Dimethyl Ether to Olefins over Modified ZSM-5 Based Catalysts Stabilized by Hydrothermal Treatment
Abstract
1. Introduction
2. Results and Discussion
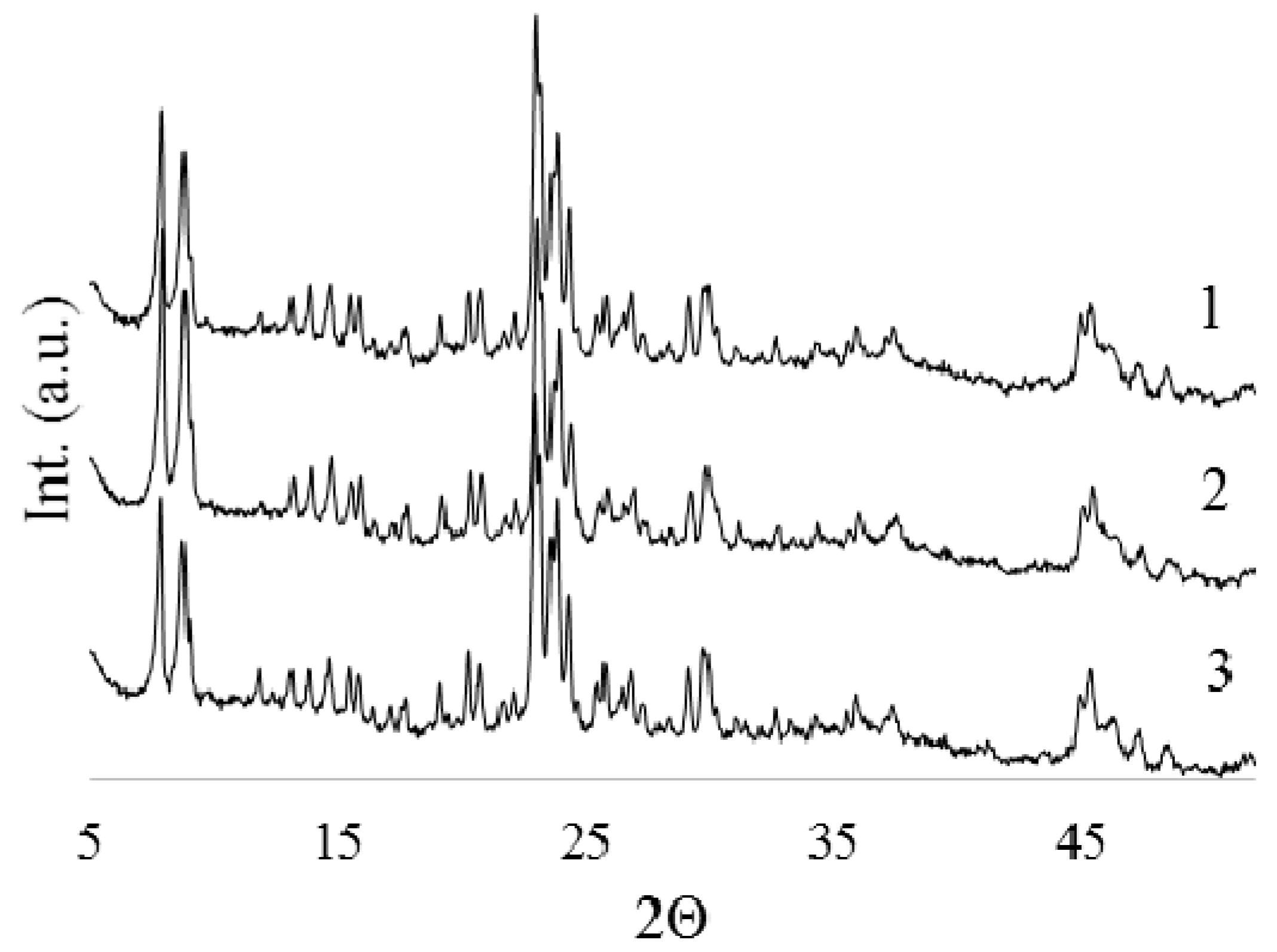
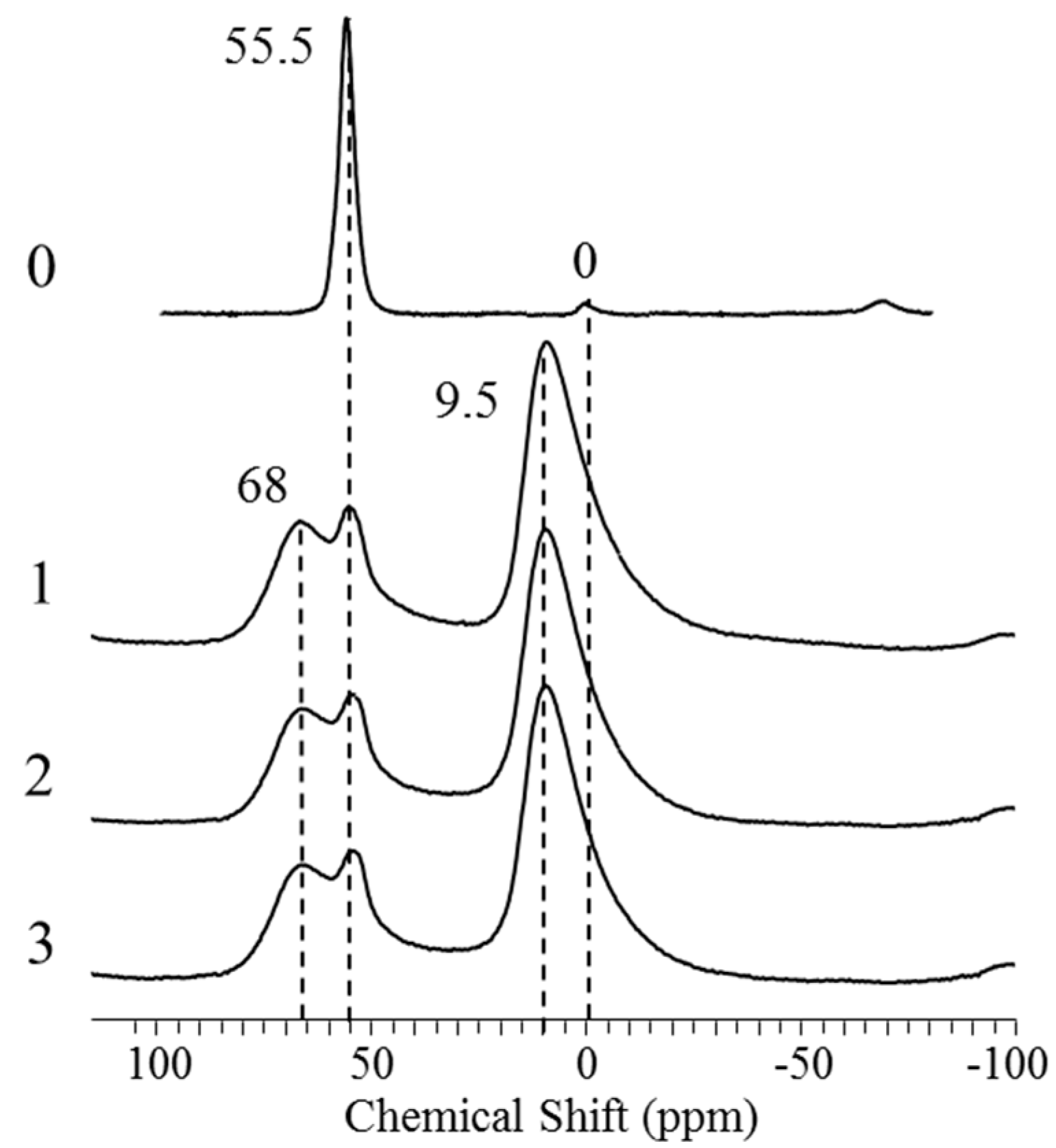
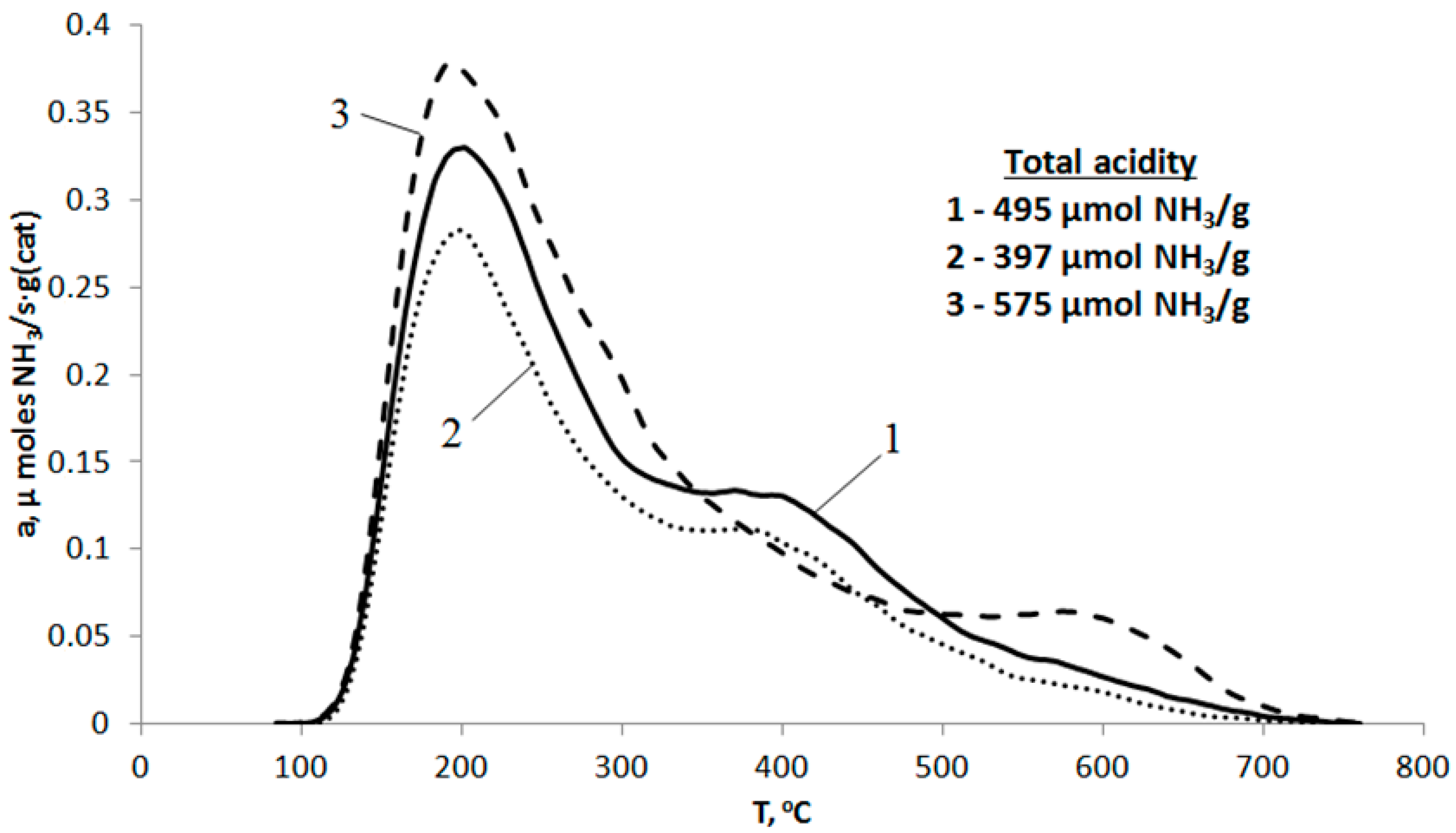
2.1. Characterization of HZSM-5/Al2O3, Zr-, and Mg-Modified Catalyst
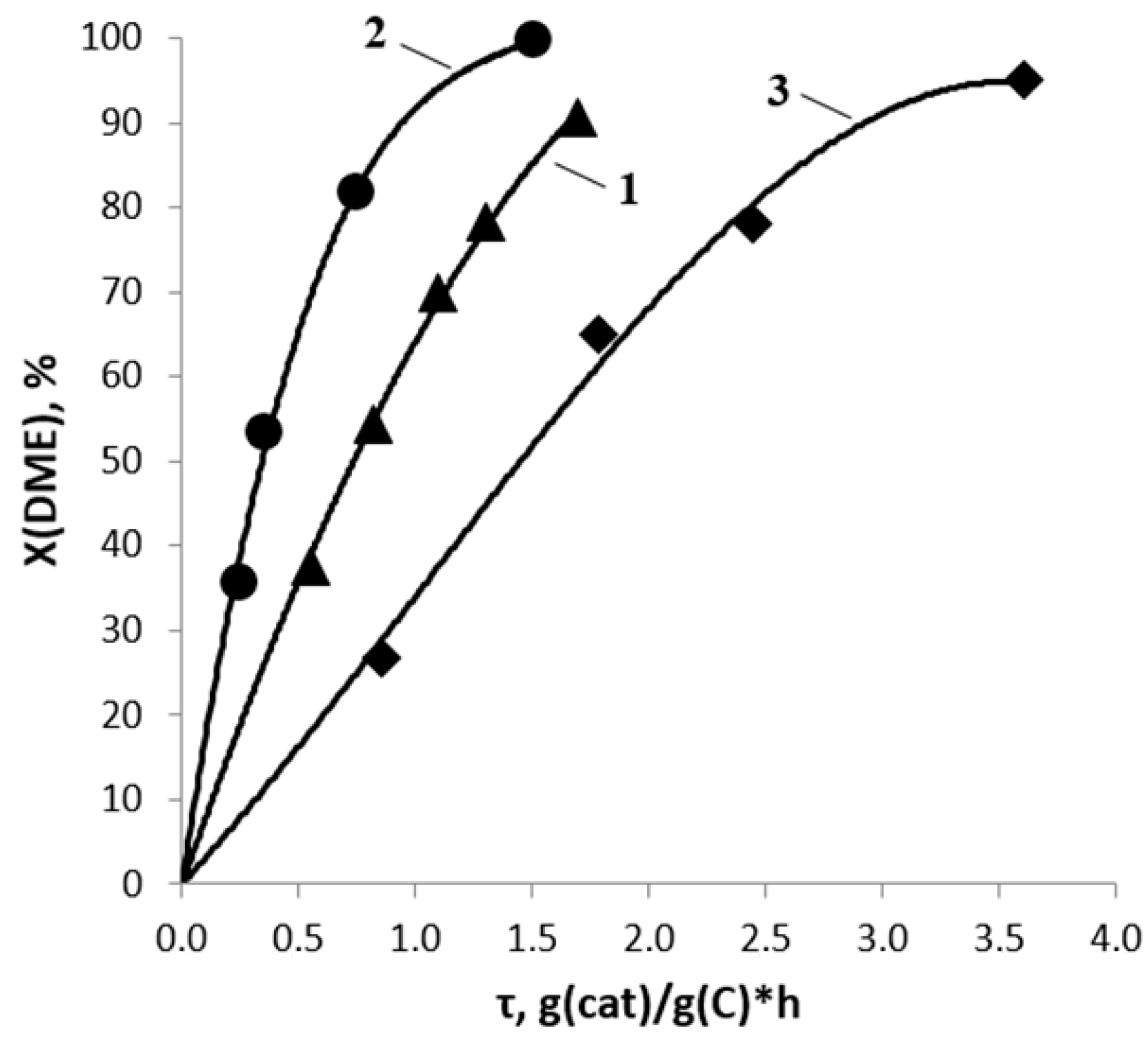
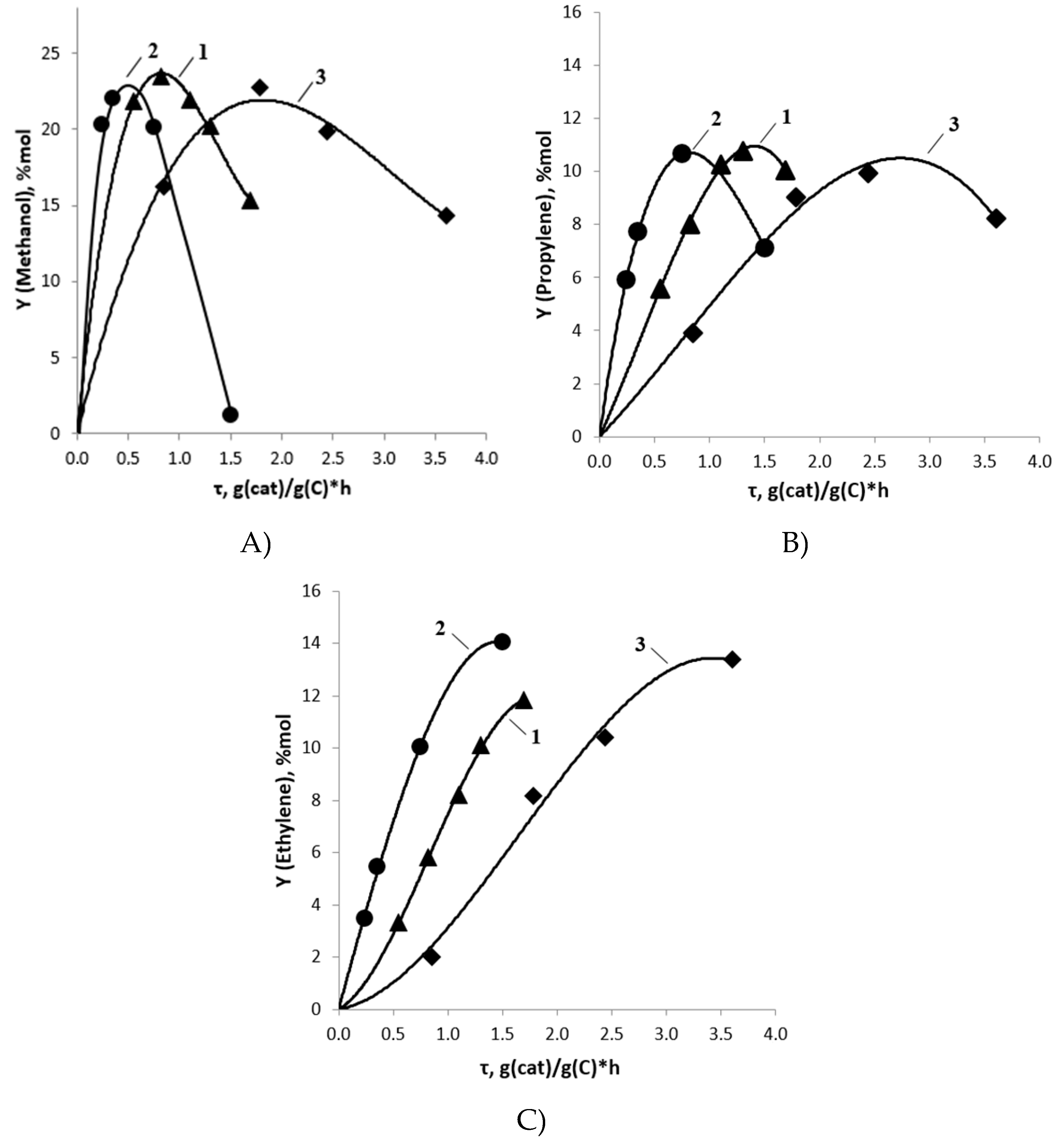
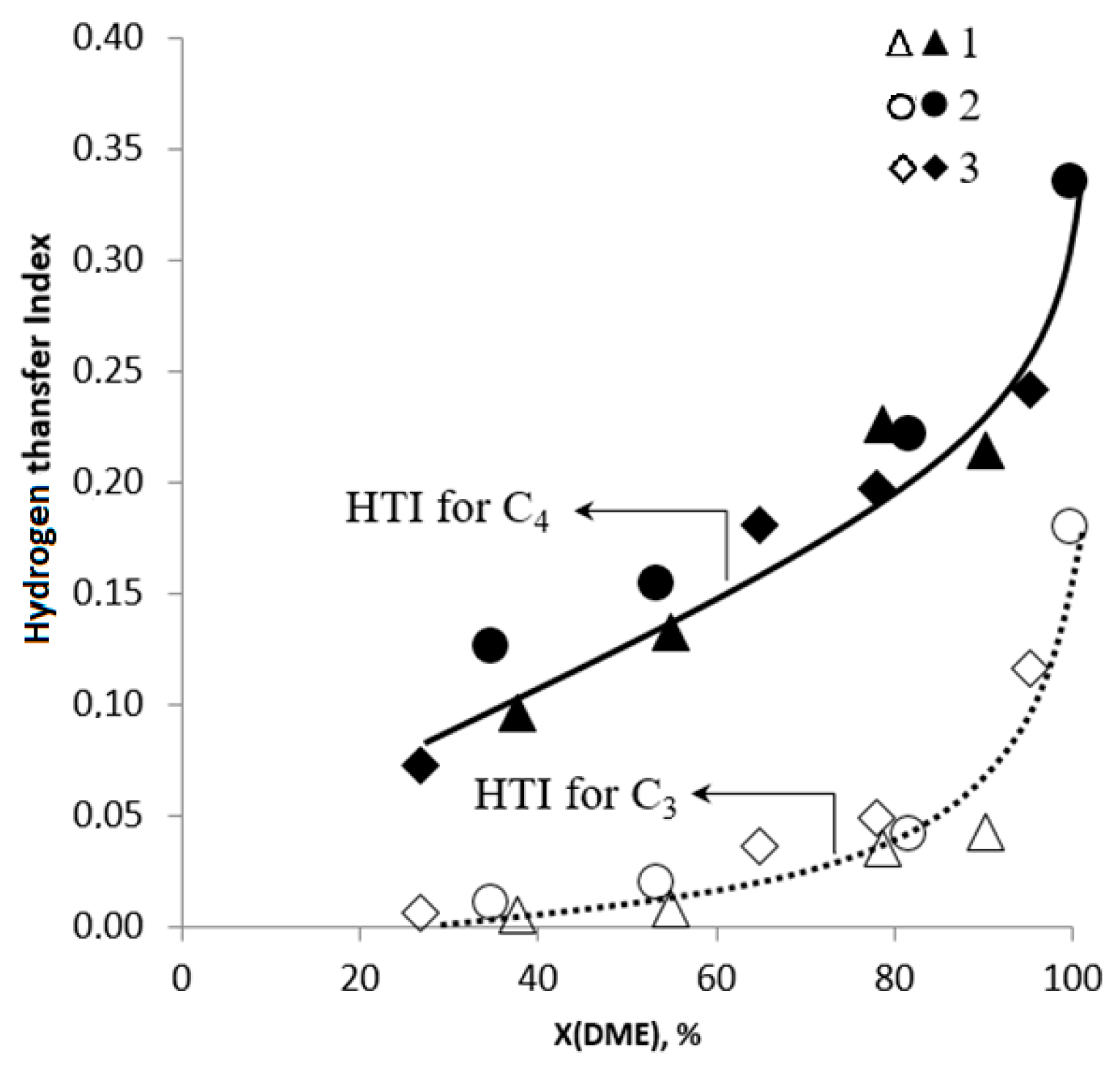
2.2. DME Conversion to Olefin
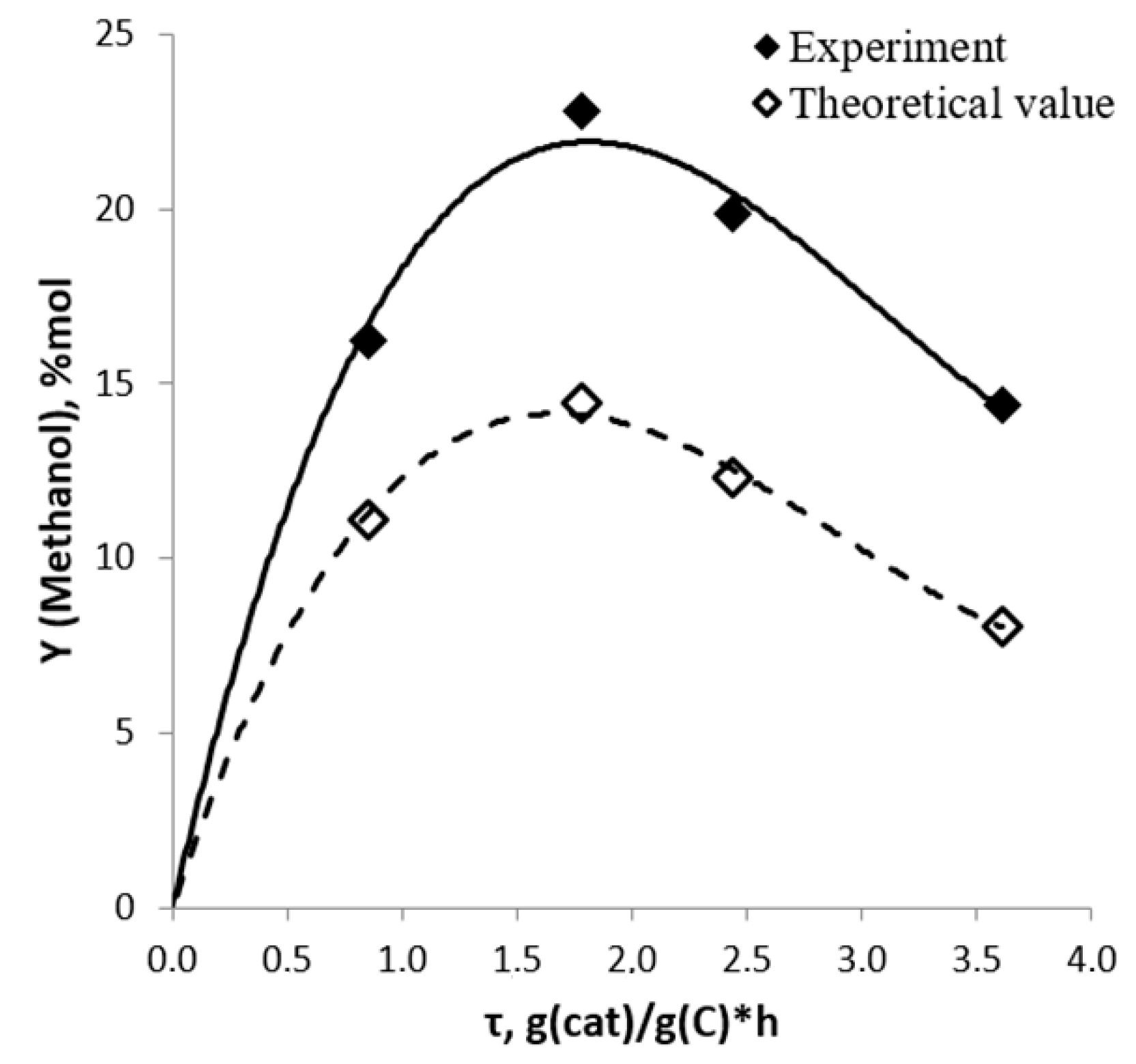
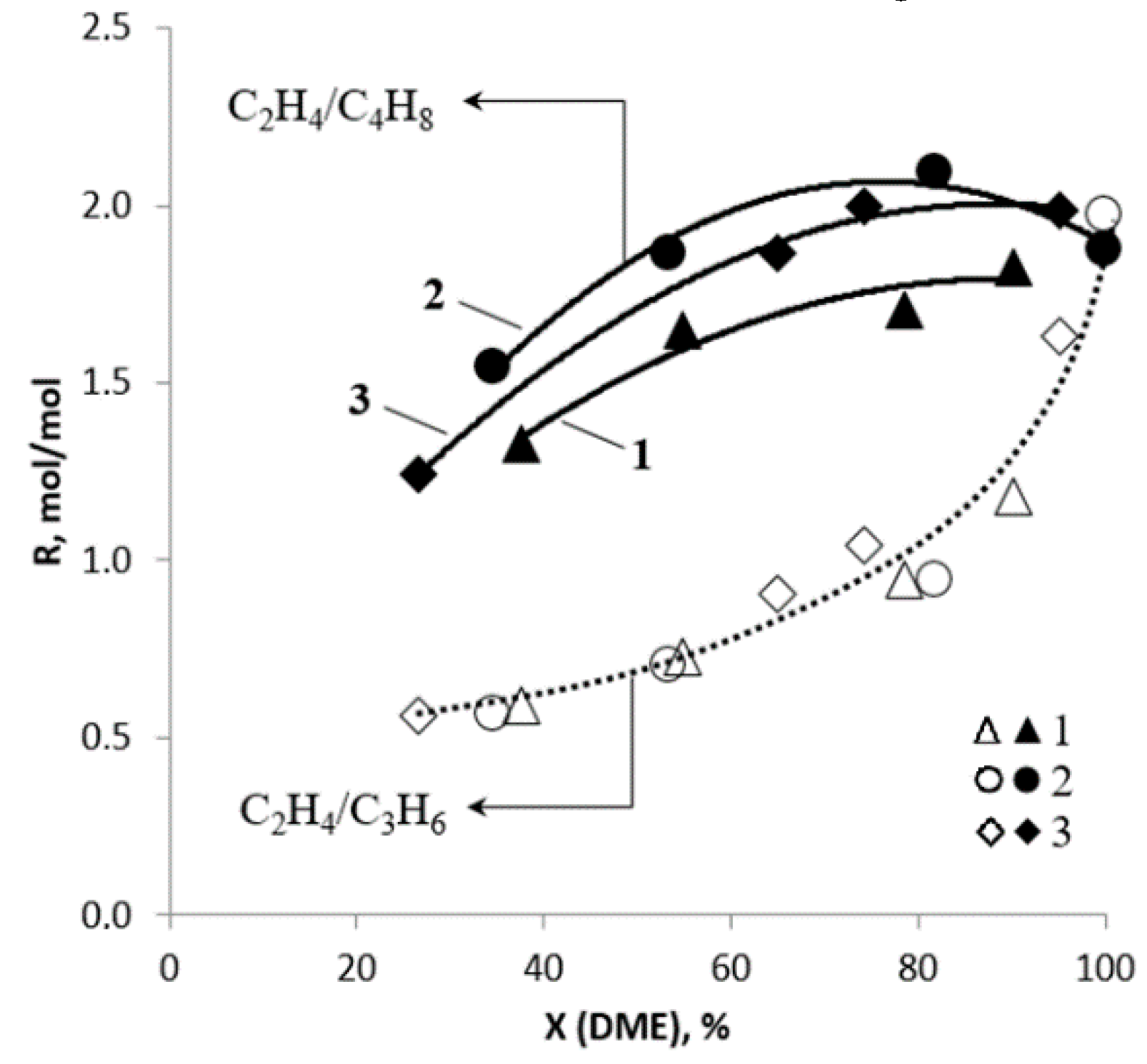
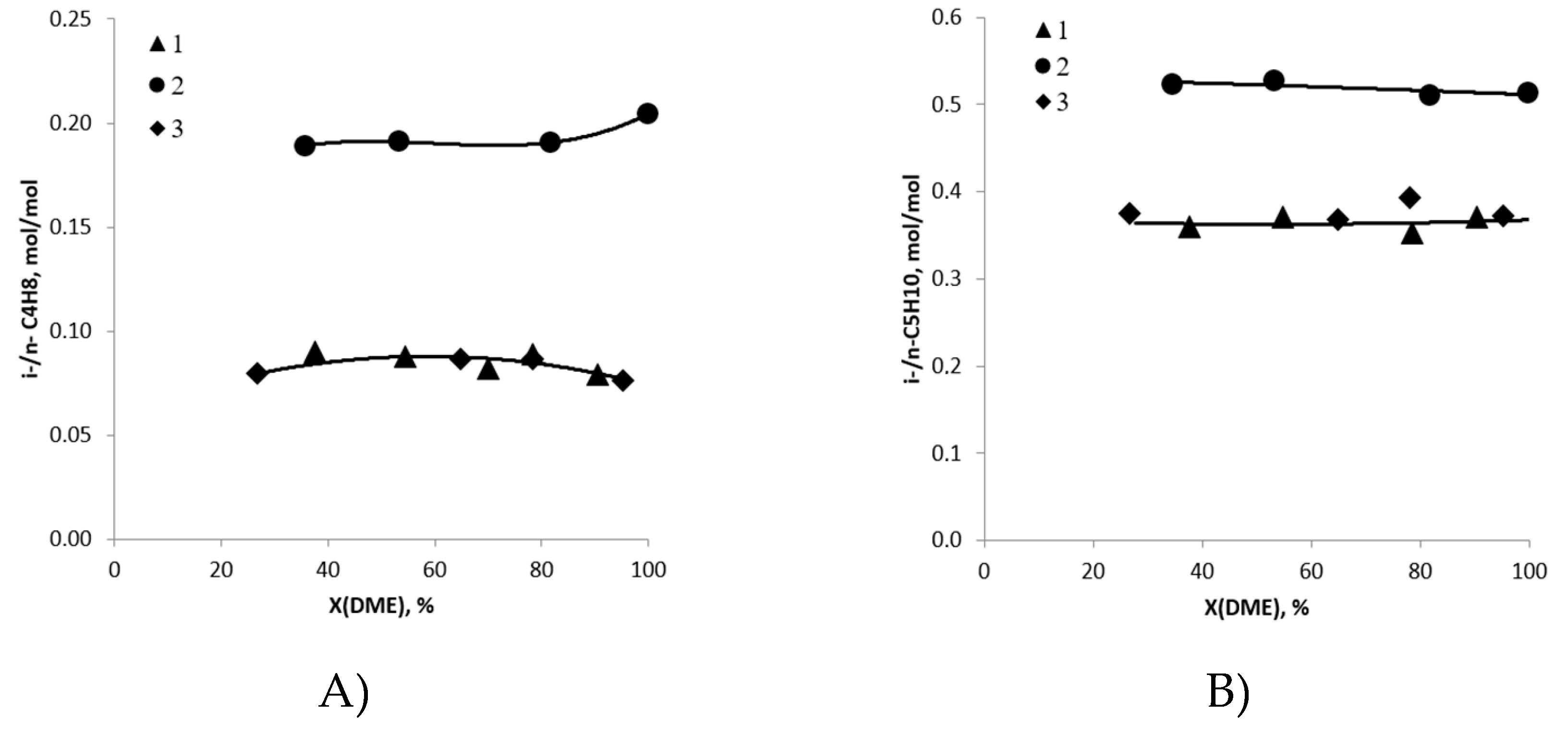
2.3. Routes of Olefin Formation from DME over Steady-State Catalyst
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Tests
3.4. Catalytic Performances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stocker, M. Methanol-to-hydrocarbons: Catalytic Materials and their behavior. Microporous Mesoporous Mater. 1999, 29, 3. [Google Scholar] [CrossRef]
- Niu, X.; Gao, J.; Wang, K.; Miao, Q.; Dong, M.; Wang, G.; Fan, W.; Qin, Z.; Wang, J. Influence of crystal size on the catalytic performance of H-ZSM-5 and Zn/H-ZSM-5 in the conversion of methanol to aromatics. Fuel Process. Technol. 2017, 157, 99. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Lee, H.-K.; Ihm, S.-K. Influence of catalyst binders on the acidity and catalytic performance of hzsm-5 zeolites for methanol-to-propylene (MTP) process: Single and binary binder system. Top. Catal. 2010, 53, 247. [Google Scholar] [CrossRef]
- Michels, N.; Mitchell, S.; Pérez-Ramírez, J. Effects of binders on the performance of shaped hierarchical MFI zeolites in methanol-to-hydrocarbons. ACS Catal. 2014, 4, 2409. [Google Scholar] [CrossRef]
- Ong, L.H.; Dömök, M.; Olindo, R.; Veen, A.C.; Lercher, J.A. Dealumination of HZSM-5 via steam-treatment. Microporous Mesoporous Mater. 2012, 164, 9. [Google Scholar] [CrossRef]
- Almutairi, S.M.T.; Mezari, B.; Pidko, E.A.; Magusin, P.C.M.M.; Hensen, E.J.M. Influence of steaming on the acidity and the methanol conversion reaction of HZSM-5 zeolite. J. Catal. 2013, 307, 194. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, Y.; Zhang, L.; Liu, Y.; Dou, T.; Xu, J.; Deng, F. Hydrothermal treatment on ZSM-5 extrudates catalyst for methanol to propylene reaction: Finely tuning the acidic property. Fuel Process. Technol. 2015, 129, 130. [Google Scholar] [CrossRef]
- Kim, H.; Jang, H.-G.; Jang, E.; Park, S.J.; Lee, T.; Jeong, Y.; Baik, H.; Cho, S.J.; Choi, J. On methanol to hydrocarbons reactions in a hierarchically structured ZSM-5 zeolite catalyst. Catal. Today 2018, 303, 150. [Google Scholar] [CrossRef]
- Al-Dughaither, A.S.; de Lasa, H. Neat dimethyl ether conversion to olefins (DTO) over HZSM-5: Effect of SiO2/Al2O3 on porosity, surface chemistry, and reactivity. Fuel 2014, 138, 52. [Google Scholar] [CrossRef]
- Pérez-Uriarte, P.; Gamero, M.; Ateka, A.; Díaz, M.; Aguayo, A.T.; Bilbao, J. Effect of the acidity of HZSM-5 zeolite and the binder in the DME transformation to olefins. Ind. Eng. Chem. Res. 2016, 55, 1513. [Google Scholar] [CrossRef]
- Hajjar, Z.; Khodadadi, A.; Mortazavi, Y.; Tayyebi, S.; Soltanali, S. Artificial intelligence modeling of DME conversion to gasoline and light olefins over modified nano ZSM-5 catalysts. Fuel 2016, 179, 79. [Google Scholar] [CrossRef]
- Fujiwara, M.; Mimura, N.; Sato, O.; Yamaguchi, A. Surface modification of HZSM-5 with organo-disilane compound for propylene production from dimethyl ether. Microporous Mesoporous Mater. 2019, 280, 219. [Google Scholar] [CrossRef]
- Park, S.; Watanabe, Y.; Nishita, Y.; Fukuoka, T.; Inagaki, S.; Kubota, Y. Catalytic conversion of dimethyl ether into propylene over MCM-68 zeolite. J. Catal. 2014, 319, 265. [Google Scholar] [CrossRef]
- Ahmed, M.H.M.; Muraza, O.; Al-Amer, A.M.; Miyake, K.; Nishiyama, N. Development of hierarchical EU-1 zeolite by sequential alkaline and acid treatments for selective dimethyl ether to propylene (DTP). Appl. Catal. A Gen. 2015, 497, 127. [Google Scholar] [CrossRef]
- Zhang, S.H.; Zhang, B.L.; Gao, Z.X.; Han, Y.Z. Methanol to olefin over Ca-modified HZSM-5 zeolites. Ind. Eng. Chem. Res. 2010, 49, 2103. [Google Scholar] [CrossRef]
- Bakare, I.A.; Muraza, O.; Sanhoob, M.A.; Miyake, K.; Hirota, Y.; Yamani, Z.H.; Nishiyama, N. Dimethyl ether-to-olefins over aluminum rich ZSM-5: The role of Ca and La as modifiers. Fuel 2018, 211, 18. [Google Scholar] [CrossRef]
- Xu, A.; Ma, H.; Zhang, H.; Ying, W.; Fang, D. Effect of boron on ZSM-5 catalyst for methanol to propylene conversion. Pol. J. Chem. Technol. 2013, 15, 95. [Google Scholar] [CrossRef]
- Barros, Z.S.; Zotin, F.M.Z.; Henriques, C.A. Conversion of natural gas to higher valued products: Light olefins production from methanol over ZSM-5 zeolites. Stud. Surf. Sci. Catal. 2007, 167, 255. [Google Scholar]
- Bjørgen, M.; Svelle, S.; Joensen, F.; Nerlov, J.; Kolboe, S.; Bonino, F.; Palumbo, L.; Bordiga, S.; Olsbye, U. Conversion of methanol to hydrocarbons over zeolite H-ZSM-5: On the origin of the olefinic species. J. Catal. 2007, 249, 195. [Google Scholar] [CrossRef]
- Olsbye, U.; Svelle, S.; Bjørgen, M.; Beato, P.; Janssens, T.V.W.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of methanol to hydrocarbons: How zeolite cavity and pore size controls product selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810. [Google Scholar] [CrossRef]
- Sun, X.; Mueller, S.; Liu, Y.; Shi, H.; Haller, G.L.; Sanchez-Sanchez, M.; van Veen, A.C.; Lercher, J.A. On reaction pathways in the conversion of methanol to hydrocarbons on HZSM-5. J. Catal. 2014, 317, 185. [Google Scholar] [CrossRef]
- Zhu, Q.; Kondo, J.N.; Setoyama, T.; Yamaguchi, M.; Domenc, K.; Tatsumi, T. Activation of hydrocarbons on acidic zeolites: Superior selectivity of methylation of ethene with methanol to propene on weakly acidic catalysts. ChemComm 2008, 41, 5164. [Google Scholar] [CrossRef]
- Wei, R.; Li, C.; Yang, C.; Shan, H. Effects of ammonium exchange and Si/Al ratio on the conversion of methanol to propylene over a novel and large partical size ZSM-5. J. Nat. Gas. Chem. 2011, 20, 261. [Google Scholar] [CrossRef]
- Ilias, S.; Khare, R.; Malek, A.; Bhan, A. A descriptor for the relative propagation of the aromatic- and olefin-based cycles in methanol-to-hydrocarbons conversion on H-ZSM-5. J. Catal. 2013, 303, 135. [Google Scholar] [CrossRef]
- Wu, W.; Guo, W.; Xiao, W.; Luo, M. Methanol conversion to olefins (MTO) over H-ZSM-5: Evidence of product distribution governed by methanol conversion. Fuel Process. Technol. 2013, 108, 19. [Google Scholar] [CrossRef]
- Magomedova, M.V.; Peresypkina, E.G.; Davydov, I.A.; Khadzhiev, S.N. Olefin synthesis from dimethyl ether in the presence of a hydrothermally treated Mg-HZSM-5/Al2O3 catalyst: Effect of reaction conditions on the product composition and ratio. Petrol. Chem. 2017, 57, 1043. [Google Scholar] [CrossRef]
- Guo, W.; Wu, W.; Luo, M.; Xiao, W. Modeling of diffusion and reaction in monolithic catalysts for the methanol-to-propylene process. Fuel Process. Technol. 2013, 108, 133. [Google Scholar] [CrossRef]
- Ortega, C.; Hessel, V.; Kolb, G. Dimethyl ether to hydrocarbons over ZSM-5: Kinetic study in an external recycle reactor. Chem. Eng. J. 2018, 354, 21. [Google Scholar] [CrossRef]
- Yaripour, F.; Shariatinia, Z.; Sahebdelfar, S.; Irandoukht, A. Effect of boron incorporation on the structure, products selectivities and lifetime of H-ZSM-5 nanocatalyst designed for application in methanol-to-olefins (MTO) reaction. Microporous Mesoporous Mater. 2015, 203, 41. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, L.; Li, G.; Shang, Y.; Zhao, X.; Ma, T.; Zhang, L.; Zhai, Y.; Gong, Y.; Xu, J.; et al. ZSM-5 extrudates modified with phosphorus as a super effective MTP catalyst: Impact of the acidity on binder. Fuel Process. Technol. 2017, 168, 105. [Google Scholar] [CrossRef]
- Rostamizadeh, M.; Taeb, A. Highly selective Me-ZSM-5 catalyst for methanol to propylene (MTP). J. Ind. Eng. Chem. 2015, 27, 297. [Google Scholar] [CrossRef]
- Goryainova, T.I.; Biryukova, E.N.; Kolesnichenko, N.V.; Khadzhiev, S.N. Study of magnesium-containing zeolite catalysts for the synthesis of lower olefins from dimethyl ether. Petrol. Chem. 2011, 51, 169. [Google Scholar] [CrossRef]
- Long, X.; Zhang, Q.; Liu, Z.-T.; Qi, P.; Lu, J.; Liu, Z.W. Magnesia modified H-ZSM-5 as an efficient acidic catalyst for steam reforming of dimethyl ether. Appl. Catal. B Environ. 2013, 134–135, 381. [Google Scholar] [CrossRef]
- Sano, T.; Kiyozumi, Y.; Shin, S. Synthesis of light olefins from methanol using ZSM-5 type zeolite catalysts. Sekiyu Gakkaishi 1992, 35, 429. [Google Scholar] [CrossRef][Green Version]
- Biryukova, E.N.; Goryainova, T.I.; Kulumbegov, R.V.; Kolesnichenko, N.V.; Khadzhiev, S.N. Conversion of dimethyl ether into lower olefins on a La-Zr-HZSM-5/Al2O3 zeolite catalyst. Petrol. Chem. 2011, 51, 49. [Google Scholar] [CrossRef]
- Zhao, T.-S.; Takemoto, T.; Tsubaki, N. Direct synthesis of propylene and light olefins from dimethyl ether catalyzed by modified H-ZSM-5. Catal. Comm. 2006, 7, 647. [Google Scholar] [CrossRef]
- Vedrine, J.C.; Auroux, A.; Dejaifve, P.; Ducarme, V.; Hoser, H.; Zhou, S. Catalytic and physical properties of phosphorus-modified ZSM-5 zeolite. J. Catal. 1982, 73, 147. [Google Scholar] [CrossRef]
- Afokin, M.I.; Davydov, I.A.; Galanova, E.G.; Magomedova, M.V. The structure and stability of the Mg-HZSM-5/Al2O3 catalyst for synthesis of olefins from DME: The influence of thermal and hydrothermal treatment. Catal. Ind. 2019, 19, 50. [Google Scholar] [CrossRef]
- Kumar, S.; Sinha, A.K.; Hegde, S.G.; Sivasanker, S. Influence of mild dealumination on physicochemical, acidicand catalytic properties of H-ZSM-5. J. Mol. Catal. A Chem. 2000, 154, 115. [Google Scholar] [CrossRef]
- Jin, L.; Hu, H.; Wang, X.; Liu, C. Methylation of 2-methylnaphthalene with methanol to 2,6-dimethylnaphthalene over ZSM-5 modified by Zr and Si. Ind. Eng. Chem. Res. 2006, 45, 3531. [Google Scholar] [CrossRef]
- Bjørgen, M.; Joensen, F.; Spangsberg Holm, M.; Olsbye, U.; Lillerud, K.; Svelle, S. Methanol to Gasoline over zeolite H-ZSM-5: Improved catalyst performance by treatment with NaOH. Appl. Catal. A Gen. 2008, 345, 43. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, Q.; Meng, Z.; Li, C.; Shan, H. Effect of magnesium modification over H-ZSM-5 in methanol to propylene reaction. Appl. Petrochem. Res. 2015, 5, 277. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X. Propylene oligomerization to produce diesel fuel on Zr-ZSM-5 catalyst. Chem. Tech. Fuel Oils 2013, 49, 156. [Google Scholar] [CrossRef]
- Mao, D.; Yang, W.; Xia, J.; Zhang, B.; Song, Q.; Chen, Q. Highly effective hybrid catalyst for the direct synthesis of dimethyl ether from syngas with magnesium oxide-modified HZSM-5 as a dehydration component. J. Catal. 2005, 230, 140. [Google Scholar] [CrossRef]
- Graça, I.; Iruretagoyena, D.; Chadwick, D. Glucose isomerisation into fructose over magnesium-impregnated NaY zeolite catalysts. Appl. Catal. B Environ. 2017, 206, 434. [Google Scholar] [CrossRef]
- Di Cosimo, J.I.; Díez, V.K.; Ferretti, C.; Apesteguía, C.R. Basic catalysis on MgO: Generation, characterization and catalytic properties of active sites. Catalysis 2014, 26, 1. [Google Scholar]
- Graça, I.; Bacariza, M.C.; Fernandes, A.; Chadwick, D. Desilicated NaY zeolites impregnated with magnesium as catalysts for glucose isomerisation into fructose. Appl. Catal. B Environ. 2018, 224, 660. [Google Scholar] [CrossRef]
- Barbera, K.; Bonino, F.; Bordiga, S.; Janssens, T.V.W.; Beato, P. Structure-deactivation relationship for ZSM-5 catalysts governed by framework defects. J. Catal. 2011, 280, 196. [Google Scholar] [CrossRef]
- Chang, C.D.; Silvestri, A.J. The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts. J. Catal. 1977, 47, 249. [Google Scholar] [CrossRef]
- Perez-Uriarte, P.; Ateka, A.; Aguayo, A.T.; Gayubo, A.G.; Bilbao, J. Kinetic model for the reaction of DME to olefins over a HZSM-5 zeolite catalyst. Chem. Eng. J. 2016, 302, 801. [Google Scholar] [CrossRef]
- Svelle, S.; Rønning, P.O.; Olsbye, U.; Kolboe, S. Kinetic studies of zeolite-catalyzed methylation reactions. Part 2. co-reaction of [12C] propene or [12C] n-butene and [13C] methanol. J. Catal. 2005, 234, 385. [Google Scholar] [CrossRef]
- Hill, I.M.; Hashimi, S.A.; Bhan, A. Kinetics and mechanism of olefin methylation reactions on zeolites. J. Catal. 2012, 285, 115. [Google Scholar] [CrossRef]
- Martinez-Espin, J.S.; Marten, M.; Janssens, T.V.W.; Svelle, S.; Beato, P.; Olsbye, U. New insights into catalyst deactivation and product distribution of zeolites in the methanol-to-hydrocarbons (MTH) reaction with methanol and dimethyl ether feeds. Catal. Sci. Technol. 2017, 7, 2700. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.; Xu, J.; Qi, G.; Wang, W.; Zhao, X.; Li, W.; Wang, Q.; Deng, F. Impact of temporal and spatial distribution of hydrocarbon pool on methanol conversion over H-ZSM-5. J. Catal. 2017, 354, 138. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, L.; Cao, Q.; Wen, Z.; Zhou, Z.; Xu, Y.; Zhu, X. Steamed Zn/ZSM-5 catalysts for improved methanol aromatization with high stability. Fuel Process. Technol. 2017, 162, 66. [Google Scholar] [CrossRef]
- Simonetti, D.A.; Carr, R.T.; Iglesia, E. Acid strength and solvation effects on methylation, hydride transfer, and isomerization rates during catalytic homologation of C1 species. J. Catal. 2012, 285, 19. [Google Scholar] [CrossRef]
- Yarulina, I.; Goetze, J.; Gücüyener, C.; Thiel, L.; Dikhtiarenko, A.; Ruiz-Martinez, J.; Weckhuysen, B.M.; Gascon, J.; Kapteijna, F. Methanol-to-olefins process over zeolite catalysts with DDR topology: Effect of composition and structural defects on catalytic performance. Catal. Sci. Technol. 2016, 6, 2663. [Google Scholar] [CrossRef]
- Simonetti, D.A.; Ahn, J.H.; Iglesia, E. Mechanistic details of acid-catalyzed reactions and their role in selective synthesis of triptane and isobutene from dimethyl ether. J. Catal. 2011, 277, 173. [Google Scholar] [CrossRef]
- Svelle, S.; Arstad, B.; Kolboe, S.; Swang, O. A theoretical investigation of the methylation of alkenes with methanol over acidic zeolites. J. Phys. Chem. B 2003, 107, 9281. [Google Scholar] [CrossRef]
- Svelle, S.; Tuma, C.; Rozanska, X.; Kerber, T.; Sauer, J. Quantum chemical modeling of zeolite-catalyzed methylation reactions: Toward chemical accuracy for barriers. J. Am. Chem. Soc. 2009, 131, 816. [Google Scholar] [CrossRef] [PubMed]
- Natal-Santiago, M.A.; Alcala, R.; Dumesic, J.A. DFT study of the isomerization of hexyl species involved in the acid-catalyzed conversion of 2-methyl-pentene-2. J. Catal. 1999, 181, 124. [Google Scholar] [CrossRef]
- Frash, M.V.; Kazanzky, V.B.; Rigby, A.M.; van-Santen, R.A. Density functional and hartree−fock calculations on the cyclopropane ring intermediates involved in the zeolite-catalyzed skeletal isomerization of hydrocarbons and in the carbon isotope scrambling in 2-propyl cation. J. Phys. Chem. B 1997, 101, 5346. [Google Scholar] [CrossRef][Green Version]
- Haw, J.F.; Nicholas, J.B.; Song, W.; Deng, F.; Wang, Z.; Xu, T.; Heneghan, C.S. Roles for cyclopentenyl cations in the synthesis of hydrocarbons from methanol on zeolite catalyst HZSM-5. J. Am. Chem. Soc. 2000, 122, 4763. [Google Scholar] [CrossRef]
- Wang, C.; Chu, Y.; Zheng, A.; Xu, J.; Wang, Q.; Gao, P.; Qi, G.; Gong, Y.; Deng, F. New insight into the hydrocarbon-pool chemistry of the methanol-to-olefins conversion over zeolite H-ZSM-5 from GCMS, solid-state NMR spectroscopy, and DFT calculations. Chem. Eur. J. 2014, 20, 12432. [Google Scholar] [CrossRef]
- Yamazaki, H.; Shima, H.; Imai, H.; Yokoi, T.; Tatsumi, T.; Kondo, J.N. Direct production of propene from methoxy species and dimethyl ether over H-ZSM-5. J. Phys. Chem. C 2012, 116, 24091. [Google Scholar] [CrossRef]
- Svelle, S.; Kolboe, S.; Swang, O.; Olsbye, U. Methylation of alkenes and methylbenzenes by dimethyl ether or methanol on acidic zeolites. J. Phys. Chem. B 2005, 109, 12874. [Google Scholar] [CrossRef]
- Svelle, S.; Visur, M.; Olsbye, U.; Bjørgen, M. Mechanistic aspects of the zeolite catalyzed methylation of alkenes and aromatics with methanol: A review. Top. Catal. 2011, 54, 897. [Google Scholar] [CrossRef]
- Rabiu, S.; Al-Khattaf, S. Kinetics of toluene methylation over ZSM-5 catalyst in a riser simulator. Ind. Eng. Chem. Res. 2008, 47, 39. [Google Scholar] [CrossRef]
- Hill, I.; Malek, A.; Bhan, A. Kinetics and mechanism of benzene, toluene, and xylene methylation over H-MFI. ACS Catal. 2013, 3, 1992. [Google Scholar] [CrossRef]
- Lesthaeghe, D.; De Sterck, B.; Van Speybroeck, V.; Marin, G.B.; Waroquier, M. Zeolite shape-selectivity in the gem-methylation of aromatic hydrocarbons. Angew. Chem. Int. Ed. 2007, 46, 1311. [Google Scholar] [CrossRef]
- Svelle, S.; Rønning, P.O.; Kolboe, S. Kinetic studies of zeolite-catalyzed methylation reactions 1. Coreaction of [12C] ethene and [13C] methanol. J. Catal. 2004, 224, 115. [Google Scholar] [CrossRef]
- Bjørgen, M.; Joensen, F.; Lillerud, K.; Olsbye, U.; Svelle, S. The mechanisms of ethene and propene formation from methanol over high silica H-ZSM-5 and H-beta. Catal. Today 2009, 142, 90. [Google Scholar] [CrossRef]
- Hutchings, G.J.; Rensburg, L.J.; Pick, W.; Hunter, R. Hydrocarbon formation from methanol and dimethyl ether using WO3/Al2O3 and H-ZSM-5 catalysts. J. Chem. Soc. Faraday Trans. I 1988, 84, 1311. [Google Scholar] [CrossRef]
- Ilias, S.; Bhan, A. Tuning the selectivity of methanol-to-hydrocarbons conversion on H-ZSM-5 by co-processing olefin or aromatic compounds. J. Catal. 2012, 290, 186. [Google Scholar] [CrossRef]
- TIPS RAS, JSC ELINP. Catalyst and Method for Olefins Synthesis from Dimethyl Ether in Its Presence. Patent RU 2445158, 2012. [Google Scholar]
- TIPS RAS. Catalyst and Method for Olefins Synthesis from Dimethyl Ether in Its Presence. RU 2323777, 2008. [Google Scholar]


| № | Sample | Elemental Composition, wt % | ||||
|---|---|---|---|---|---|---|
| Si | Al | Zr | Mg | Na | ||
| 1 | HZSM-5/Al2O3 | 28.77 ± 0.11 | 19.85 ± 0.13 | 0.06 ± 0.005 | 0.11 ± 0.006 | 0.03 ± 0.012 |
| 2 | Zr-HZSM-5/Al2O3 | 28.13 ± 0.11 | 19.91 ± 0.13 | 1.19 ± 0.05 | 0.10 ± 0.006 | 0.03 ± 0.012 |
| 3 | Mg-HZSM-5/Al2O3 | 29.20 ± 0.11 | 18.66 ± 0.13 | 0.02 ± 0.002 | 1.03 ± 0.04 | 0.03 ± 0.013 |
| № | Sample | SBET, m2/g | Vpore, cm3/g | ||
|---|---|---|---|---|---|
| Total | Total | Micro | Meso | ||
| 1 | HZSM-5/Al2O3 | 293 | 0.198 | 0.057 | 0.142 |
| 2 | Zr-HZSM-5/Al2O3 | 276 | 0.197 | 0.056 | 0.141 |
| 3 | Mg-HZSM-5/Al2O3 | 267 | 0.247 | 0.067 | 0.181 |
| № | Sample | Acidity of Fresh Catalyst, μmol NH3/g (cat) | B/L * | |||
|---|---|---|---|---|---|---|
| Total | Weak Sites | Strong Sites | ||||
| T1 = 200 °C | T2 = 400 °C | T3 = 600 °C | ||||
| 1 | HZSM-5/Al2O3 | 495 | 337 (68.1%) | 138 (27.9%) | 20 (4.0%) | 0.42 |
| 2 | Zr-HZSM-5/Al2O3 | 397 | 280 (70.5%) | 105 (26.4%) | 12 (3.0%) | 0.34 |
| 3 | Mg-HZSM-5/Al2O3 | 575 | 390 (67.8%) | 131 (22.8%) | 54 (9.4%) | 0.16 |
| Product | S, mol % | |
|---|---|---|
| X = 55% | X = 78% | |
| CH3OH | 51.2 | 38.0 |
| C2H4 | 12.7 | 17.5 |
| C3H6 | 17.4 | 18.6 |
| ΣC4H8 | 7.7 | 10.5 |
| ΣC5H10 | 0.9 | 1.2 |
| CH4 | 0.5 | 0.6 |
| C2H6 | 0.1 | 0.1 |
| C3H8 | 0.3 | 0.8 |
| ΣC4H10 | 1.0 | 2.7 |
| ΣC5H12 | 0.5 | 1.3 |
| ΣC6 | 2.8 | 3.2 |
| ΣC7+ | 5.0 | 5.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magomedova, M.; Galanova, E.; Davidov, I.; Afokin, M.; Maximov, A. Dimethyl Ether to Olefins over Modified ZSM-5 Based Catalysts Stabilized by Hydrothermal Treatment. Catalysts 2019, 9, 485. https://doi.org/10.3390/catal9050485
Magomedova M, Galanova E, Davidov I, Afokin M, Maximov A. Dimethyl Ether to Olefins over Modified ZSM-5 Based Catalysts Stabilized by Hydrothermal Treatment. Catalysts. 2019; 9(5):485. https://doi.org/10.3390/catal9050485
Chicago/Turabian StyleMagomedova, Maria, Ekaterina Galanova, Ilya Davidov, Mikhail Afokin, and Anton Maximov. 2019. "Dimethyl Ether to Olefins over Modified ZSM-5 Based Catalysts Stabilized by Hydrothermal Treatment" Catalysts 9, no. 5: 485. https://doi.org/10.3390/catal9050485
APA StyleMagomedova, M., Galanova, E., Davidov, I., Afokin, M., & Maximov, A. (2019). Dimethyl Ether to Olefins over Modified ZSM-5 Based Catalysts Stabilized by Hydrothermal Treatment. Catalysts, 9(5), 485. https://doi.org/10.3390/catal9050485





