Controllable Acid/Base Propriety of Sulfate Modified Mixed Metal Oxide Derived from Hydrotalcite for Synthesis of Propylene Carbonate
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Mixed Metal Oxide Derived from Hydrotalcite
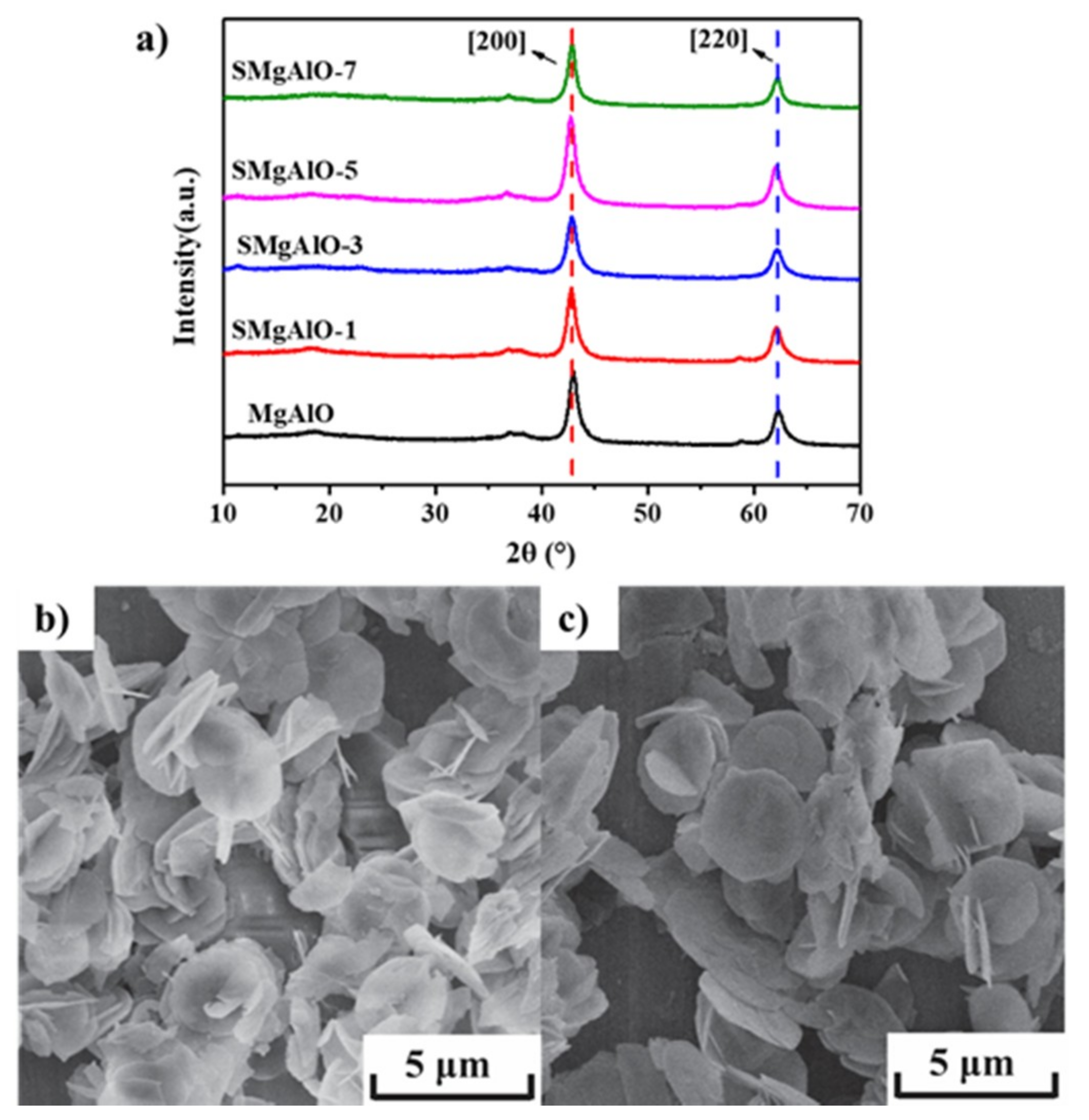
2.1.1. Mophologic Structure
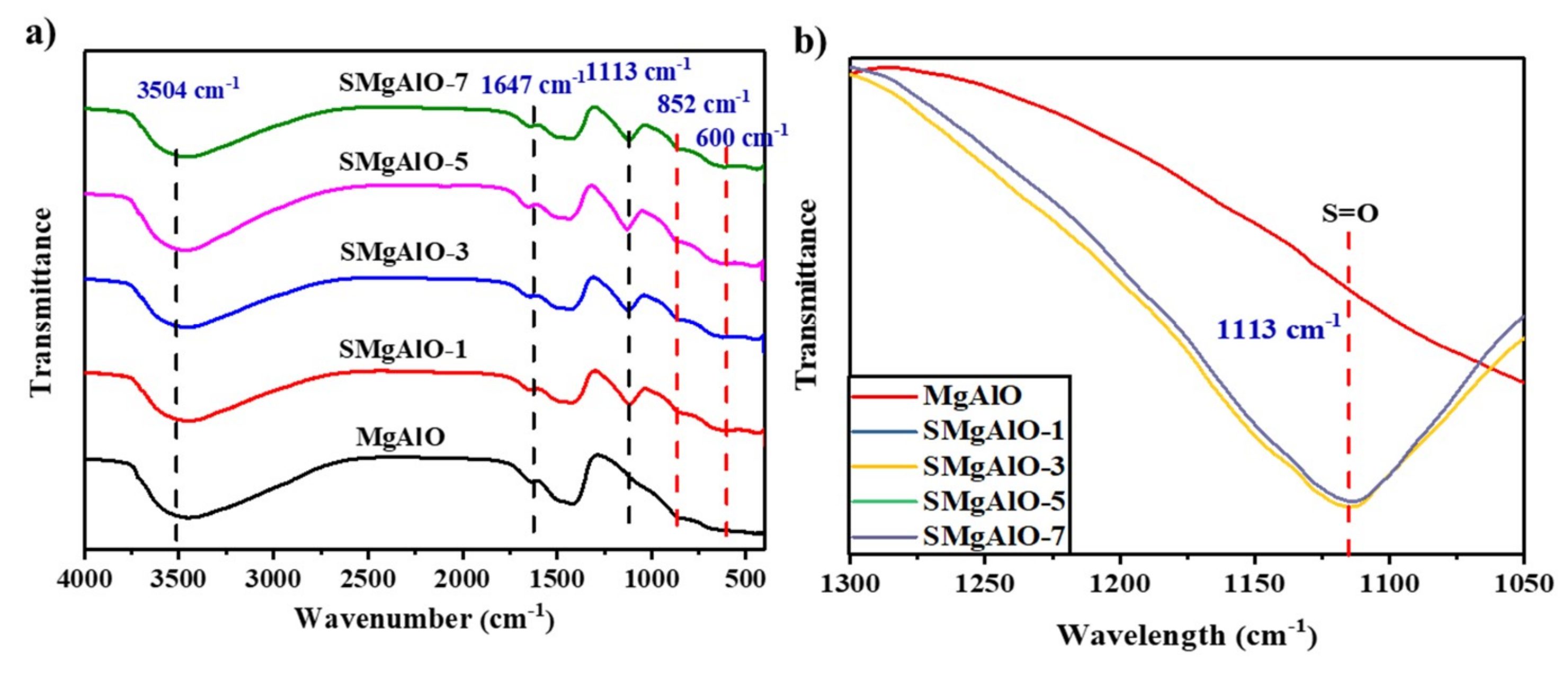
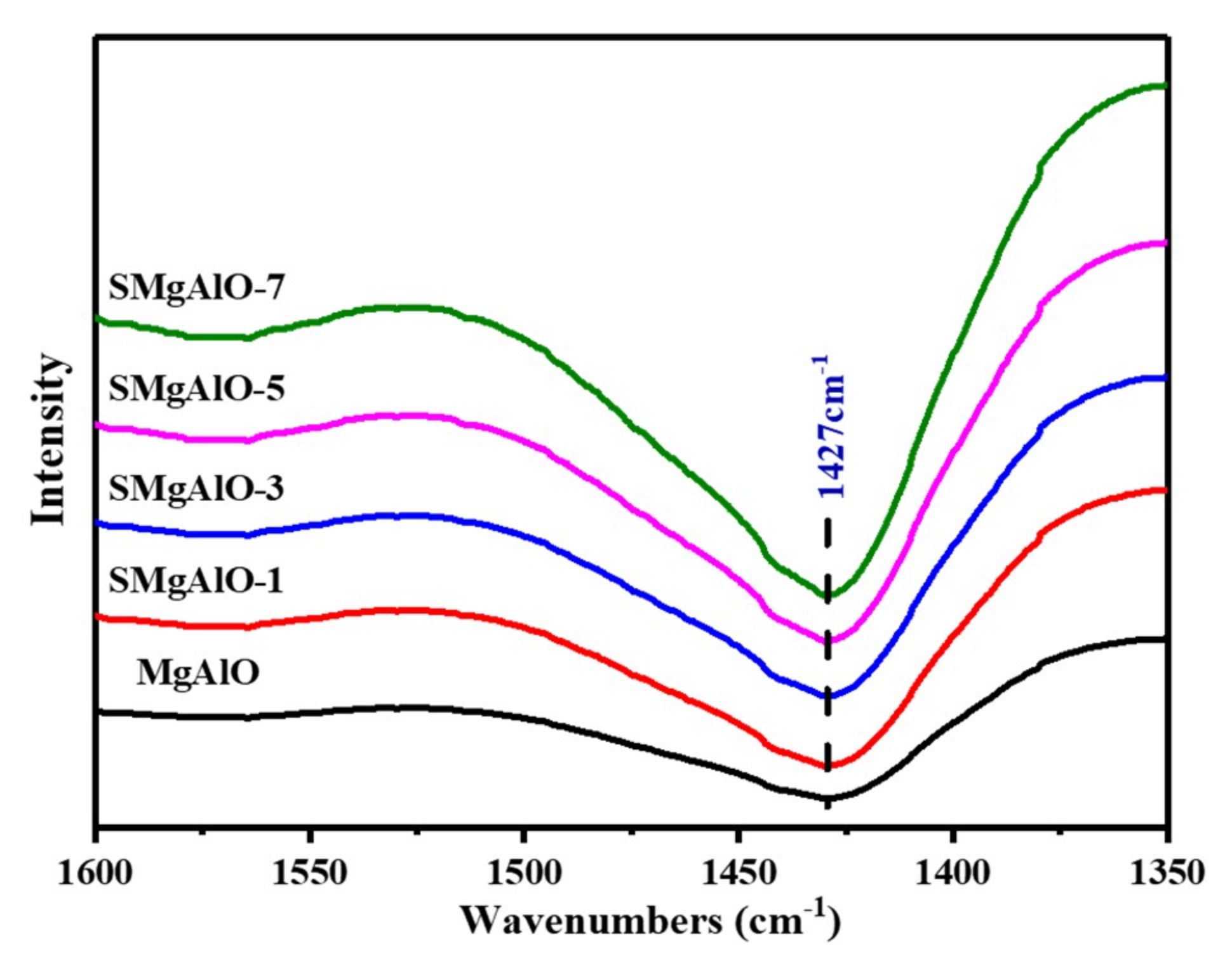
2.1.2. FT-IR Spectra
2.1.3. Textural and Composition Properties
2.1.4. XPS Characterization
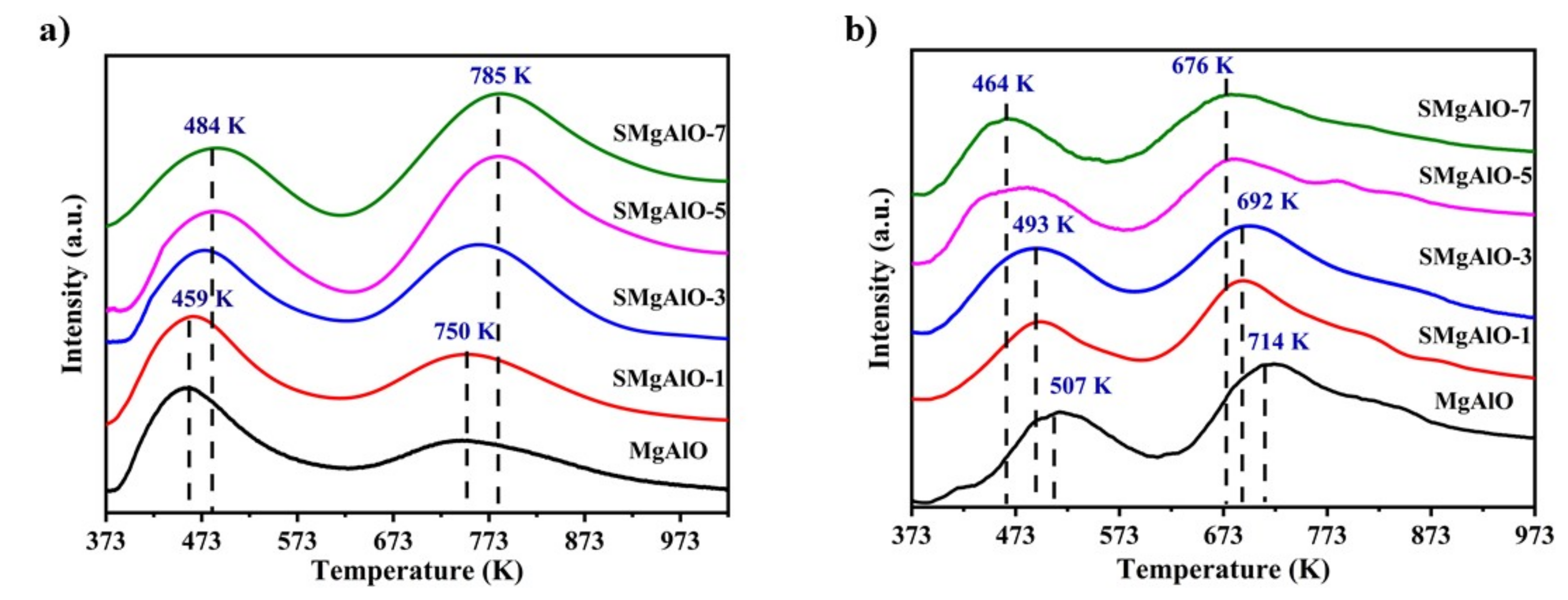
2.2. Acid-Base Site Characterization
2.3. PC Catalytic Synthesis
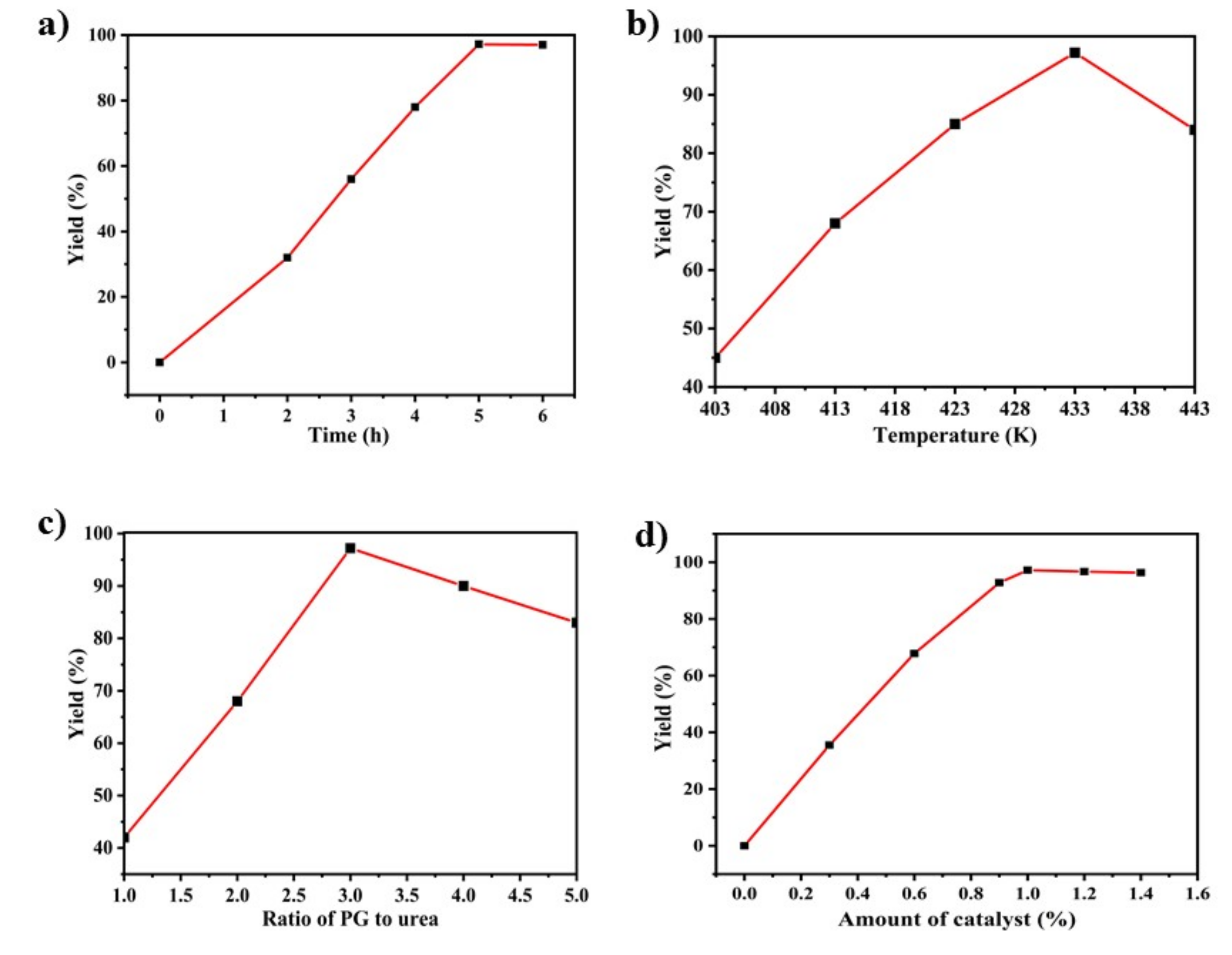
2.3.1. Catalytic Activity of the Synthesis of PC
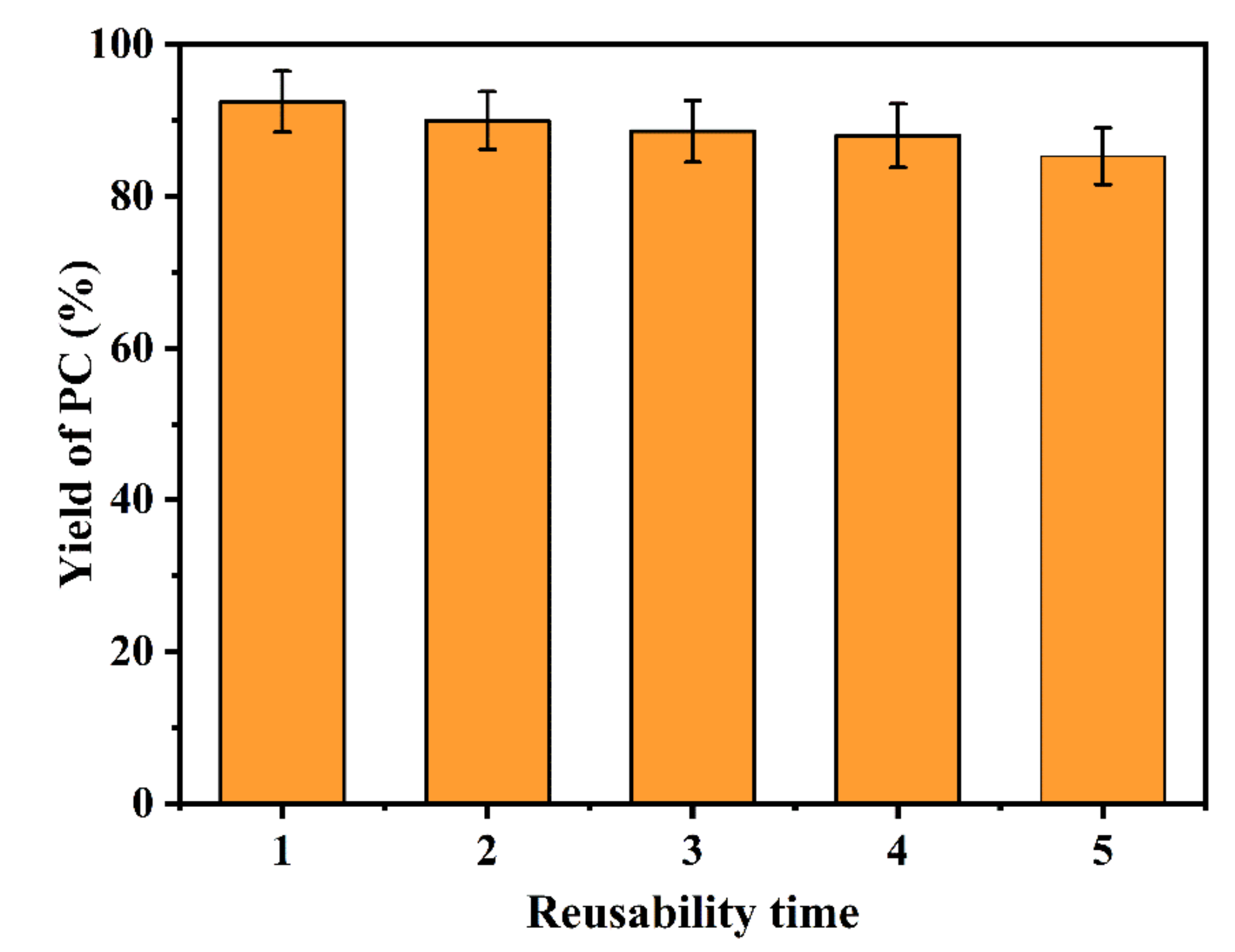
2.3.2. Reusability of Catalyst
2.4. Mechanism Discussion
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.2.1. General Tests
3.2.2. Titration Characterization
3.2.3. IGC Characterization
3.3. Catalyst Test
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Li, H.Y.; Li, C.; Zhang, M.; Wang, K.; Xie, B. Removal of V(V) from aqueous Cr(VI)-bearing solution using anion exchange resin: Equilibrium and kinetics in batch studies. Hydrometallurgy 2016, 165, 381–389. [Google Scholar] [CrossRef]
- An, H.L.; Ma, Y.H.; Zhao, X.Q.; Wang, Y.J. Preparation of Zn-Al oxide catalyst and its catalytic performance in propylene carbonate synthesis from urea and propylene glycol on a fixed-bed reactor. Catal. Today 2016, 264, 136–143. [Google Scholar] [CrossRef]
- Jiang, H.-F.; Yuan, B.-Z.; Qi, C.-R. Coupling of carbon dioxide with epoxides catalyzed by amino acid hydrochloride salts. Chin. J. Chem. 2008, 26, 1305–1308. [Google Scholar] [CrossRef]
- Ma, L.; Song, L.N.; Li, F.; Wang, H.; Liu, B.H. Preparation and properties of poly (propylene carbonate)-based waterborne polyurethane-acrylate composite emulsion. Colloid Polym. Sci. 2017, 295, 2299–2307. [Google Scholar] [CrossRef]
- Kong, J.J.; Li, Z.L.; Cao, Z.W.; Han, C.Y.; Dong, L. The excellent gas barrier properties and unique mechanical properties of poly(propylene carbonate)/organo-montmorillonite nanocomposites. Polym. Bull. 2017, 74, 5065–5082. [Google Scholar] [CrossRef]
- Calderon, B.A.; Sobkowicz, M.J. Evidence of compatibility and thermal stability improvement of poly(propylene carbonate) and polyoxymethylene blends. J. Appl. Polym. Sci. 2018, 135, 45823. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, N.; Wei, W.; Sun, Y. Catalytic performance of metal oxides for the synthesis of propylene carbonate from urea and 1,2-propanediol. J. Mol. Catal. a-Chemical. 2007, 270, 44–49. [Google Scholar] [CrossRef]
- Gao, Z.W.; Wang, S.F.; Xia, C.G. Synthesis of propylene carbonate from urea and 1,2-propanediol. Chin. J. Chem. 2009, 20, 131–135. [Google Scholar] [CrossRef]
- Du, Z.; Chen, F.; Lin, Z.; Li, X.; Yuan, H.; Wu, Y. Effect of MgO on the catalytic performance of MgTiO3 in urea alcoholysis to propylene carbonate. Chem. Eng. J. 2015, 278, 79–84. [Google Scholar] [CrossRef]
- Murugan, C.; Bajaj, H.C.; Jasra, R.V. Transesterification of propylene carbonate by methanol using KF/Al2O3 as an efficient base catalyst. Catal. Lett. 2010, 137, 224–231. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Shi, F.; Liu, S.; He, Y.; Lu, L.; Ma, X.; Deng, Y. Quaternary ammonium ionic liquids as bi-functional catalysts for one-step synthesis of dimethyl carbonate from ethylene oxide, carbon dioxide and methanol. Catal. Lett. 2011, 141, 339–346. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; He, X.; Yang, Y. Gel polymer electrolyte based on poly(methyl methacrylate-maleic anhydride)-poly(ethylene glycol) monomethyl ether and organophilic rectorite clay. Clay Miner. 2010, 45, 431–440. [Google Scholar] [CrossRef]
- Da Silva, E.; Dayoub, W.; Mignani, G.; Raoul, Y.; Lemaire, M. Propylene carbonate synthesis from propylene glycol, carbon dioxide andbenzonitrile by alkali carbonate catalysts. Catal. Comm. 2012, 29, 58–62. [Google Scholar] [CrossRef]
- Doya, M.; Ohkawa, T.; Kanbara, T.; Okamoto, A.; Kimizuka, K. A process for producing alkylene carbonates. European Patent No. EP2657214, 30 March 1994. [Google Scholar]
- Huang, S.; Liu, S.; Li, J.; Zhao, N.; Wei, W.; Sun, Y. Modified zinc oxide for the direct synthesis of propylene carbonate from propylene glycol and carbon dioxide. Catal. Lett. 2007, 118, 290–294. [Google Scholar] [CrossRef]
- Yu, G.L.; Chen, X.R.; Chen, C.L. Synthesis of propylene carbonate from urea and 1,2-propylene glycol over ZnO/NaY catalyst. Catal. Lett. 2009, 97, 69–75. [Google Scholar] [CrossRef]
- Trost, B.M. The atom economy—a search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Gryglewicz, S.; Oko, F.A.; Gryglewicz, G. Synthesis of modern synthetic oils based on dialkyl carbonates. Ind. Eng. Chem. Res. 2003, 42, 5007–5010. [Google Scholar] [CrossRef]
- Meher, L.C.; Sagar, D.V.; Naik, S.N. Technical aspects of biodiesel production by transesterification—A review. Renew. Sust. Energ. Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Zhang, R.; Hua, L.; Guo, L.; Song, B.N.; Chen, J.Z.; Hou, Z.S. Calcined Mg-Al layered double hydroxide as a heterogeneous catalyst for the synthesis of urea derivatives from amines and CO2. Chin. J. Chem. 2013, 31, 381–387. [Google Scholar] [CrossRef]
- Meloni, D.; Monaci, R.; Cutrufello, M.G.; Rombi, E.; Ferino, I. Adsorption microcalorimetry characterization of K-doped mgal mixed oxide catalysts for soybean oil transesterification synthesized by impregnation and ball milling techniques. J. Therm. Anal. Calorim. 2015, 119, 1023–1036. [Google Scholar] [CrossRef]
- Luo, R.C.; Zhang, W.Y.; Zhou, X.T.; Ji, H.B. Tannic acid as a polyphenol material-assisted synthesis of cyclic carbonates using CO2 as a feedstock: Kinetic characteristic and mechanism studies. Chin. J. Chem 2017, 35, 659–664. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, J.; Zhou, Y.; Meng, L.; Wang, Y.; Ren, X.; Liang, J.; Jiang, M. Synergistic interactions between Ti-OOH and (PO4 WO2(O-2) (4))(3-) of HPW/Zn-Ti hydrotalcites: Efficient heterogeneous catalysts for the epoxidation of fatty acid methyl ester. J. Brazil. Chem. Soc. 2018, 29, 67–73. [Google Scholar] [CrossRef]
- Perotti, G.F.; Barud, H.S.; Ribeiro, S.J.L.; Constantino, V.R.L. Bacterial cellulose as a template for preparation of hydrotalcite-like compounds. J. Brazil. Chem. Soc. 2014, 25, 1647–1655. [Google Scholar] [CrossRef]
- Nakagaki, S.; Castro, K.A.D.F.; Ucoski, G.M.; Halma, M.; Prevot, V.; Forano, C.; Wypych, F. Anionic iron(III) porphyrin immobilized on/into exfoliated macroporous layered double hydroxides as catalyst for oxidation reactions. J. Brazil. Chem. Soc. 2014, 25, 2329–2338. [Google Scholar] [CrossRef]
- Marcato, P.D.; Parizotto, N.V.; Martinez, D.S.T.; Paula, A.J.; Ferreira, I.R.; Melo, P.S.; Duran, N.; Alves, O.L. New hybrid material based on layered double hydroxides and biogenic silver nanoparticles: Antimicrobial activity and cytotoxic effect. J. Brazil. Chem. Soc. 2013, 24, 266–272. [Google Scholar] [CrossRef]
- Ren, X.Q.; Hu, X.; Zhang, F.; Wang, J.E.; Liang, J.H.; Wu, W.L.; Jiang, M.; Wang, J. Synthesis of sulphur-modified bifunctional hydrotalcites and study of their surface characteristics by inverse gas chromatography. Catal. Sci. Technol. 2015, 5, 4813–4820. [Google Scholar] [CrossRef]
- Ju, X.H.; Xiao, H.M.; Xia, Q.Y. Theoretical study on intermolecular interactions and thermodynamic properties of difluoroamine complex. Chin. J. Chem. 2003, 21, 1440–1446. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wu, Y.T.; Wang, Y.; Dai, L.Y. Experimental and theoretical investigation of one-pot synthesis of 2-amino-4H-chromenes catalyzed by basic-functionalized ionic liquids. Chin. J. Chem. 2012, 30, 1709–1714. [Google Scholar] [CrossRef]
- Zhou, R.Y.; Jiang, W.; Zhang, L.N.; Wang, L.; Liu, C.L. Extra copper-mediated enhancement of the DNA cleavage activity supported with wild-type Cu, Zn superoxide dismutase. Chin. J. Chem. 2008, 26, 564–570. [Google Scholar] [CrossRef]
- Yasir, A.; Shukla, K.; Srivastava, V.C. Synthesis of propylene carbonate from propane-1,2-diol and urea using hydrotalcite-derived catalysts. Energ Fuel 2017, 31, 9890–9897. [Google Scholar] [CrossRef]
- Ionescu, R.; Pavel, O.D.; Birjega, R.; Zavoianu, R.; Angelescu, E. Epoxidation of cyclohexene with H2O2 and acetonitrile catalyzed by Mg-Al hydrotalcite and cobalt modified hydrotalcites. Catal. Lett. 2010, 134, 309–317. [Google Scholar] [CrossRef]
- Du, Z.P.; Liu, L.; Yuan, H.; Xiong, J.; Zhou, B.; Wu, Y.X. Synthesis of propylene carbonate from alcoholysis of urea catalyzed by modified hydroxyapatites. Chin. J. Catal. 2010, 31, 371–373. [Google Scholar] [CrossRef]
- Zhang, X.G.; Ke, X.B.; Zhu, H.Y. Zeolite-supported gold nanoparticles for selective photooxidation of aromatic alcohols under visible-light irradiation. Chem.-Eur. J. 2012, 18, 8048–8056. [Google Scholar] [CrossRef] [PubMed]
- Osatiashtiani, A.; Lee, A.F.; Brown, D.R.; Melero, J.A.; Morales, G.; Wilson, K. Bifunctional SO4/ZrO2 catalysts for 5-hydroxymethylfufural (5-HMF) production from glucose. Catal. Sci. Tech. 2014, 4, 333–342. [Google Scholar] [CrossRef]
- Alhassan, F.H.; Rashid, U.; Al-Qubaisi, M.S.; Rasedee, A.; Taufiq-Yap, Y.H. The effect of sulfate contents on the surface properties of iron-manganese doped sulfated zirconia catalysts. Powder Technol. 2014, 253, 809–813. [Google Scholar] [CrossRef]
- Wang, X.S.; Ma, S.Q.; Sun, D.F.; Parkin, S.; Zhou, H.C. A mesoporous metal-organic framework with permanent porosity. J. Am. Chem. Soc. 2006, 128, 16474–16475. [Google Scholar] [CrossRef]
- Zhou, H.R.; Li, X.G.; Ma, J.; Dong, C.F.; Huang, Y.Z. Dependence of the corrosion behavior of aluminum alloy 7075 on the thin electrolyte layers. Sci. Eng. B-Adv. 2009, 162, 1–8. [Google Scholar] [CrossRef]
- Ishii, D.; Tatsumi, D.; Matsumoto, T. Effect of aluminum sulfate on dispersion state of sodium carboxymethylcellulose in aqueous solution. Nihon Reoroji Gakk. 2012, 40, 267–272. [Google Scholar] [CrossRef]
- Ye, X.; Jiang, Z.; Li, L.; Xie, Z.-H. In-situ growth of nial-layered double hydroxide on AZ31 Mg alloy towards enhanced corrosion protection. Nanomaterials 2018, 8, 411–420. [Google Scholar] [CrossRef]
- Wang, X.-F.; Xiong, S.-M. Oxidation behavior of molten magnesium in atmospheres containing SO2 and air in a sealed furnace. Corros. Sci. 2013, 66, 300–307. [Google Scholar] [CrossRef]
- Delgado, M.G.; Garcia-Galvan, F.R.; Llorente, I.; Perez, P.; Adeva, P.; Feliu, S., Jr. Influence of aluminium enrichment in the near-surface region of commercial twin-roll cast AZ31 alloys on their corrosion behaviour. Corros. Sci. 2017, 123, 182–196. [Google Scholar] [CrossRef]
- Shen, L.J.; Zheng, X.H.; Lei, G.C.; Li, X.; Cao, Y.N.; Jiang, L.L. Hierarchically porous γ-Al2O3 nanosheets: Facile template-free preparation and reaction mechanism for H2S selective oxidation. Chem. Eng. J. 2018, 346, 238–248. [Google Scholar] [CrossRef]
- Stephan, D.W.; Erker, G. Frustrated lewis pairs: Metal-free hydrogen activation and more. Angew. Chem.-Int. Edit. 2010, 49, 46–76. [Google Scholar] [CrossRef]
- Zhang, T.T.; Zhang, B.S.; Li, L.; Zhao, N.; Xiao, F.K. Zn-Mg mixed oxide as high-efficiency catalyst for the synthesis of propylene carbonate by urea alcoholysis. Catal. Commun. 2015, 66, 38–41. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Zhang, Y.; Wang, Y.J. Synthesis of propylene carbonate from urea and 1,2-propylene glycol over a zinc acetate catalyst. Ind. Eng. Chem. Res. 2004, 43, 4038–4042. [Google Scholar] [CrossRef]
- Jung, S.M.; Grange, P. Evidence of correlation between electronic density and surface acidity of sulfated TiO2. Catal. Lett. 2001, 76, 27–30. [Google Scholar] [CrossRef]
- Kost, T.; Sigalov, M.; Goldberg, I.; Ben-Asuly, A.; Lemcoff, N.G. Latent sulfur chelated ruthenium catalysts: Steric acceleration effects on olefin metathesis. J. Organomet. Chem. 2008, 693, 2200–2203. [Google Scholar] [CrossRef]
- Wang, S.P.; Shi, Y.; Ma, X.B. Effect of sulfate modification on structure properties, surface acidity, and transesterification catalytic performance of titanium-submitted mesoporous molecular sieve. Ind. Eng. Chem. Res. 2012, 51, 5737–5742. [Google Scholar] [CrossRef]
- Fugel, M.; Hesse, M.F.; Pal, R.; Beckmann, J.; Jayatilaka, D.; Turner, M.J.; Karton, A.; Bultinck, P.; Chandler, G.S.; Grabowsky, S. Covalency and ionicity do not oppose each other-relationship between Si-O bond character and basicity of siloxanes. Chem.-Eur. J. 2018, 24, 15275–15286. [Google Scholar] [CrossRef]
- Rao, M.M.; Reddy, B.R.; Jayalakshmi, M.; Jaya, V.S.; Sridhar, B. Hydrothermal synthesis of Mg-Al hydrotalcites by urea hydrolysis. Mater. Res. Bull. 2005, 40, 347–359. [Google Scholar] [CrossRef]
- Costantino, U.; Marmottini, F.; Nocchetti, M.; Vivani, R. New synthetic routes to hydrotalcite-like compounds - characterisation and properties of the obtained materials. Eur. J. Inorg. Chem. 1998, 1439–1446. [Google Scholar] [CrossRef]


| Samples | Sulfur Content (wt%) a | ICP | SBET b (m2/g) | VT c (cm3/g) |
|---|---|---|---|---|
| Mg: Al | ||||
| MgAlO | - | 4.01:1 | 75.5 | 0.26 |
| SMgAlO-1 | 0.37 | 3.93: 1 | 81.5 | 0.25 |
| SMgAlO-3 | 1.21 | 4.01: 1 | 89.8 | 0.28 |
| SMgAlO-5 | 2.24 | 3.96: 1 | 101.4 | 0.31 |
| SMgAlO-7 | 3.19 | 3.98: 1 | 90.6 | 0.22 |
| Samples | Distribution of Acidic Sites | Total Acidic Sites/mmol·g−1 | Distribution of Alkaline Sites | Total Alkaline Sites/mmol·g−1 | ||
|---|---|---|---|---|---|---|
| Aw | As | Bw | Bs | |||
| MgAlO | 0.096 | 0.026 | 0.122 | 0.435 | 0.527 | 0.962 |
| SMgAlO-1 | 0.102 | 0.036 | 0.138 | 0.396 | 0.478 | 0.874 |
| SMgAlO-3 | 0.125 | 0.146 | 0.271 | 0.422 | 0.413 | 0.835 |
| SMgAlO-5 | 0.143 | 0.215 | 0.358 | 0.403 | 0.388 | 0.791 |
| SMgAlO-7 | 0.124 | 0.191 | 0.315 | 0.387 | 0.341 | 0.728 |
| Catalyst | Alkali Number/mmol·g−1 | ||
|---|---|---|---|
| phenolphthalein H_ ≥ 9.3 | 2,4,6-trinitroaniline H_ ≥ 12.2 | Total | |
| MgAlO | 2.31 | 0.45 | 2.76 |
| SMgAlO-1 | 2.17 | 0.30 | 2.47 |
| SMgAlO-3 | 2.13 | 0.28 | 2.41 |
| SMgAlO-5 | 2.12 | 0.26 | 2.38 |
| SMgAlO-7 | 2.08 | 0.24 | 2.32 |
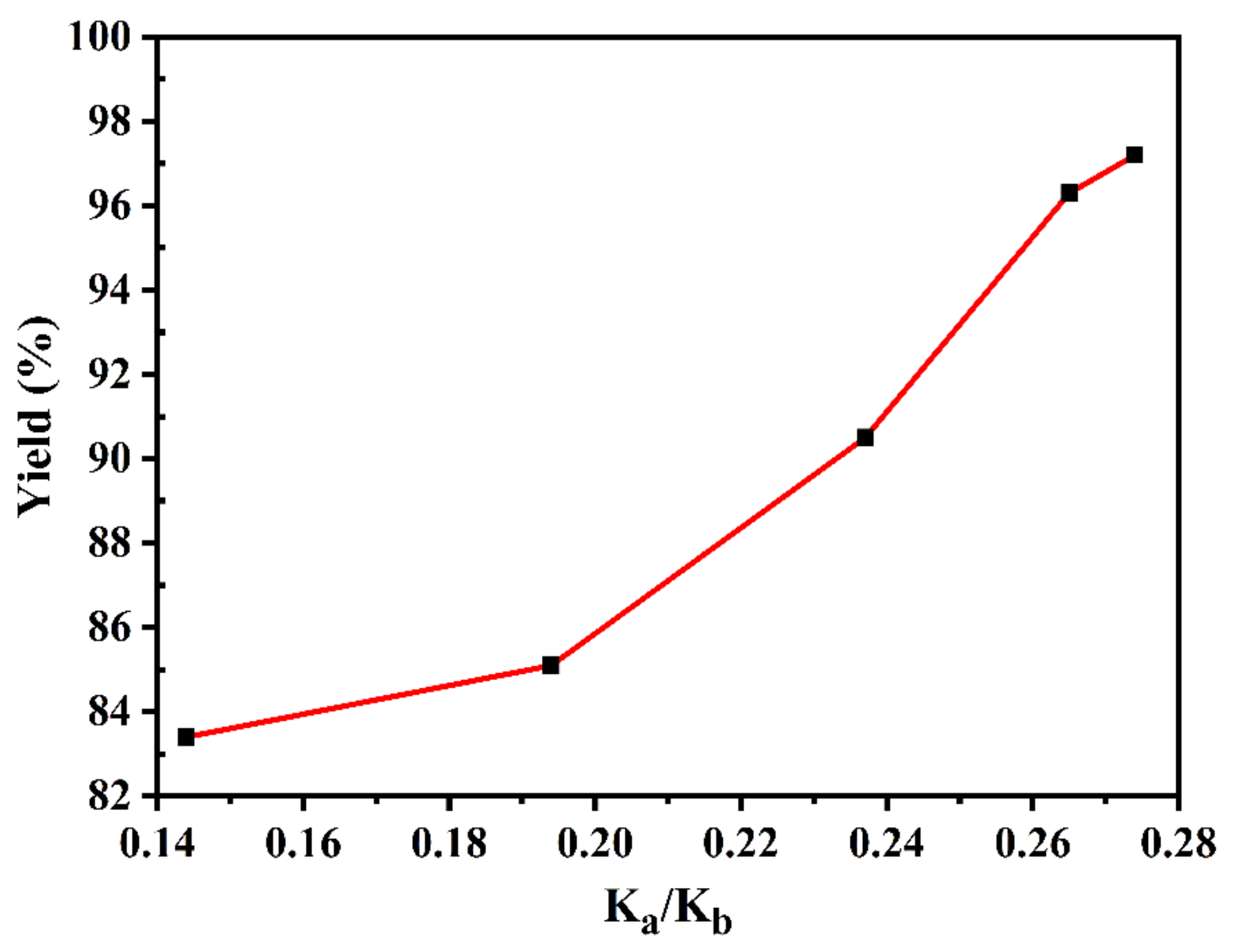
| Sample | Ka | Kb | Ka + Kb | Ka/Kb | Yield of PC (%) |
|---|---|---|---|---|---|
| MgAlO | 0.098 | 0.681 | 0.779 | 0.144 | 83.4 ± 0.4 |
| SMgAlO-1 | 0.132 | 0.679 | 0.811 | 0.194 | 85.1 ± 0.6 |
| SMgAlO-3 | 0.156 | 0.659 | 0.815 | 0.237 | 90.5 ± 0.2 |
| SMgAlO-5 | 0.179 | 0.653 | 0.832 | 0.274 | 97.2 ± 0.3 |
| SMgAlO-7 | 0.172 | 0.649 | 0.821 | 0.265 | 96.3 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Z.; Xu, W.; Zhang, X.; Liu, Z.; Shen, J.; Liang, J.; Jiang, M.; Ren, X. Controllable Acid/Base Propriety of Sulfate Modified Mixed Metal Oxide Derived from Hydrotalcite for Synthesis of Propylene Carbonate. Catalysts 2019, 9, 470. https://doi.org/10.3390/catal9050470
Ding Z, Xu W, Zhang X, Liu Z, Shen J, Liang J, Jiang M, Ren X. Controllable Acid/Base Propriety of Sulfate Modified Mixed Metal Oxide Derived from Hydrotalcite for Synthesis of Propylene Carbonate. Catalysts. 2019; 9(5):470. https://doi.org/10.3390/catal9050470
Chicago/Turabian StyleDing, Zhongxie, Wenlong Xu, Xingguang Zhang, Zhen Liu, Jiecan Shen, Jinhua Liang, Min Jiang, and Xiaoqian Ren. 2019. "Controllable Acid/Base Propriety of Sulfate Modified Mixed Metal Oxide Derived from Hydrotalcite for Synthesis of Propylene Carbonate" Catalysts 9, no. 5: 470. https://doi.org/10.3390/catal9050470
APA StyleDing, Z., Xu, W., Zhang, X., Liu, Z., Shen, J., Liang, J., Jiang, M., & Ren, X. (2019). Controllable Acid/Base Propriety of Sulfate Modified Mixed Metal Oxide Derived from Hydrotalcite for Synthesis of Propylene Carbonate. Catalysts, 9(5), 470. https://doi.org/10.3390/catal9050470