Synthesis of MCM-41 Immobilized (Phenoxy)Imine Palladium(II) Complexes as Recyclable Catalysts in the Methoxycarbonylation of 1-Hexene
Abstract
1. Introduction
2. Results and Discussion
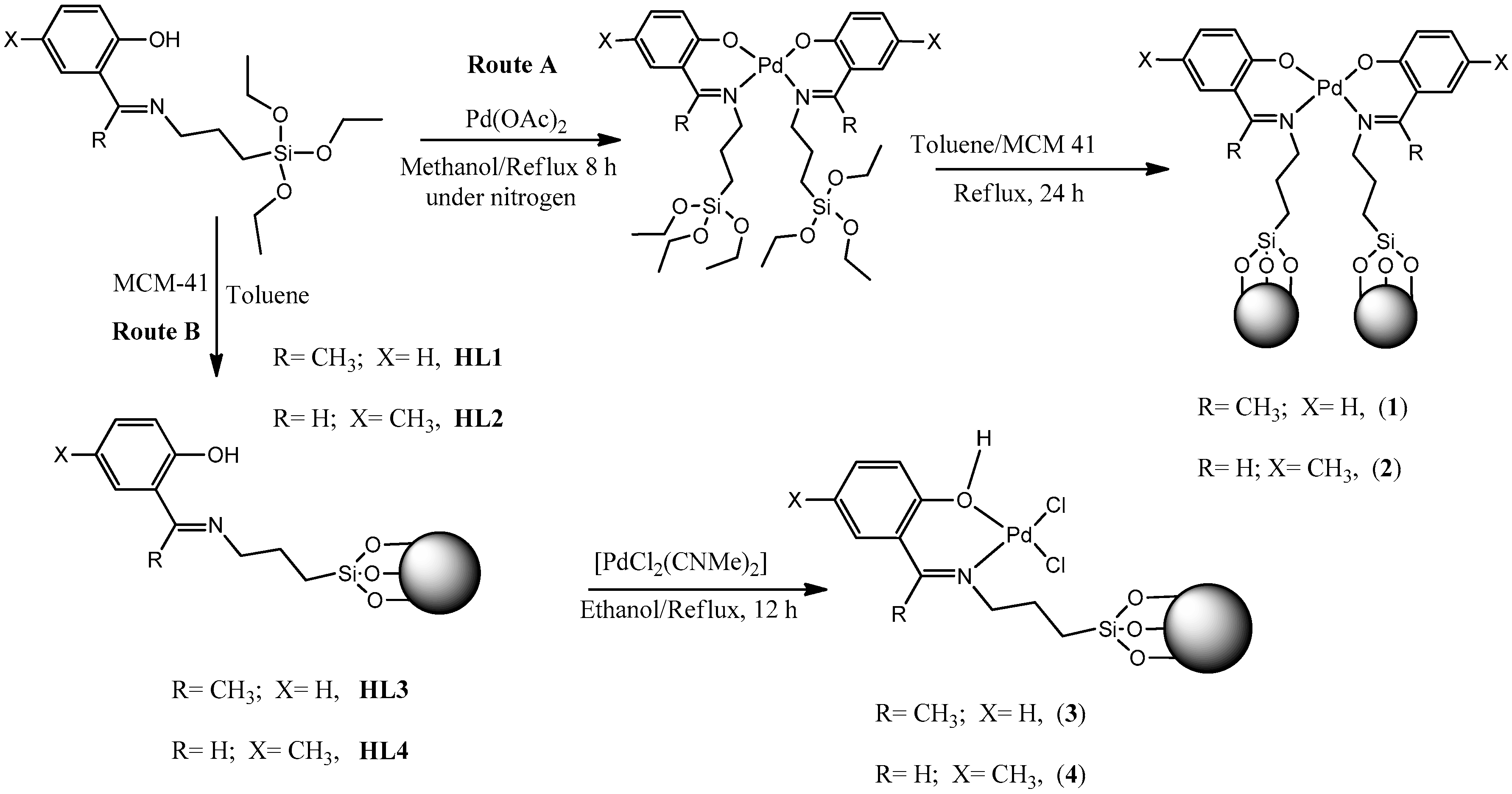
2.1. Synthesis of Immobilized (Phenoxy)Imine Ligands and their Palladium(II) Complexes
2.2. Catalytic Studies of the Immobilized Catalysts in the Methoxycarbonylation of Olefins
2.2.1. Methoxycarbonylation of 1-Hexene Catalysed by Complexes 1–4
2.2.2. The Effect of Acid Promoter on the Methoxycarbonylation of 1-Hexene
2.2.3. The Effect of Reaction Conditions on Methoxycarbonylation Reactions
2.2.4. Catalyst Recycling and Leaching Studies
3. Experimental Section and Methods
3.1. General Instrumentation and Materials
3.2. Synthesis of (Phenoxy)Imine Immobilized Ligands and Their Palladium(II) Complexes
3.2.1. Synthesis of HL1-MCM-41 (HL3)
3.2.2. Synthesis of HL2-MCM 41 (HL4)
3.2.3. Synthesis of (Pd(L1)2)-MCM-41 (1)
3.2.4. Synthesis of (Pd(L2)2)-MCM-41 (2)
3.2.5. Synthesis of Pd(HL3)(Cl2) (3)
3.2.6. Synthesis of Pd(HL4)(Cl2) (4)
3.3. General Procedure for the Methoxycarbonylation Reactions
3.4. General Procedure for Catalyst Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marr, A.; Marr, P. Entrapping homogeneous catalysts by sol–gel methods: The bottom-up synthesis of catalysts that recycle and cascade. Dalton Trans. 2011, 40, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, R.K.; Sleight, A. Structure-Activity and Selectivity Relationships in Heterogeneous Catalysis; Elsevier: Amsterdam, The Netherlands, 1991; Volume 67. [Google Scholar]
- De Pater, J.J.; Deelman, B.J.; Elsevier, C.J.; van Koten, G. Multiphase systems for the recycling of alkoxycarbonylation catalysts. Adv. Synth. Catal. 2006, 348, 1447–1458. [Google Scholar] [CrossRef]
- Brintzinger, H.H.; Fischer, D.; Mülhaupt, R.; Rieger, B.; Waymouth, R.M. Stereospecific olefin polymerization with chiral metallocene catalysts. Angew. Chem. Int. Ed. Engl. 1995, 34, 1143–1170. [Google Scholar] [CrossRef]
- Shylesh, S.; Schünemann, V.; Thiel, W.R. Magnetically separable nanocatalysts: Bridges between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2010, 49, 3428–3459. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.H.; Macquarrie, D.J.; Mubofu, E.B. Preparation of a novel silica-supported palladium catalyst and its use in the Heck reaction. Green Chem. 2000, 2, 53–56. [Google Scholar] [CrossRef]
- Mukhopadhyay, K.; Sarkar, B.R.; Chaudhari, R.V. Anchored Pd complex in MCM-41 and MCM-48: Novel heterogeneous catalysts for hydrocarboxylation of aryl olefins and alcohols. J. Am. Chem. Soc. 2002, 124, 9692–9693. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Liao, S.; Yu, D. Polymer-supported palladium–manganese bimetallic catalyst for the oxidative carbonylation of amines to carbamate esters. Appl. Catal. A 1999, 183, 81–84. [Google Scholar] [CrossRef]
- McMorn, P.; Hutchings, G.J. Heterogeneous enantioselective catalysts: Strategies for the immobilisation of homogeneous catalysts. Chem. Soc. Rev. 2004, 33, 108–122. [Google Scholar] [CrossRef]
- Horváth, I.T.; Rábai, J. Facile catalyst separation without water: Fluorous biphase hydroformylation of olefins. Science 1994, 266, 72–75. [Google Scholar] [CrossRef]
- Collis, A.E.; Horvath, I.T. Heterogenization of homogeneous catalytic systems. Catal. Sci. Technol. 2011, 1, 912–919. [Google Scholar] [CrossRef]
- Standfest-Hauser, C.M.; Lummerstorfer, T.; Schmid, R.; Hoffmann, H.; Kirchner, K.; Puchberger, M.; Trzeciak, A.M.; Mieczyńska, E.; Tylus, W.; Ziółkowski, J.J. Rhodium phosphine complexes immobilized on silica as active catalysts for 1-hexene hydroformylation and arene hydrogenation. J. Mol. Catal. A Chem. 2004, 210, 179–187. [Google Scholar] [CrossRef]
- Thomas, J.M.; Raja, R. Catalytic significance of organometallic compounds immobilized on mesoporous silica: Economically and environmentally important examples. J. Organomet. Chem. 2004, 689, 4110–4124. [Google Scholar] [CrossRef]
- Ma, Z.; Sun, W.H.; Zhu, N.; Li, Z.; Shao, C.; Hu, Y. Preparation of silica-supported late transition metal catalyst and ethylene polymerization. Polym. Int. 2002, 51, 349–352. [Google Scholar] [CrossRef]
- Zhi, J.; Song, D.; Li, Z.; Lei, X.; Hu, A. Palladium nanoparticles in carbon thin film-lined SBA-15 nanoreactors: Efficient heterogeneous catalysts for Suzuki–Miyaura cross coupling reaction in aqueous media. Chem. Commun. 2011, 47, 10707–10709. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Cui, Z.-M.; Niu, F.; Jiang, L.; Song, W.-G. Pd nanoparticles in silica hollow spheres with mesoporous walls: A nanoreactor with extremely high activity. Chem. Commun. 2010, 46, 6524–6526. [Google Scholar] [CrossRef] [PubMed]
- Sadjadi, S.; Heravi, M.M.; Masoumi, B.; Kazemi, S.S. Pd (0) nanoparticles immobilized on multi-nitrogen functionalized Halloysite for promoting Sonogashira reaction: Studying the role of the number of surface nitrogens in catalytic performance. J. Coord. Chem. 2019, in press. [Google Scholar] [CrossRef]
- Sadjadi, S.; Koohestani, F.; Bahri-Laleh, N.; Didehban, K. Halloysite nanoclay decorated with 2-amino pyrimidine functionalized poly glycidyl methacrylate: An efficient support for the immobilization of Pd nanoparticles. J. Solid State Chem. 2019, 271, 59–66. [Google Scholar] [CrossRef]
- Oliveira, R.L.; He, W.; Gebbink, R.J.K.; de Jong, K.P. Palladium nanoparticles confined in thiol-functionalized ordered mesoporous silica for more stable Heck and Suzuki catalysts. Catal. Sci. Technol. 2015, 5, 1919–1928. [Google Scholar] [CrossRef]
- Espinoza, R.; Snel, R.; Korf, C.; Nicolaides, C. Catalytic oligomerization of ethene over nickel-exchanged amorphous silica-aluminas; effect of the acid strength of the support. Appl. Catal. 1987, 29, 295–303. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tomishige, K. Total hydrogenation of furan derivatives over silica-supported Ni–Pd alloy catalyst. Catal. Commun. 2010, 12, 154–156. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, B.; Lee, K.R.; Yi, M.H.; Hur, N.H. Dual Pd and CuFe2O4 nanoparticles encapsulated in a core/shell silica microsphere for selective hydrogenation of arylacetylenes. Chem. Commun. 2012, 48, 4414–4416. [Google Scholar]
- Khedkar, M.V.; Sasaki, T.; Bhanage, B.M. Immobilized palladium metal-containing ionic liquid-catalyzed alkoxycarbonylation, phenoxycarbonylation, and aminocarbonylation reactions. ACS Catal. 2013, 3, 287–293. [Google Scholar] [CrossRef]
- Mane, R.S.; Sasaki, T.; Bhanage, B.M. Silica supported palladium-phosphine as a reusable catalyst for alkoxycarbonylation and aminocarbonylation of aryl and heteroaryl iodides. RSC Adv. 2015, 5, 94776–94785. [Google Scholar] [CrossRef]
- Bredenkamp, T.; Holzapfel, C. The Pd-catalysed hydromethoxycarbonylation of aliphatic internal alkenes with minimal double bond isomerisation. Catal. Commun. 2017, 96, 74–78. [Google Scholar] [CrossRef]
- Kosswig, K.; Schaefer, W. Hydrocarboxymethylation-an Attractive Route from Olefins to Fatty Acid Esters? Ind. Eng. Chem. Prod. Res. Dev. 1980, 19, 330–334. [Google Scholar] [CrossRef]
- Saphan, O.A.; Stephen, O.O. Methoxycarbonylation of olefins catalysed by homogeneous palladium(II) complexes of (phenoxy)imine ligands bearing alkoxy silane groups. Inorg. Chim. Acta 2019. Under review. [Google Scholar]
- Knapen, J.W.; van der Made, A.W.; de Wilde, J.C.; van Leeuwen, P.W.; Wijkens, P.; Grove, D.M.; van Koten, G. Homogeneous catalysts based on silane dendrimers functionalized with arylnickel (II) complexes. Nature 1994, 372, 659. [Google Scholar] [CrossRef]
- Anwander, R. Immobilization of molecular catalysts. In Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 583–614. [Google Scholar]
- Singh, G.; Saroa, A.; Khullar, S.; Mandal, S.K. Schiff bases of N-(2-aminoethyl)-3-aminopropyltrimethoxysilane and its silatranes: Synthesis and characterization. J. Chem. Sci. 2015, 127, 679–685. [Google Scholar] [CrossRef]
- Bhunia, S.; Jana, S.; Saha, D.; Dutta, B.; Koner, S. Catalytic olefin epoxidation over cobalt (II)-containing mesoporous silica by molecular oxygen in dimethylformamide medium. Catal. Sci. Technol. 2014, 4, 1820–1828. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Molnár, Á. Silica-supported Pd catalysts for Heck coupling reactions. Tetrahedron 2007, 63, 6949–6976. [Google Scholar] [CrossRef]
- Bhunia, S.; Saha, D.; Koner, S. MCM-41-Supported Oxo-vanadium (IV) Complex: A Highly Selective Heterogeneous Catalyst for the Bromination of Hydroxy Aromatic Compounds in Water. Langmuir 2011, 27, 15322–15329. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, S.; Ghasemzadeh, M.S.; Honarmand, M.; Zarifi, F. Acetamidine–palladium complex immobilized on γ-Fe2O3 nanoparticles: A novel magnetically separable catalyst for Heck and Suzuki coupling reactions. RSC Adv. 2014, 4, 44166–44174. [Google Scholar] [CrossRef]
- Huo, C.; Ouyang, J.; Yang, H. CuO nanoparticles encapsulated inside Al-MCM-41 mesoporous materials via direct synthetic route. Sci. Rep. 2014, 4, 3682. [Google Scholar] [CrossRef] [PubMed]
- Hajjami, M.; Ghorbani, F.; Bakhti, F. MCM-41-N-propylsulfamic acid: An efficient catalyst for one-pot synthesis of 1-amidoalkyl-2-naphtols. Appl. Catal. A 2014, 470, 303–310. [Google Scholar] [CrossRef]
- Durand, J.; Zangrando, E.; Stener, M.; Fronzoni, G.; Carfagna, C.; Binotti, B.; Kamer, P.C.; Müller, C.; Caporali, M.; van Leeuwen, P.W. Long-Lived Palladium Catalysts for CO/Vinyl Arene Polyketones Synthesis: A Solution to Deactivation Problems. Chem. Eur. J. 2006, 12, 7639–7651. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, P.A.; Lagos, C.A.; Moya, S.A.; Zúñiga, C.; Vera-Oyarce, C.; Sola, E.; Peris, G.; Bayón, J.C. Methoxycarbonylation of olefins catalyzed by palladium complexes bearing P, N-donor ligands. Dalton Trans. 2007, 46, 5419–5426. [Google Scholar] [CrossRef]
- Tshabalala, T.A.; Ojwach, S.O.; Akerman, M.A. Palladium complexes of (benzoimidazol-2-ylmethyl) amine ligands as catalysts for methoxycarbonylation of olefins. J. Mol. Catal. A Chem. 2015, 406, 178–184. [Google Scholar] [CrossRef]
- Zolezzi, S.; Moya, S.A.; Valdebenito, G.; Abarca, G.; Parada, J.; Aguirre, P. Methoxycarbonylation of olefins catalyzed by palladium (II) complexes containing naphthyl (diphenyl) phosphine ligands. Appl. Organomet. Chem. 2014, 28, 364–371. [Google Scholar] [CrossRef]
- Kumar, K.; Darkwa, J. Palladium (II) complexes bearing mixed N^ N^ X (X= O and S) tridentate ligands as pre-catalysts for the methoxycarbonylation of selected 1-alkenes. Polyhedron 2017, 138, 249–257. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Wang, T.; Li, C.; Yan, L.; Jiang, M.; Liu, J.; Sun, X.; Jiang, Z.; Ding, Y. The 2V-P, N polymer supported palladium catalyst for methoxycarbonylation of acetylene. J. Mol. Catal. A Chem. 2016, 414, 37–46. [Google Scholar] [CrossRef]
- Tshabalala, T.A.; Ojwach, S.O. Tuning the regioselectivity of (benzimidazolylmethyl) amine palladium (II) complexes in the methoxycarbonylation of hexenes and octenes. Transit. Metal Chem. 2018, 43, 339–346. [Google Scholar] [CrossRef]
- Alam, M.G.; Tshabalala, T.A.; Ojwach, S.O. Metal-Catalyzed Alkene Functionalization Reactions Towards Production of Detergent and Surfactant Feedstocks. J. Surfact. Deterg. 2017, 20, 75–81. [Google Scholar] [CrossRef]
- Cypryk, M.; Apeloig, Y. Mechanism of the Acid-Catalyzed Si–O Bond Cleavage in Siloxanes and Siloxanols. A Theoretical Study. Organometallics 2002, 21, 2165–2175. [Google Scholar] [CrossRef]
- Tang, C.-M.; Li, X.-L.; Wang, G.-Y. A highly efficient catalyst for direct synthesis of methyl acrylate via methoxycarbonylation of acetylene. Korean J. Chem. Eng. 2012, 29, 1700–1707. [Google Scholar] [CrossRef]
- Hendriksen, B.; Frenken, J. CO oxidation on Pt (110): Scanning tunneling microscopy inside a high-pressure flow reactor. Phys. Rev. Lett. 2002, 89, 046101. [Google Scholar] [CrossRef] [PubMed]
- Mägerlein, W.; Beller, M.; Indolese, A.F. Palladium-catalyzed carbonylation of aryl halides—A detailed investigation of the alkoxycarbonylation of 4-bromoacetophenone. J. Mol. Catal. A Chem. 2000, 156, 213–221. [Google Scholar] [CrossRef]
- Takegami, Y.; Yokokawa, C.; Watanabe, Y.; Masada, H.; Okuda, Y. Studies of the Organic Reactions of Metal Carbonyls. VI. The Isomerization of Acylcobalt Carbonyls—The Effects of Solvents and of the Structure of the Acyl Group. Bull. Chem. Soc. Jpn. 1965, 38, 787–791. [Google Scholar] [CrossRef]
- Crocker, M.; Herold, R. Carbomethoxylation of ethylene catalysed by Pd (II) complexes intercalated in smectite clay. J. Mol. Catal. 1991, 70, 209–216. [Google Scholar] [CrossRef]
- Dyson, P.J.; Jessop, P.G. Solvent effects in catalysis: Rational improvements of catalysts via manipulation of solvent interactions. Catal. Sci. Technol. 2016, 6, 3302–3316. [Google Scholar] [CrossRef]
- Ziccarelli, I.; Neumann, H.; Kreyenschulte, C.; Gabriele, B.; Beller, M. Pd-Supported on N-doped carbon: Improved heterogeneous catalyst for base-free alkoxycarbonylation of aryl iodides. Chem. Commun. 2016, 52, 12729–12732. [Google Scholar] [CrossRef]
- Ünver, H.; Yılmaz, F. Synthesis, Characterization, and Catalytic Hydrogenation Activity of New N-Acyl-Benzotriazole Rh (I) and Ru (III) Complexes in [bmim][BF4]. Catalysts 2016, 6, 147. [Google Scholar] [CrossRef]
- Widegren, J.A.; Finke, R.G. A review of the problem of distinguishing true homogeneous catalysis from soluble or other metal-particle heterogeneous catalysis under reducing conditions. J. Mol. Catal. A Chem. 2003, 198, 317–341. [Google Scholar] [CrossRef]
- Yu, K.; Sommer, W.; Weck, M.; Jones, C.W. Silica and polymer-tethered Pd–SCS-pincer complexes: Evidence for precatalyst decomposition to form soluble catalytic species in Mizoroki–Heck chemistry. J. Catal. 2004, 226, 101–110. [Google Scholar] [CrossRef]
- Williams, D.B.G.; Lawton, M. Drying of organic solvents: Quantitative evaluation of the efficiency of several desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef] [PubMed]
- Burfield, D.R.; Smithers, R.H. Desiccant efficiency in solvent drying. 3. Dipolar aprotic solvents. J. Org. Chem. 1978, 43, 3966–3968. [Google Scholar] [CrossRef] [PubMed]








| Entry | Catalyst | Conv (%) b | l/b (%) c | TOF d |
|---|---|---|---|---|
| 1 | 1 | 76 | 60/40 | 6.3 |
| 2 | 2 | 78 | 62/38 | 6.5 |
| 3 | 3 | 59 | 63/37 | 4.9 |
| 4 | 4 | 61 | 63/37 | 5.0 |
| 5 e | - | 0 | - | - |
| 6 f | 2 | 0 | 0 | 0 |
| 7 | Pd(OAc)2/PPh3 | 20 | 65/35 | 1.7 |
| Entry | PCO (bar) | Temp (°C) | Time (h) | Pd% | Conv (%) b | l/b (%) c | TOF d |
|---|---|---|---|---|---|---|---|
| 1 | 60 | 90 | 24 | 0.5 | 78 | 62/38 | 6.5 |
| 2 | 60 | 90 | 24 | 0.25 | 27 | 64/36 | 4.5 |
| 3 | 60 | 90 | 24 | 0.75 | 94 | 65/35 | 5.2 |
| 4 | 60 | 90 | 24 | 1.0 | 87 | 62/38 | 3.6 |
| 5 | 60 | 90 | 24 | 1.5 | 62 | 61/39 | 1.7 |
| 6 | 60 | 90 | 12 | 0.5 | 34 | 66/34 | 5.4 |
| 7 | 40 | 90 | 24 | 0.5 | 66 | 64/36 | 5.5 |
| 8 | 60 | 60 | 24 | 0.5 | 59 | 61/39 | 4.9 |
| 9 | 60 | 90 | 36 | 0.5 | 90 | 60/40 | 5.0 |
| 10 | 60 | 100 | 24 | 0.5 | 51 | 63/37 | 4.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akiri, S.O.; Ojwach, S.O. Synthesis of MCM-41 Immobilized (Phenoxy)Imine Palladium(II) Complexes as Recyclable Catalysts in the Methoxycarbonylation of 1-Hexene. Catalysts 2019, 9, 143. https://doi.org/10.3390/catal9020143
Akiri SO, Ojwach SO. Synthesis of MCM-41 Immobilized (Phenoxy)Imine Palladium(II) Complexes as Recyclable Catalysts in the Methoxycarbonylation of 1-Hexene. Catalysts. 2019; 9(2):143. https://doi.org/10.3390/catal9020143
Chicago/Turabian StyleAkiri, Saphan O., and Stephen O. Ojwach. 2019. "Synthesis of MCM-41 Immobilized (Phenoxy)Imine Palladium(II) Complexes as Recyclable Catalysts in the Methoxycarbonylation of 1-Hexene" Catalysts 9, no. 2: 143. https://doi.org/10.3390/catal9020143
APA StyleAkiri, S. O., & Ojwach, S. O. (2019). Synthesis of MCM-41 Immobilized (Phenoxy)Imine Palladium(II) Complexes as Recyclable Catalysts in the Methoxycarbonylation of 1-Hexene. Catalysts, 9(2), 143. https://doi.org/10.3390/catal9020143







