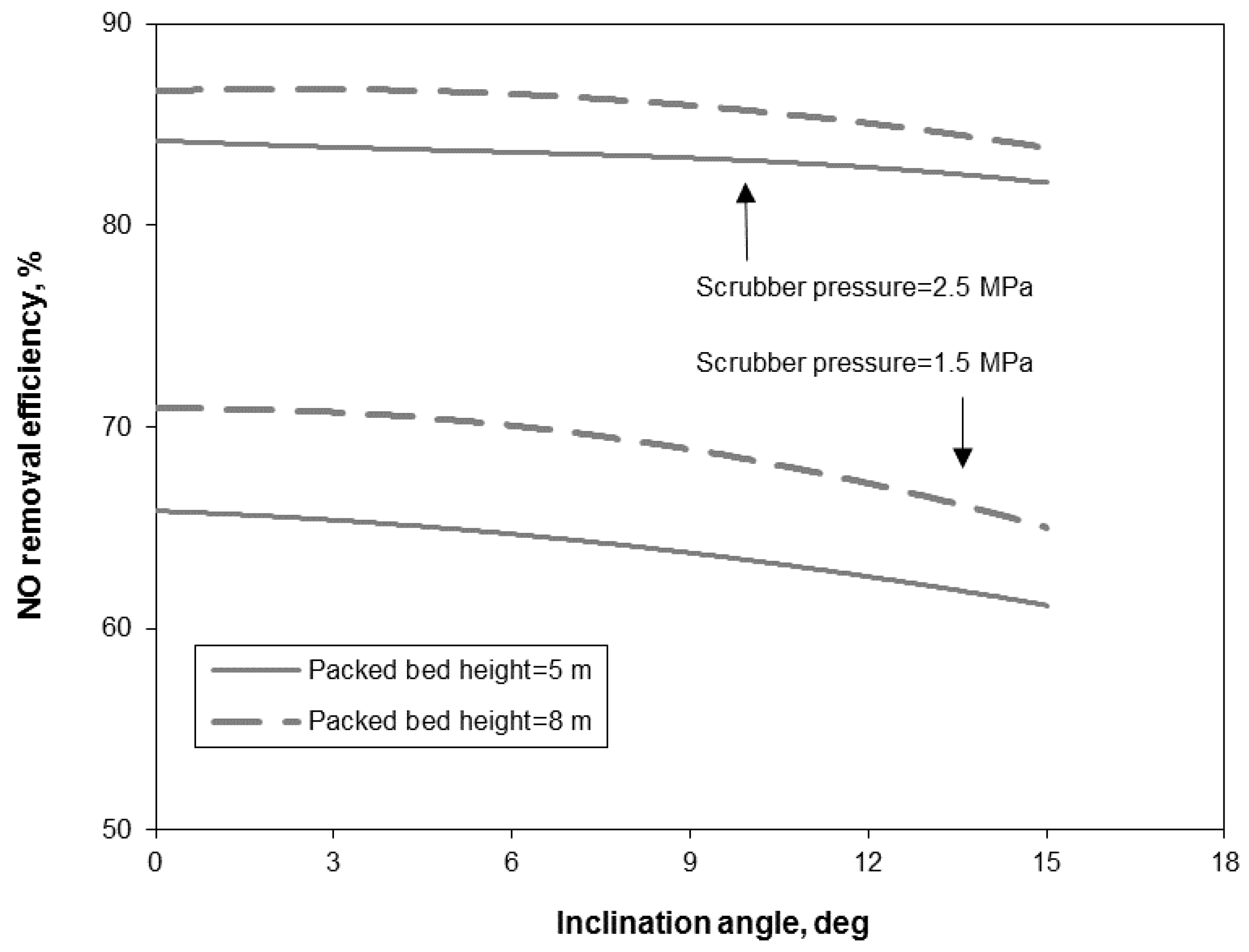
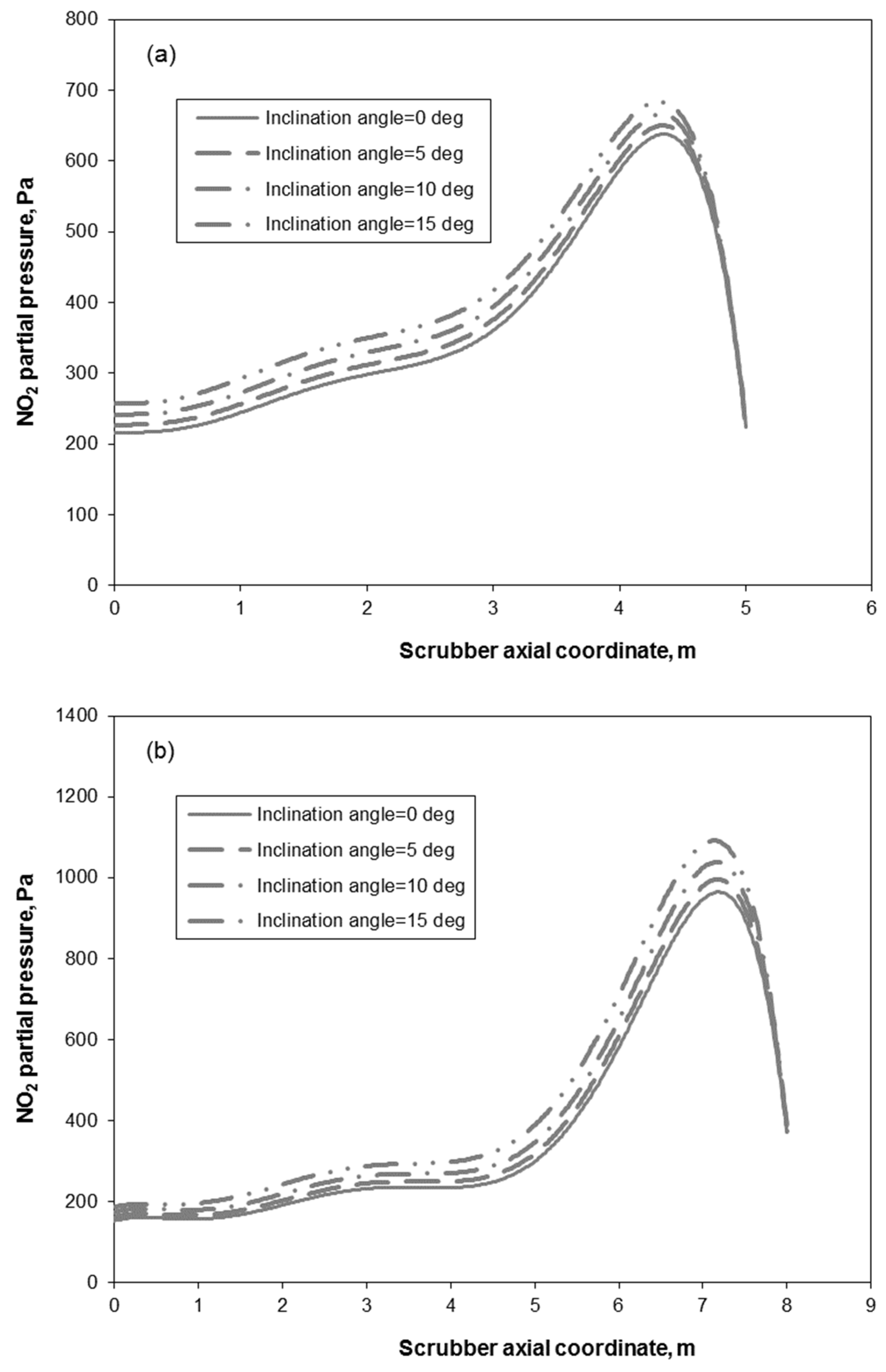
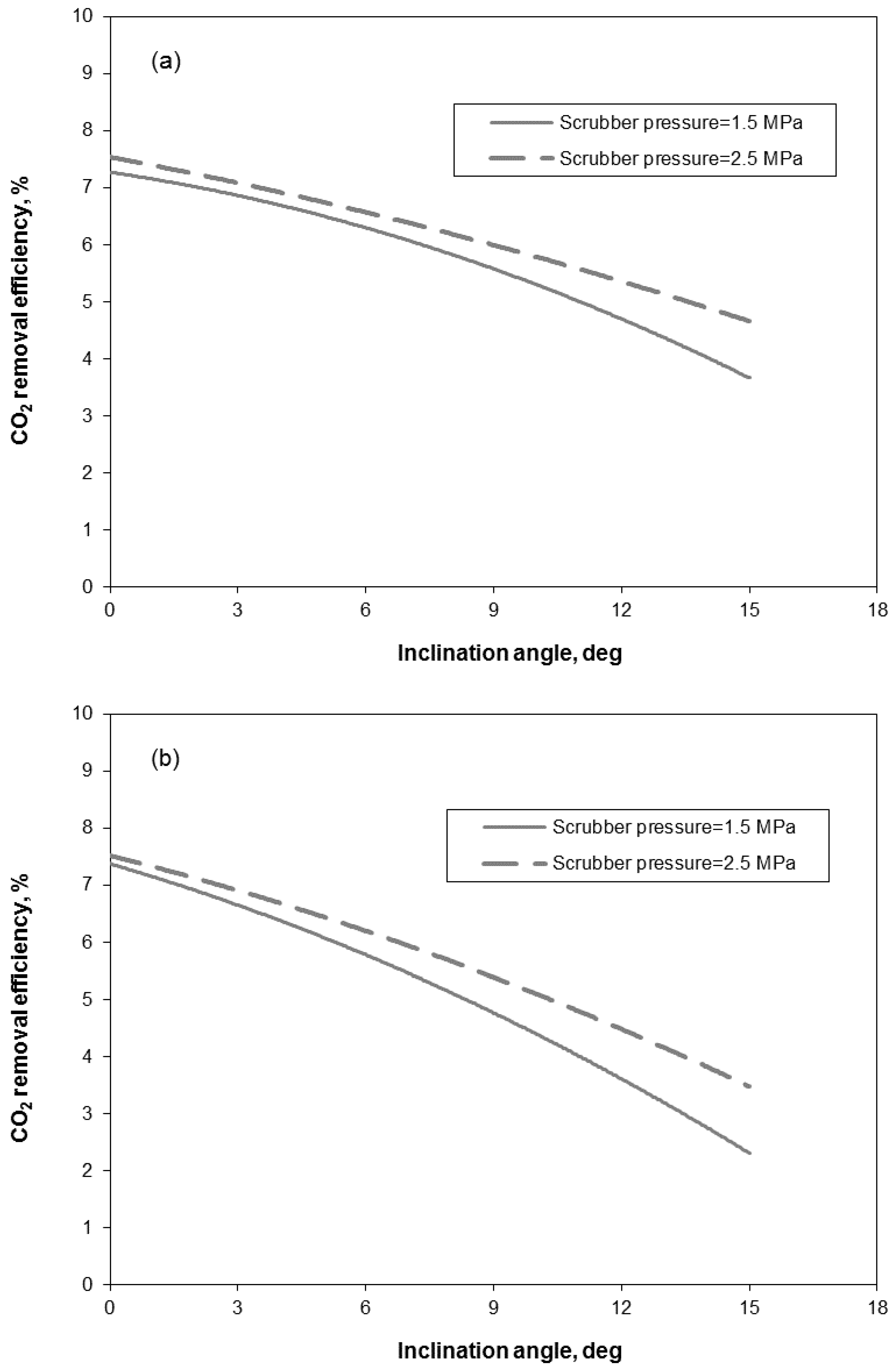
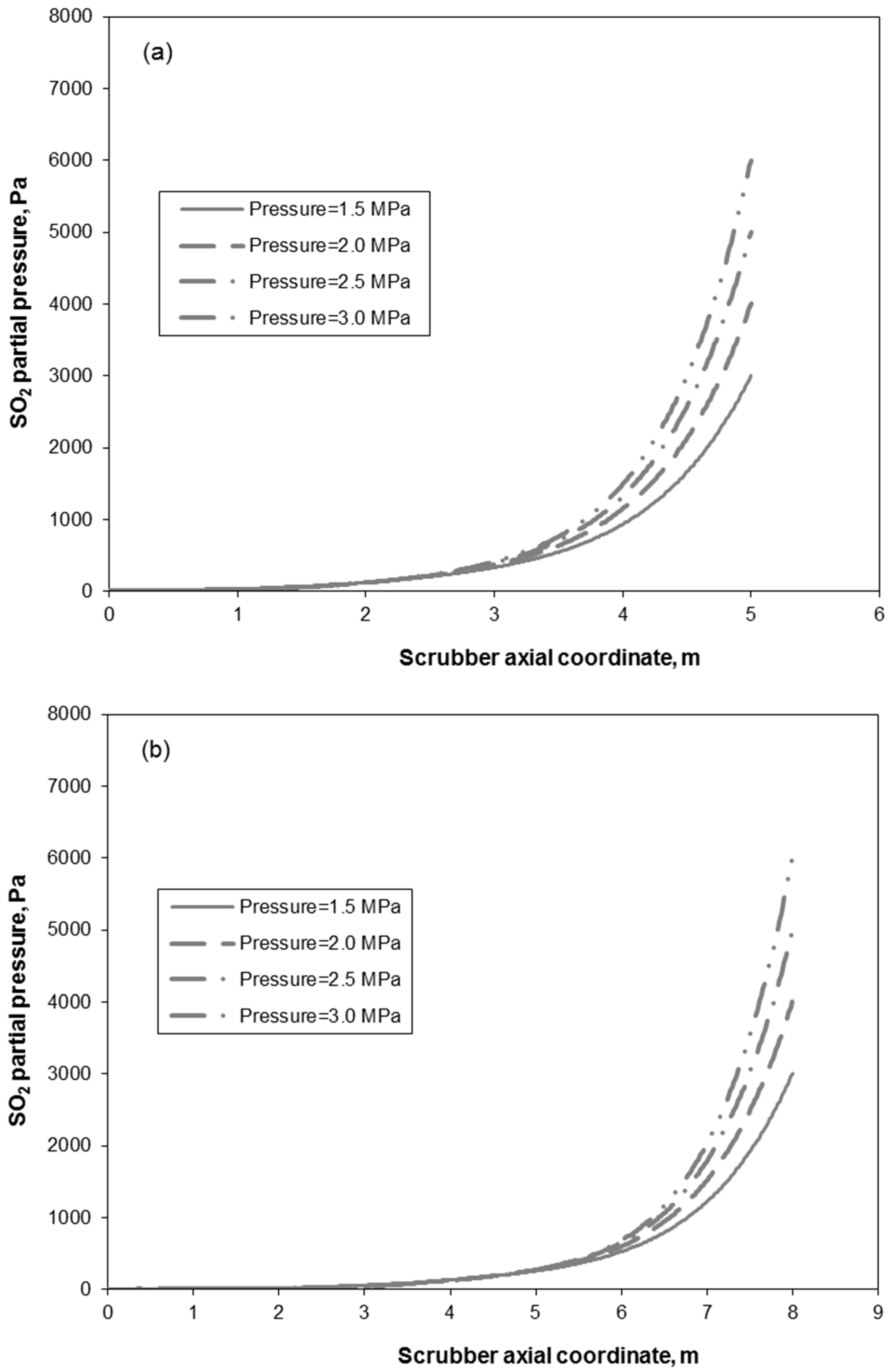
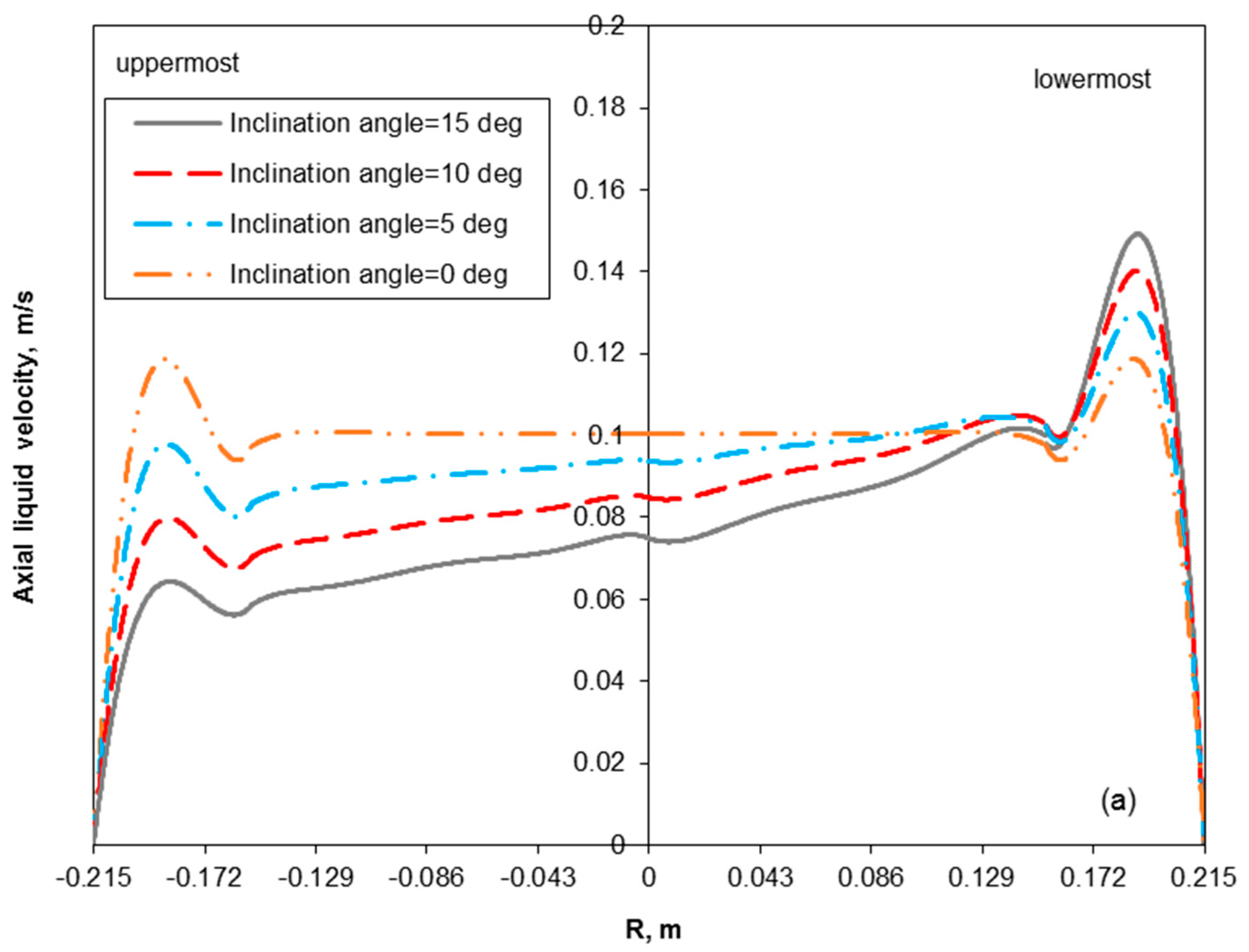
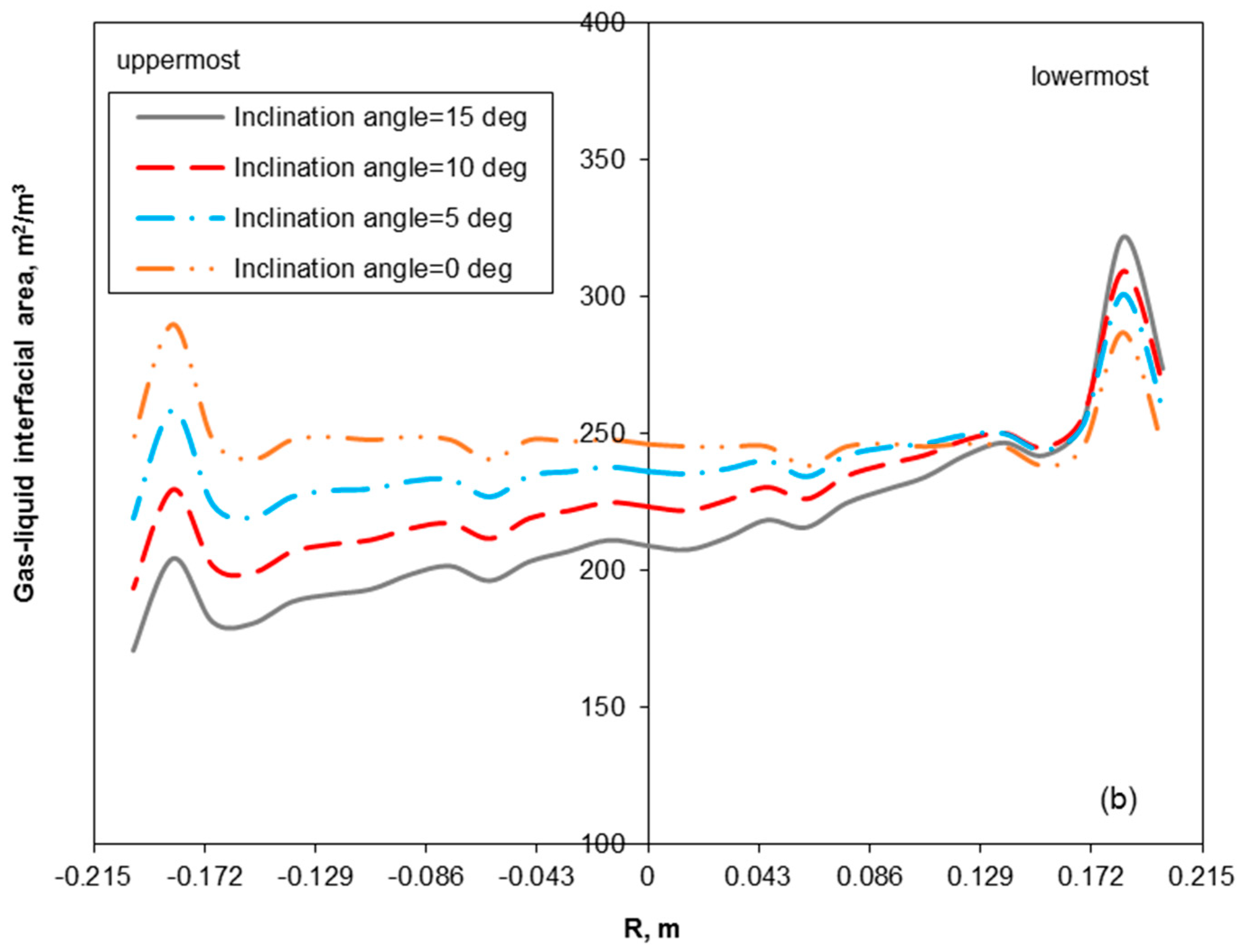
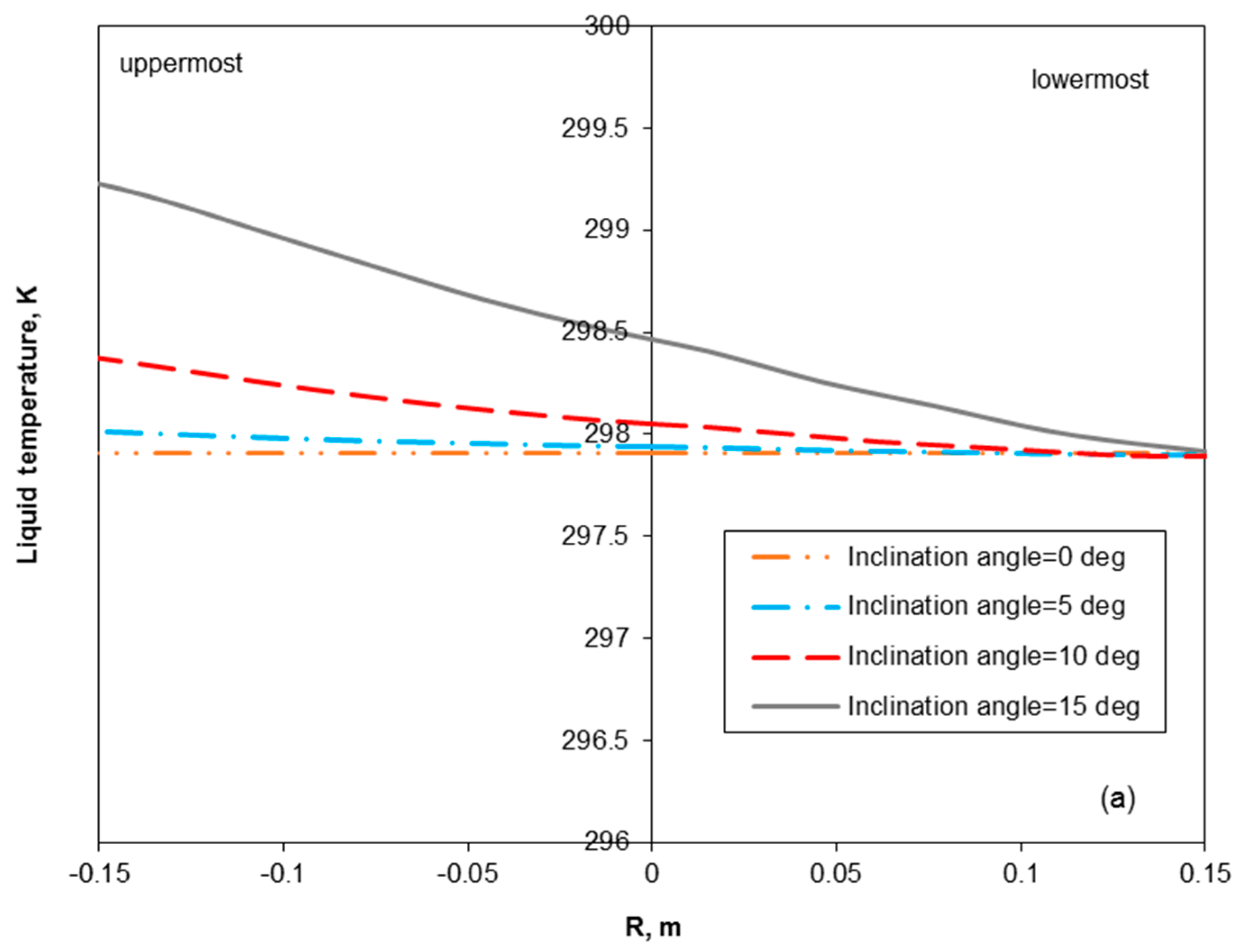
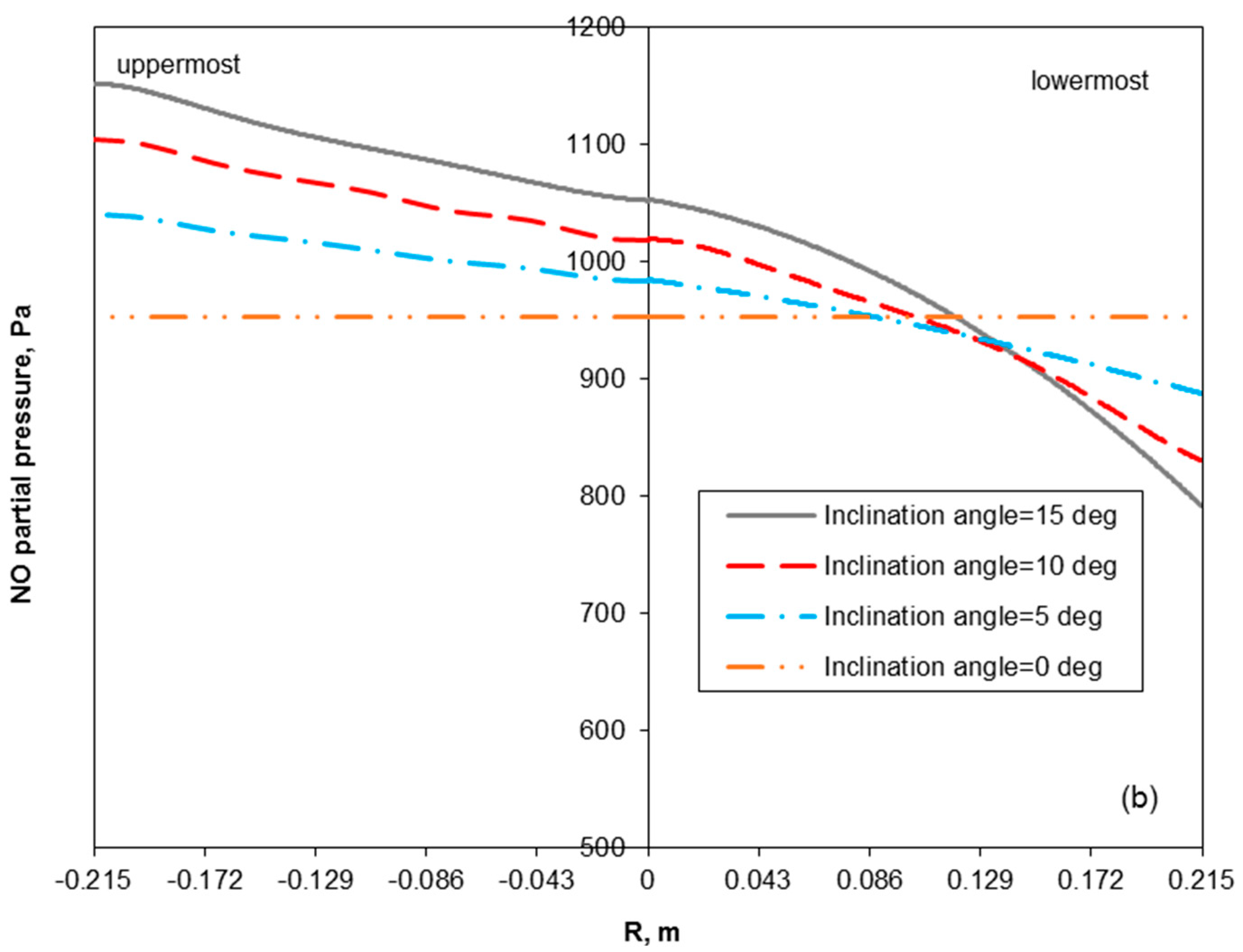
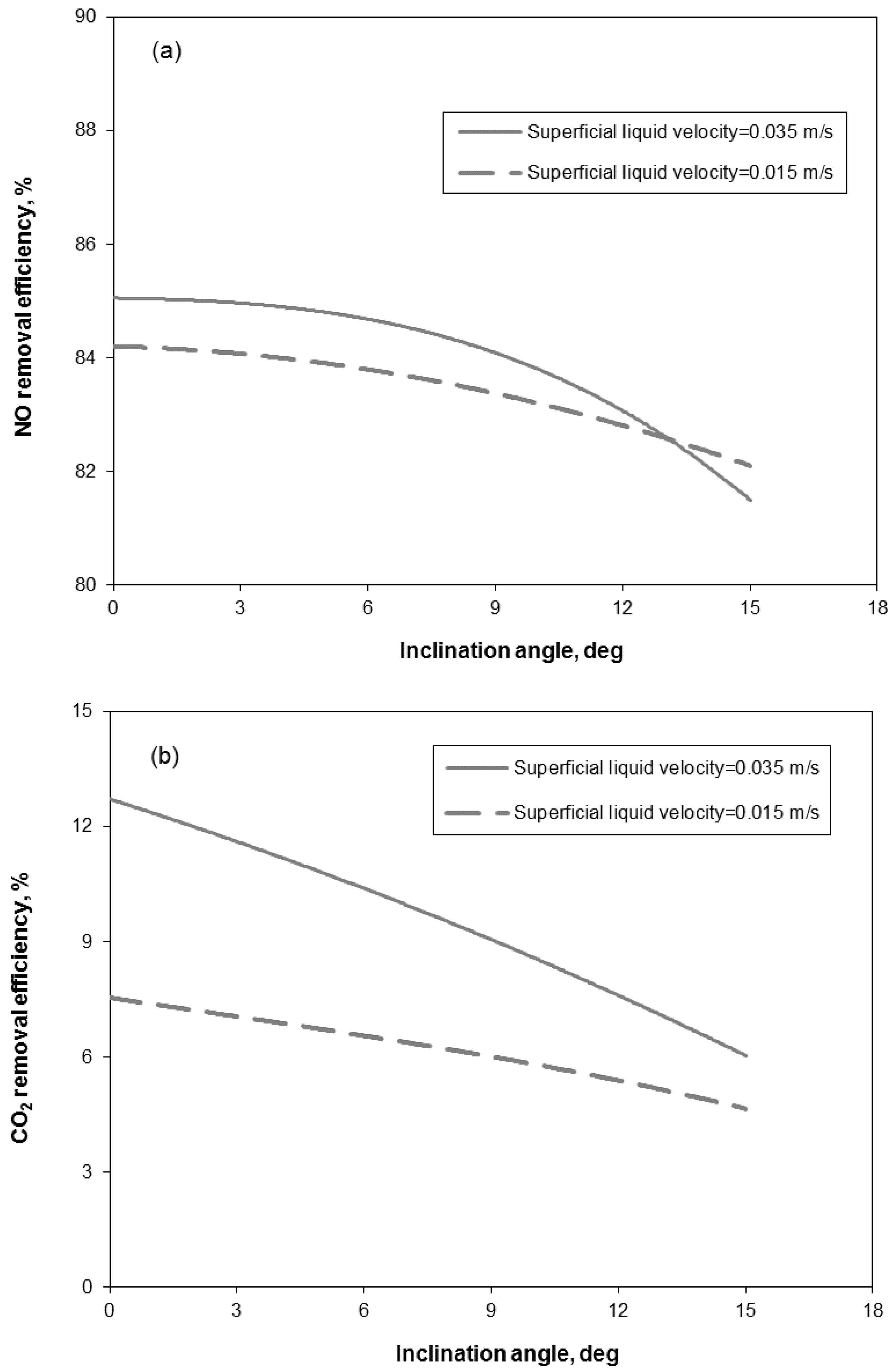
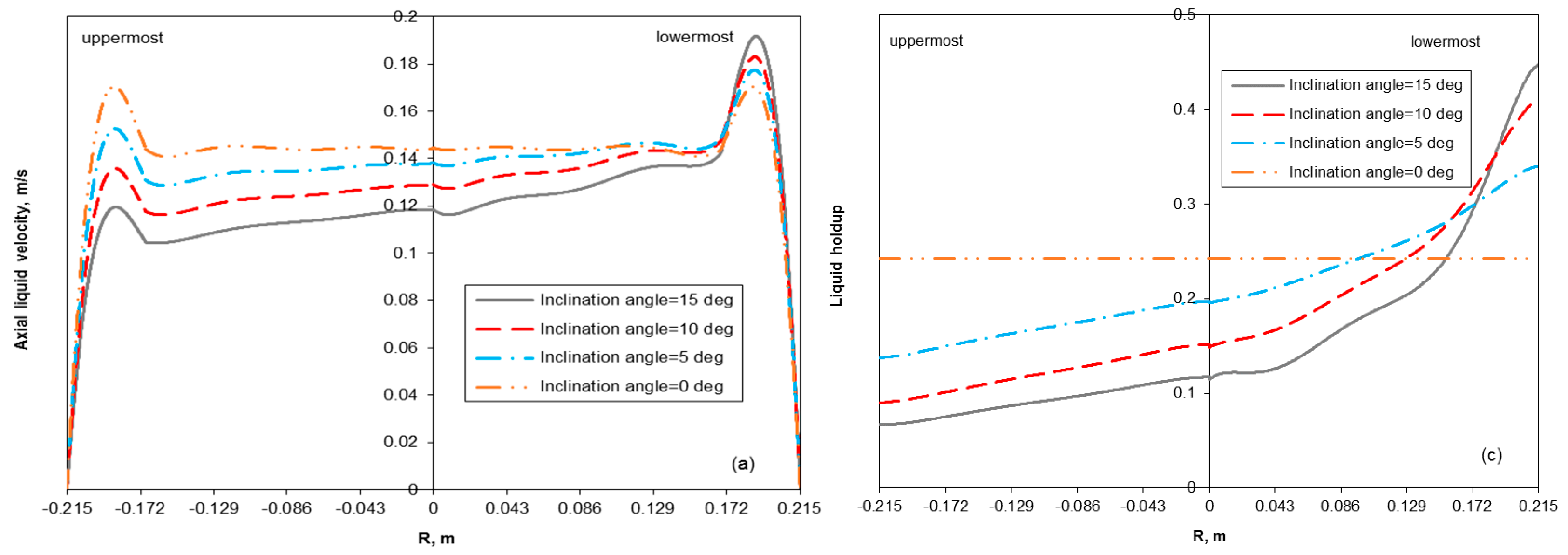
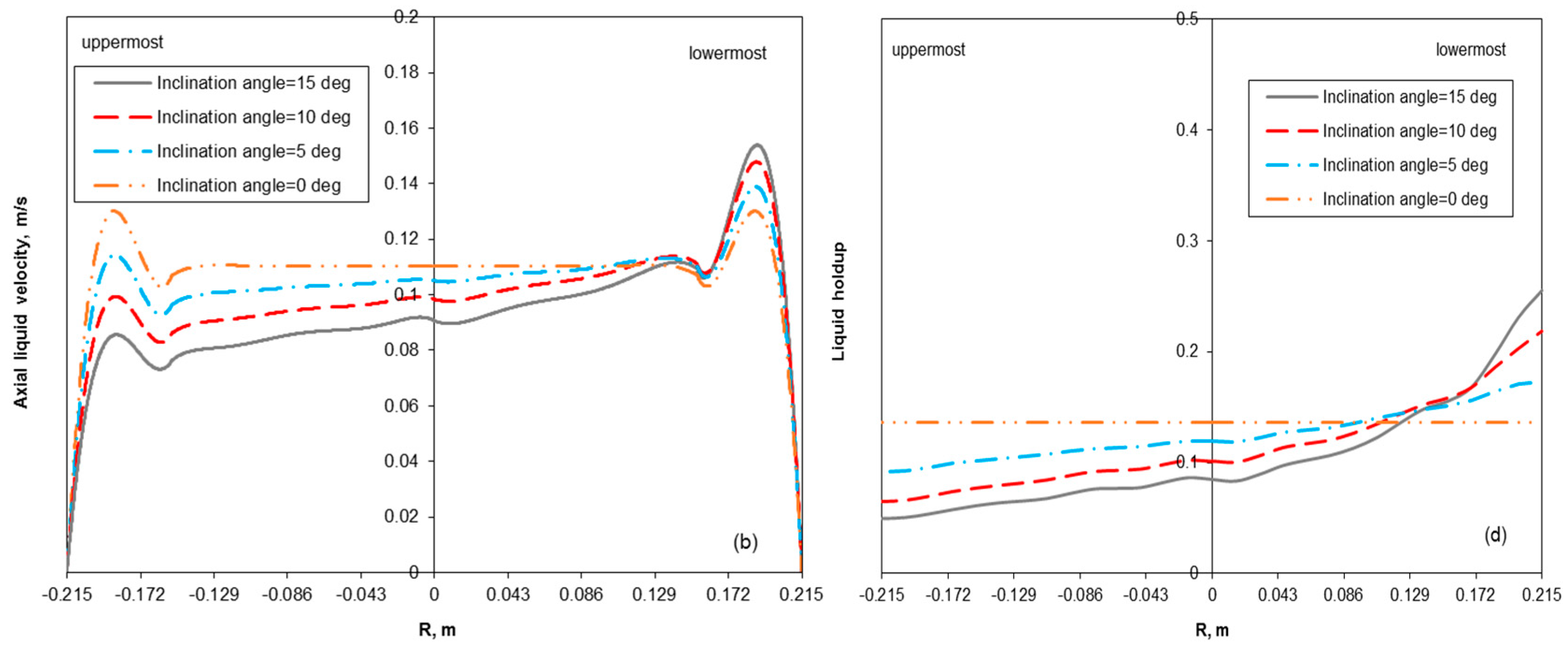
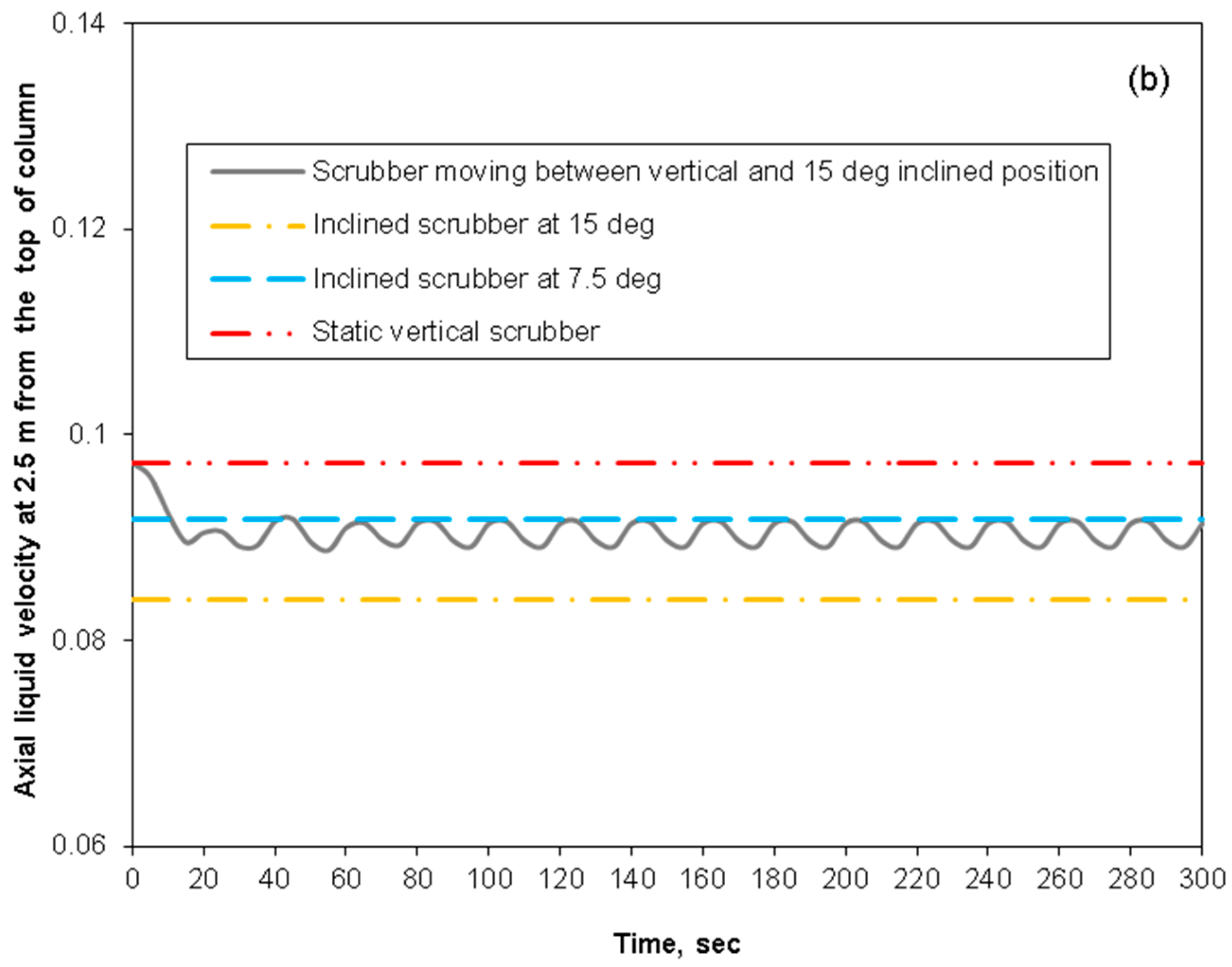
3.2. Removal Performance in Vertical and Inclined Packed-Bed Columns
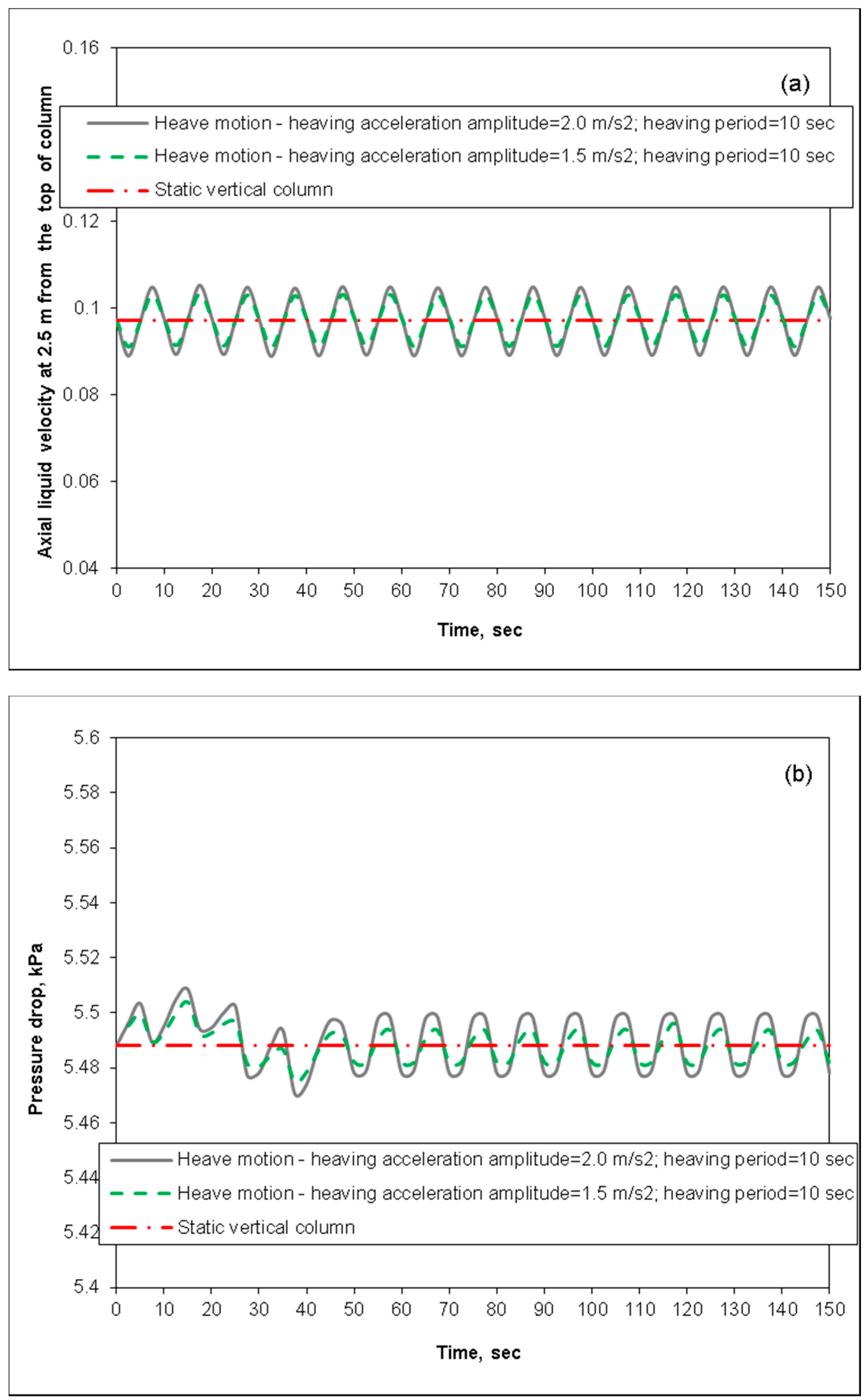
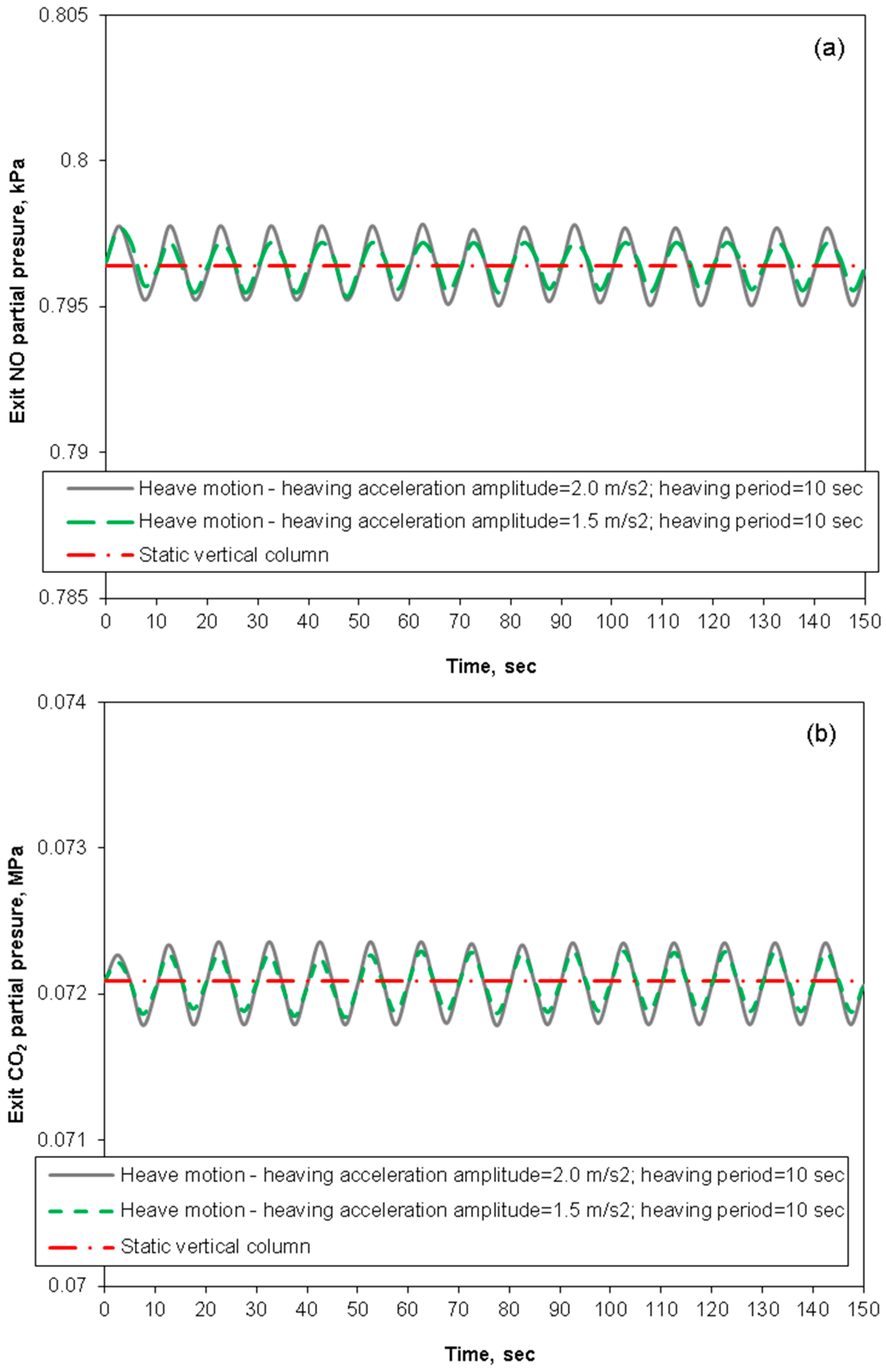
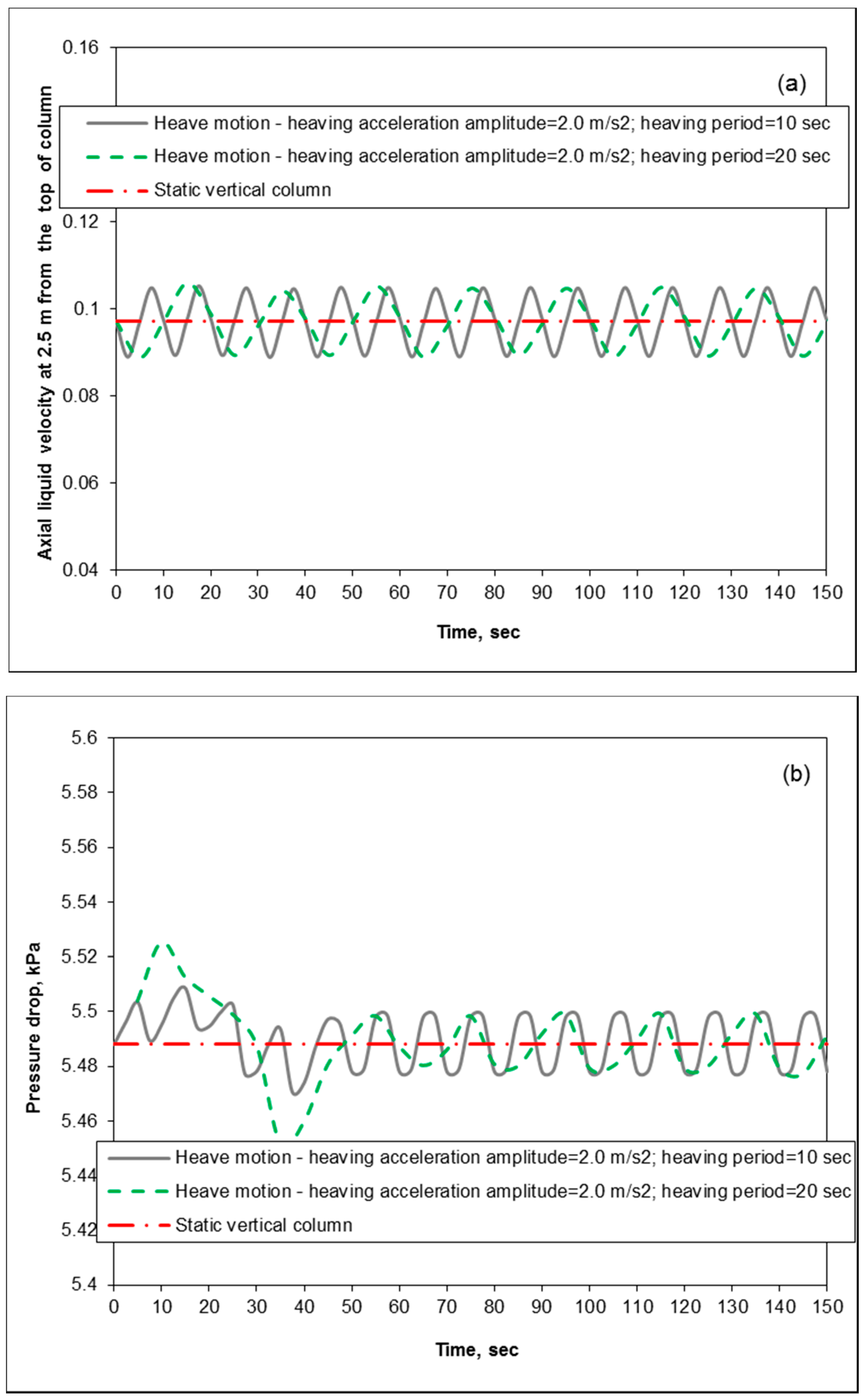
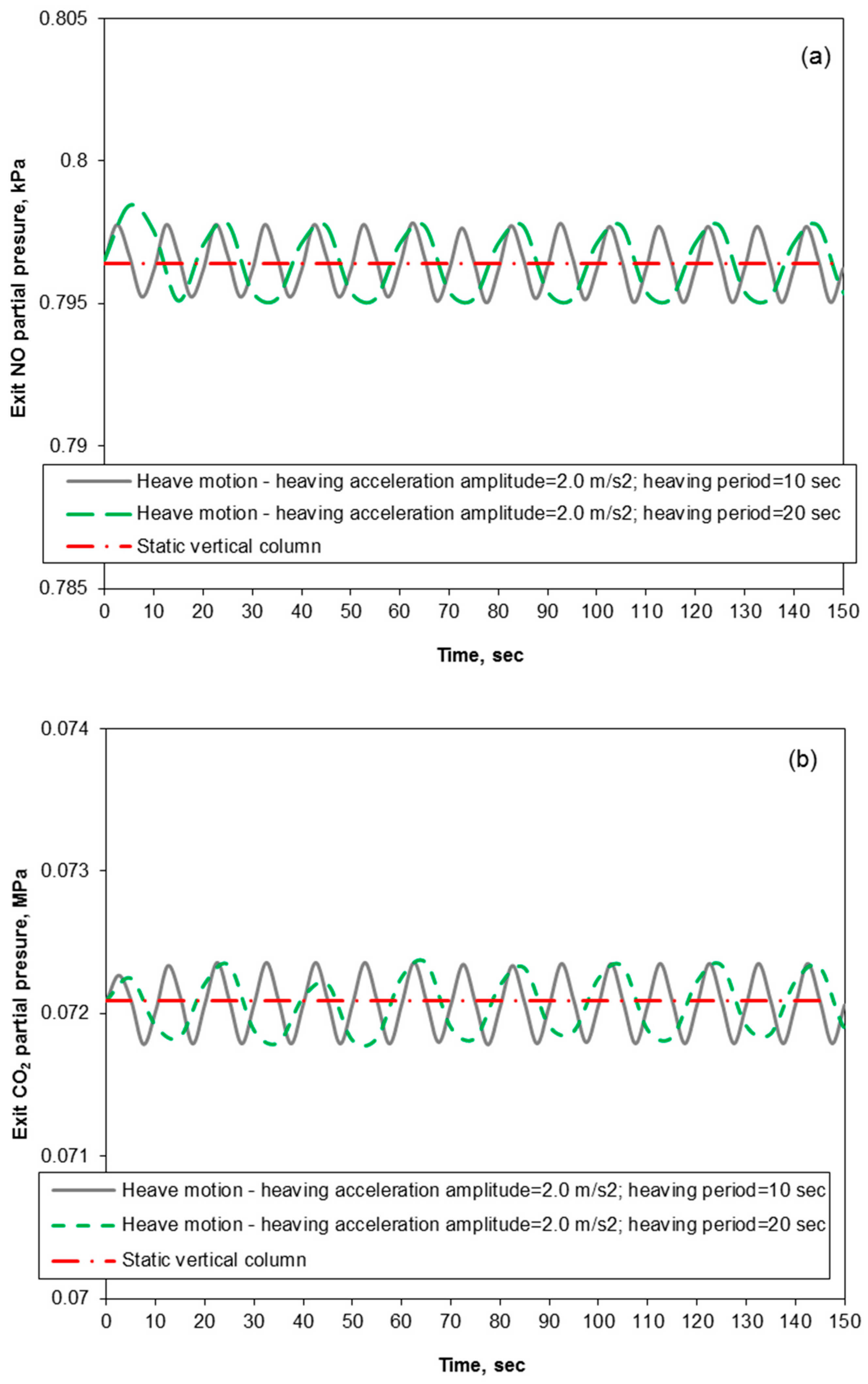
3.3. Removal Performance in Packed-Bed Column under Heaving Motion Conditions
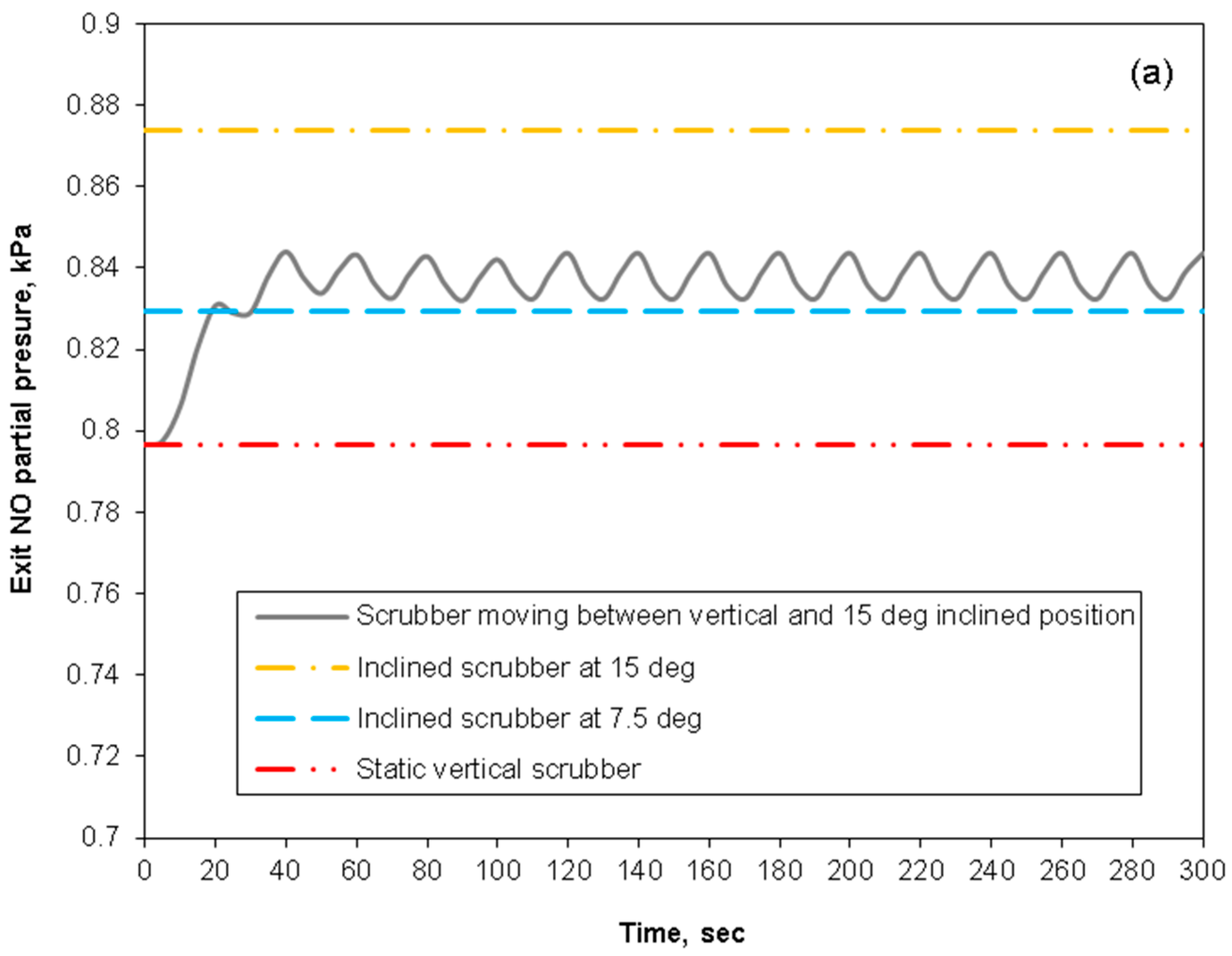
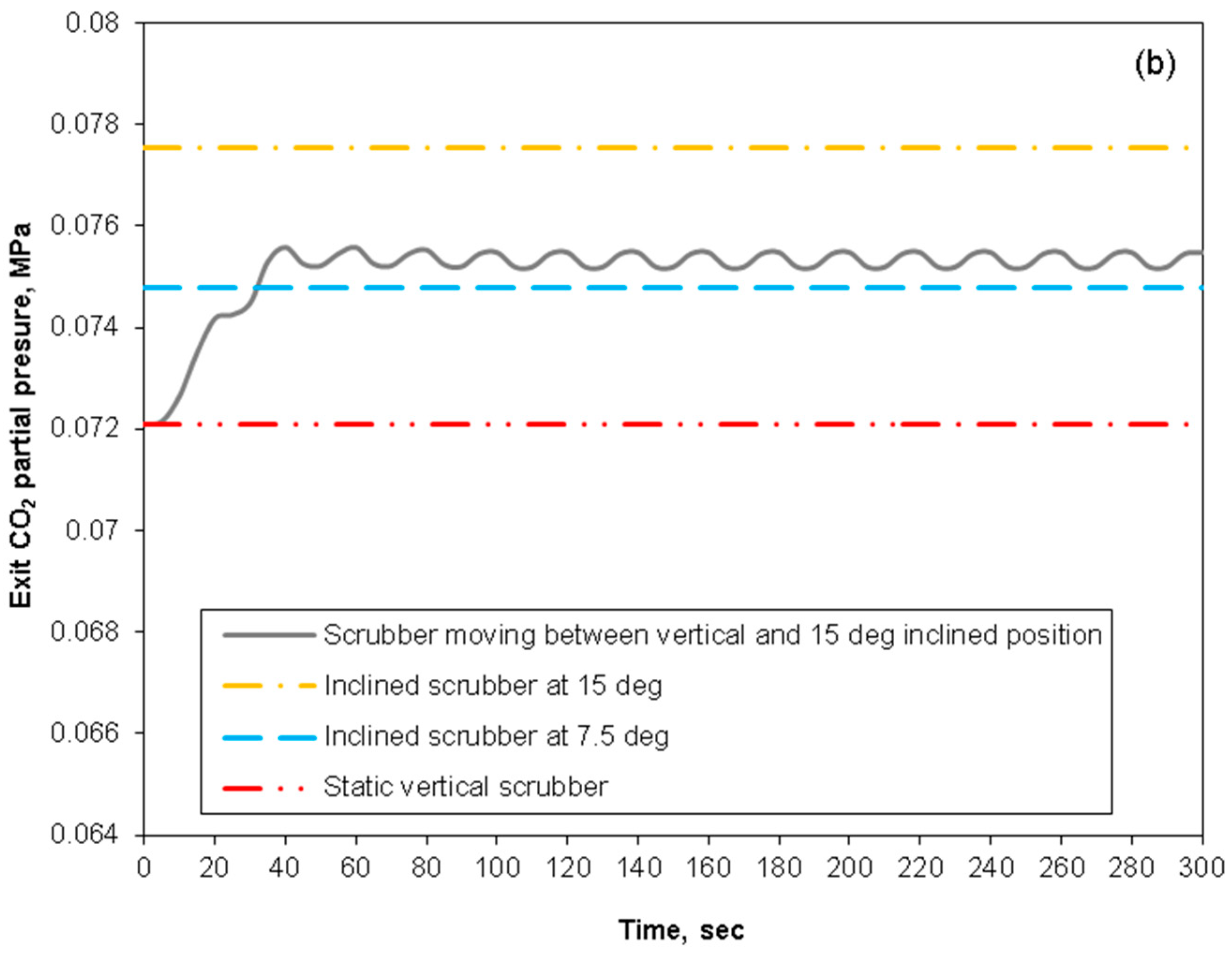
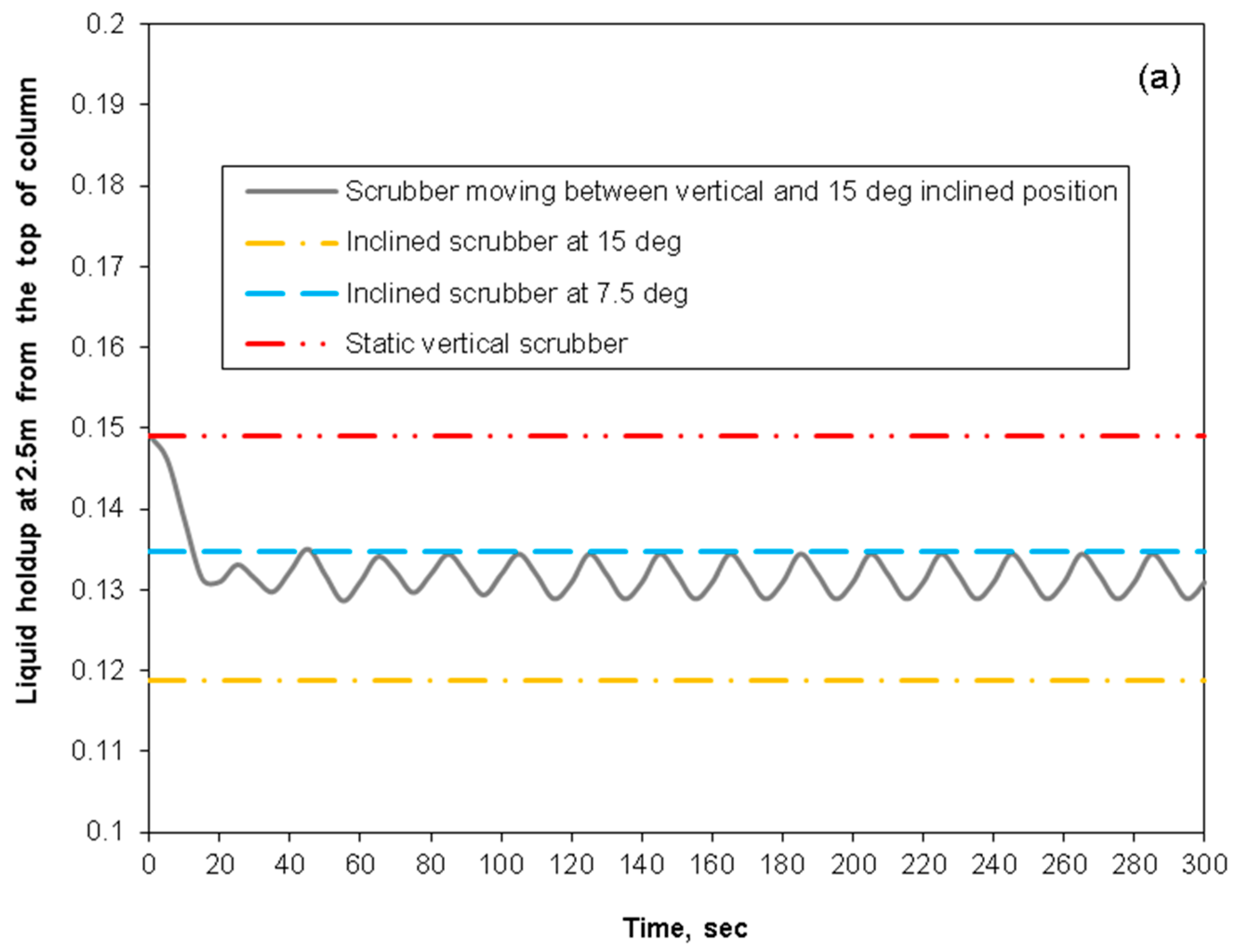
3.4. Removal Performance in Asymmetric/Symmetric Oscillating Packed-Bed Column
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Notation
| a | gas-liquid interfacial area, m2/m3 |
| ah | acceleration of heaving, m/s2 |
| heaving acceleration amplitude, m/s2 | |
| as | packing specific surface area (surface packing area/column volume), m2/m3 |
| cp,α | specific heat capacity of α-phase (), J/kgK |
| Cj | concentration of species j, kmol/m3 |
| dp | effective particle diameter, m |
| D | column diameter, m |
| molecular diffusivity coefficient of species j in the liquid phase, m2/s | |
| liquid and gas dispersion coefficients, m2/s | |
| E | mass transfer enhancement factor |
| E1, E2 | Ergun constants |
| fe | wetted fraction of the packing area |
| interaction force exerted on gas phase, N/m3, | |
| interaction force exerted on liquid phase, N/m3, | |
| . | |
| mechanical dispersion force in gas, N/m3, | |
| mechanical dispersion force in liquid, N/m3, | |
| gas-liquid drag force, N/m3, | |
| gas-solid drag force, N/m3, | |
| liquid-solid drag force, N/m3, | |
| g | gravitational acceleration, m/s2 |
| H | scrubber height, m |
| molar enthalpy of species j in α-phase, kJ/kmol | |
| Henry’s law constant in molal unit | |
| k4,5 | pseudo first-order reaction rate constant for N2O4 hydrolysis (Reaction 19), s−1 |
| kg | gas phase mass transfer coefficient, m/s |
| volumetric liquid phase mass transfer coefficient, 1/s | |
| Kc | effective equilibrium constant of the SO2 absorption reaction, mol/L |
| mj | molal concentration of component j, kmol/kg, |
| Mj | molecular mass of component j, kg/kmol |
| Nj | interfacial molar flux of component j, kmol/m2s, |
| P | reactor pressure, Pa |
| Pj | partial pressure of species j, Pa |
| Pc | capillary pressure, Pa |
| r | radial coordinate, m |
| R | ideal-gas constant or reactor radius, m |
| R1 | reaction rate, kmol/m3s |
| S | spreading factor, m |
| t | time, s |
| T | temperature, K |
| T | period of heaving/oscillating motion |
| uα | interstitial velocity of α-fluid, m/s |
| drift velocity for phase α, m/s, | |
| α-phase superficial velocity, m/s | |
| z | axial coordinate, m |
| Greek Letters | |
| α | angle of packed-bed column inclination with respect to the horizontal plane |
| heat transfer coefficient at the gas-liquid interface, J/m2sK | |
| αmax | amplitude of the angular motion |
| ε | packed bed porosity, - |
| εα | α-phase holdup, - |
| SO2 absorption reaction enthalpy, kJ/Kmol | |
| N2O4 absorption reaction enthalpy, kJ/Kmol | |
| radial effective thermal conductivity of -phase, J/msK | |
| μα | α-phase dynamic viscosity, kg/ms |
| α-phase effective viscosity (a combination of bulk and shear terms), kg/m s | |
| ρα | α-phase density, kg/m3 |
| σℓ | surface tension, N/m |
| θ | azimuthal coordinate, m |
| Subscripts/Superscripts | |
| g | gas phase |
| i | gas-liquid interface |
| in | scrubber inlet |
| ℓ | liquid phase |
| r | radial direction |
| trans | transfer |
| z | axial direction |
| w | scrubber wall |
References
- Nielsen, R.F.; Haglind, F.; Larsen, U. Design and modeling of an advanced marine machinery system including waste heat recovery and removal of sulphur oxides. Energy Convers. Manag. 2014, 85, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Zhu, M.; Chen, S.; Sperling, D. Pollution: Three steps to a green shipping industry. Nature 2016, 530, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Goldsworthy, L. Exhaust emissions from ship engines–significance, regulations, control technologies. Aust. N. Z. Marit. Law J. 2010, 24, 21–30. [Google Scholar]
- Klara, J.M.; Woods, M.C.; Capicotto, P.J.; Haslbeck, J.L.; Kuehn, N.J.; Matuszewski, M.; Pinkerton, L.L.; Rutkowski, M.D.; Schoff, R.L.; Vaysman, V. Cost and Performance Baseline for Fossil Energy Plants: Volume 1: Bituminous Coal and Natural Gas to Electricity; DOE/NETL-2007/1281. Available online: https://www.nrc.gov/docs/ML1217/ML12170A423.pdf (accessed on 28 May 2019).
- Iloeje, C.; Field, R.; Ghoniem, A.F. Modeling and parametric analysis of nitrogen and sulfur oxide removal. Fuel 2015, 160, 178–188. [Google Scholar] [CrossRef]
- Yang, S.; Pan, X.; Han, Z.; Zhao, D.; Zheng, D.; Yan, Z. Removal of NOx and SO2 from simulated ship emissions using wet scrubbing based on seawater electrolysis technology. Chem. Eng. J. 2018, 331, 8–15. [Google Scholar] [CrossRef]
- Keilin, B.; Walitt, A.L. Method of Converting Sulfur Dioxide to Sulfuric Acid. U.S. Patent 3,649,188, 14 March 1972. [Google Scholar]
- White, V.; Torrente-Murciano, L.; Sturgeon, D.; Chadwick, D. Purification of oxyfuel-derived CO2. Int. J. Greenhouse Gas Control 2010, 4, 137–142. [Google Scholar] [CrossRef]
- Flagiello, D.; Erto, A.; Lancia, A.; Di Natale, F. Experimental and modelling analysis of seawater scrubbers for Sulphur dioxide removal from flue-gas. Fuel 2018, 214, 254–263. [Google Scholar] [CrossRef]
- Makkonen, M.; Repka, S. The innovation inducement impact of environmental regulations on maritime transport: A literature review. Int. J. Innov. Sustain. Dev. 2015, 10, 69–86. [Google Scholar] [CrossRef]
- IMO. Marpol Annex VI and NTC 2008, with Guidelines for Implementation, 2017 ed.; International Maritime Organization: London, UK, 2017. [Google Scholar]
- Andreasen, A.; Mayer, S. Use of seawater scrubbing for SO2 removal from marine engine exhaust gas. Energy Fuels 2007, 21, 3274–3279. [Google Scholar] [CrossRef]
- Darake, S.; Rahimi, A.; Hatamipour, M.S.; Hamzeloui, P. SO2 removal by seawater in a packed-bed tower: Experimental study and mathematical modeling. Sep. Sci. Technol. 2014, 49, 988–998. [Google Scholar] [CrossRef]
- Tang, X.-J.; Li, T.; Yu, H.; Zhu, Y.-M. Prediction model for desulphurization efficiency of onboard magnesium-base seawater scrubber. Ocean Eng. 2014, 76, 98–104. [Google Scholar] [CrossRef]
- Kim, Y.; Nam, B.W.; Kim, D.W.; Kim, Y.S. Study on coupling effects of ship motion and sloshing. Ocean Eng. 2007, 34, 2176–2187. [Google Scholar] [CrossRef]
- Gu, Y.; Ju, Y. LNG-FPSO: Offshore LNG solution. Front. Energy Power Eng. Chin. 2008, 2, 249–255. [Google Scholar] [CrossRef]
- Iliuta, I.; Larachi, F. Three-dimensional simulations of gas-liquid cocurrent downflow in vertical, inclined and oscillating packed beds. AIChE J. 2016, 62, 916–927. [Google Scholar] [CrossRef]
- Assima, G.P.; Dashliborun, A.M.; Larachi, F. Emulation of gas-liquid flow in packed beds for offshore floating applications using a swell simulation hexapod. AIChE J. 2015, 61, 2354–2367. [Google Scholar] [CrossRef]
- Iliuta, I.; Petre, C.F.; Larachi, F. Hydrodynamic continuum model for structured-packing-containing columns. Chem. Eng. Sci. 2004, 59, 879–888. [Google Scholar] [CrossRef]
- Lappalainen, K.; Mannienen, M.; Alopaeus, V. CFD modeling of radial spreading of flow in trickle-bed reactors due to mechanical and capillary dispersion. Chem. Eng. Sci. 2009, 64, 207–218. [Google Scholar] [CrossRef]
- Iliuta, I.; Larachi, F.; Fourati, M.; Raynal, L.; Roig, V. Flooding limit in countercurrent gas-liquid structured packed beds–Prediction from a linear stability analysis of an Eulerian two-fluid model. Chem. Eng. Sci. 2014, 120, 49–58. [Google Scholar] [CrossRef]
- Iliuta, I.; Larachi, F. Enzyme-mediated CO2 capture in oscillating structured packed-bed columns-Hydrodynamics and process performance for offshore applications. Ocean Eng. 2017, 144, 157–174. [Google Scholar] [CrossRef]
- Attou, A.; Ferschneider, G. A two-fluid hydrodynamic model for the transition between trickle and pulse flow in a cocurrent gas-liquid packed-bed reactor. Chem. Eng. Sci. 2000, 55, 491–511. [Google Scholar] [CrossRef]
- Iliuta, I.; Larachi, F. CO2 abatement in oscillating packed-bed scrubbers—Hydrodynamics and reaction performances for marine applications. AIChE J. 2016, 63, 1064–1076. [Google Scholar] [CrossRef]
- Iliuta, I.; Larachi, F. Thermal regeneration of amines in vertical, inclined and oscillating CO2 packed-bed strippers for offshore floating applications. Int. J. Greenhouse Gas Control 2018, 74, 229–250. [Google Scholar] [CrossRef]
- Iliuta, I.; Larachi, F. CO2 and H2S absorption by MEA solution in packed-bed columns under inclined and heaving motion conditions-Hydrodynamics and reactions performance for marine applications. Int. J. Greenhouse Gas Control. 2018, 79, 1–3. [Google Scholar] [CrossRef]
- Miller, D.N. Mass transfer in nitric acid absorption. AIChE J. 1987, 33, 1351–1358. [Google Scholar] [CrossRef]
- Bodenstein, M. The rate of the reaction between nitric oxide and oxygen. Zeit. Elektrochem. 1918, 34, 183–191. [Google Scholar]
- Bodenstein, M. Formation and dissociation of the higher nitrogen Oxide. Zeitschrift für Physikalische Chemie 1922, 100, 68–75. [Google Scholar]
- Dekker, W.W.; Snoeck, E.; Kramers, H. The rate of absorption of NO2 in water. Chem. Eng. Sci. 1959, 11, 61–71. [Google Scholar] [CrossRef]
- Eigen, M.; Kustin, K.; Maas, G. Die Geschwindigkeit der Hydratation von SO2 in Wassriger Losung. Zeitschrift für Physikalische Chemie 1961, 30, 130–136. [Google Scholar] [CrossRef]
- Chang, C.S.; Rochelle, G.T. SO2 absorption into aqueous solutions. AIChE J. 1981, 27, 292–298. [Google Scholar] [CrossRef]
- Ellison, T.K.; Eckert, C.A. The oxidation of aqueous SO2. The influence of nitrogen dioxide at low pH. J. Phys. Chem. 1984, 88, 2335–2339. [Google Scholar] [CrossRef]
- Taylor, R.; Krishna, R. Multicomponent Mass Transfer; John Wiley & Sons: New York, NY, USA, 1993. [Google Scholar]
- Dankworth, D.C.; Kevrekidis, I.G.; Sundaresan, S. Dynamics of pulsing in trickle beds. AIChE J. 1990, 36, 605–621. [Google Scholar] [CrossRef]
- Hikita, H.; Asai, S.; Nose, H. Absorption of sulfur dioxide into water. AIChE J. 1978, 24, 147–149. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connell, J.P. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Rodriguez-Sevilla, J.; Alvarez, M.; Diaz, M.C.; Marrero, M.C. Absorption equilibria of dilute SO2 in seawater. J. Chem. Eng. Data 2004, 49, 1710–1716. [Google Scholar] [CrossRef]
- Plummer, L.N.; Busenberg, E. The solubilities of calcite, aragonite and vaterite in CO2–H2O solutions between 0 and 90oC and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochimica et Cosmochimica Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Rodriguez-Sevilla, J.; Alvarez, M.; Liminana, G.; Diaz, M.C. Dilute SO2 absorption equilibria in aqueous HCl and NaCl solutions at 298.15 K. J. Chem. Eng. Data 2002, 47, 1339–1345. [Google Scholar] [CrossRef]
- Schumpe, A. The estimation of gas solubilities in salt solutions. Chem. Eng. Sci. 1993, 48, 153–158. [Google Scholar] [CrossRef]
- Weisenberger, S.; Schumpe, A. Estimation of gas solubilities in salt solutions at temperatures from 273 K to 363 K. AIChE J. 1996, 42, 298–300. [Google Scholar] [CrossRef]
- Armor, J.N. Influence of pH and ionic strength upon solubility of NO in aqueous solution. J. Chem. Eng. Data 1974, 19, 82–89. [Google Scholar] [CrossRef]
- Weekman, V.W.; Myers, J.E. Heat transfer characteristics of concurrent gas-liquid flow in packed beds. AIChE J. 1965, 11, 13–17. [Google Scholar] [CrossRef]
- Onda, K.; Takeuchi, H.; Okumoto, Y. Mass transfer coefficients between gas and liquid phases in packed columns. J. Chem. Eng. Jpn. 1968, 1, 56–63. [Google Scholar] [CrossRef]
- Sater, V.E.; Levenspiel, O. Two-phase flow in packed beds. Evaluation of axial dispersion and holdup by moment analysis. Ind. Eng. Chem. Fundam. 1966, 5, 86–92. [Google Scholar] [CrossRef]
- Otake, T.; Kunugita, E. Axial dispersion of the gas phase in countercurrent packed bed columns. Kagaku Kogaku 1958, 22, 144–152. [Google Scholar] [CrossRef]
- Ziolkowski, D.; Szustek, S. Effect of fluid velocity radial profile on the radial mass dispersion in a fluid stream in a packed bed tubular apparatus. Chem. Eng. Sci. 1989, 44, 1195–1204. [Google Scholar] [CrossRef]
- Tontiwachwuthikul, P.; Meisenz, A.; Lim, C.J. CO2 absorption by NaOH, monoethanolamine and 2-amino-2-methyl-1-propanol solutions in a packed column. Chem. Eng. Sci. 1992, 47, 381–390. [Google Scholar] [CrossRef]
- Rambo, B.M.; Bucholz, T.L.; Powell, D.C.; Weber, L.E.; Linder, A.J.; Duesing, C.M.H.; Zaks, A. Polysilicate-Polysilicone Enzyme Immobilization Materials. U.S. Patent 8,895,280, 25 November 2014. [Google Scholar]
- Chen, W.Z.; Yu, L.; Hao, J.L. Thermal Hydraulics of Nuclear Power Plants; Chinese Atomic Energy Press: Bejing, China, 2013.
- Iliuta, I.; Larachi, F. Fischer-Tropsch synthesis in vertical, inclined and oscillating trickle-bed reactors for offshore floating applications. Chem. Eng. Sci. 2018, 177, 509–522. [Google Scholar] [CrossRef]





| Enzyme Loading | CO2 Conversion Experimental | CO2 ConversionModel |
|---|---|---|
| 1.898 | 0.74 | 0.752 |
| 2.301 | 0.73 | 0.7525 |
| 4.931 | 0.73 | 0.753 |
| 5.003 | 0.72 | 0.7533 |
| 5.699 | 0.68 | 0.753 |
| 6.147 | 0.72 | 0.753 |
| Operating Conditions | Data |
|---|---|
| Scrubber height | 5.0 and 8 m |
| Scrubber diameter | 0.43 m |
| Packing specific area (Raschig super-Rings No. 0.3)/Bed porosity | 315 m2/m3/0.96 |
| Packing specific area (I-Rings)/Bed porosity | 305 m2/m3/0.97 |
| Packing specific area (Pall-Rings)/Bed porosity | 360 m2/m3/0.92 |
| Scrubber pressure | 0.1–3 MPa |
| Scrubber inlet temperature | 298 K |
| Superficial gas velocity | 0.4 m/s |
| Superficial liquid velocity | 0.015 m/s |
| Inlet gas composition (mole fraction): | - |
| SO2 | 0.002 |
| NO | 0.0015 |
| NO2 | 0.00015 |
| CO2 | 0.05 |
| O2 | 0.15 |
| Ionic composition of seawater, mol/L | - |
| Cl− | 0.56 |
| Na+ | 0.5 |
| SO42− | 0.03 |
| HCO3− | 0.003 |
| Seawater salinity | 36.87 g/kg |
| Seawater ionic strength | 0.775 mol/kg |
| Spreading factor | 0.0074 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iliuta, I.; Larachi, F. Modeling and Simulations of NOx and SO2 Seawater Scrubbing in Packed-Bed Columns for Marine Applications. Catalysts 2019, 9, 489. https://doi.org/10.3390/catal9060489
Iliuta I, Larachi F. Modeling and Simulations of NOx and SO2 Seawater Scrubbing in Packed-Bed Columns for Marine Applications. Catalysts. 2019; 9(6):489. https://doi.org/10.3390/catal9060489
Chicago/Turabian StyleIliuta, Ion, and Faïçal Larachi. 2019. "Modeling and Simulations of NOx and SO2 Seawater Scrubbing in Packed-Bed Columns for Marine Applications" Catalysts 9, no. 6: 489. https://doi.org/10.3390/catal9060489
APA StyleIliuta, I., & Larachi, F. (2019). Modeling and Simulations of NOx and SO2 Seawater Scrubbing in Packed-Bed Columns for Marine Applications. Catalysts, 9(6), 489. https://doi.org/10.3390/catal9060489




