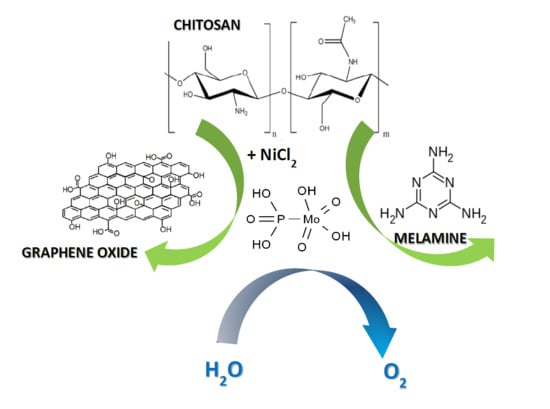
Ni-Based Composites from Chitosan Biopolymer a One-Step Synthesis for Oxygen Evolution Reaction
Abstract
:1. Introduction
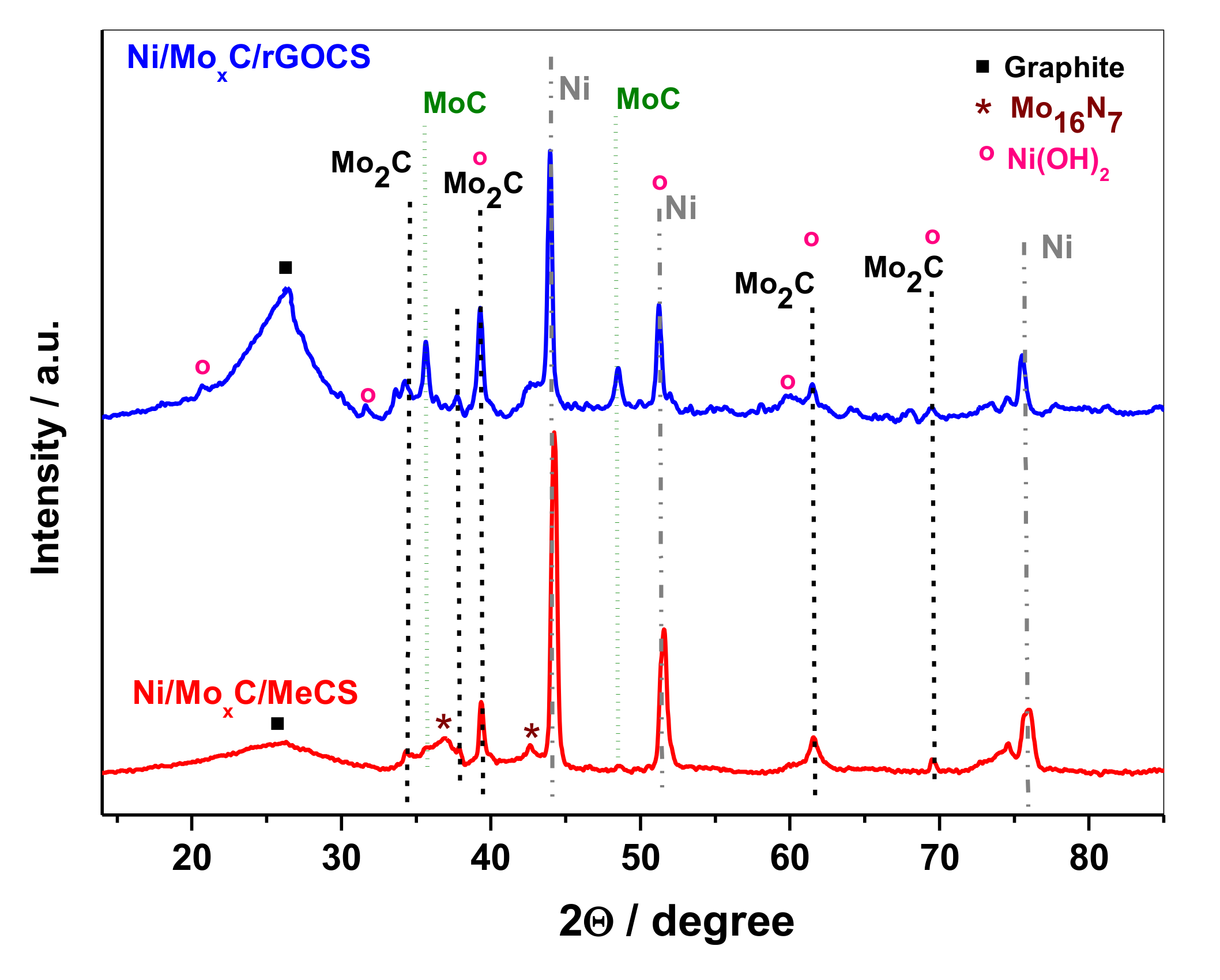
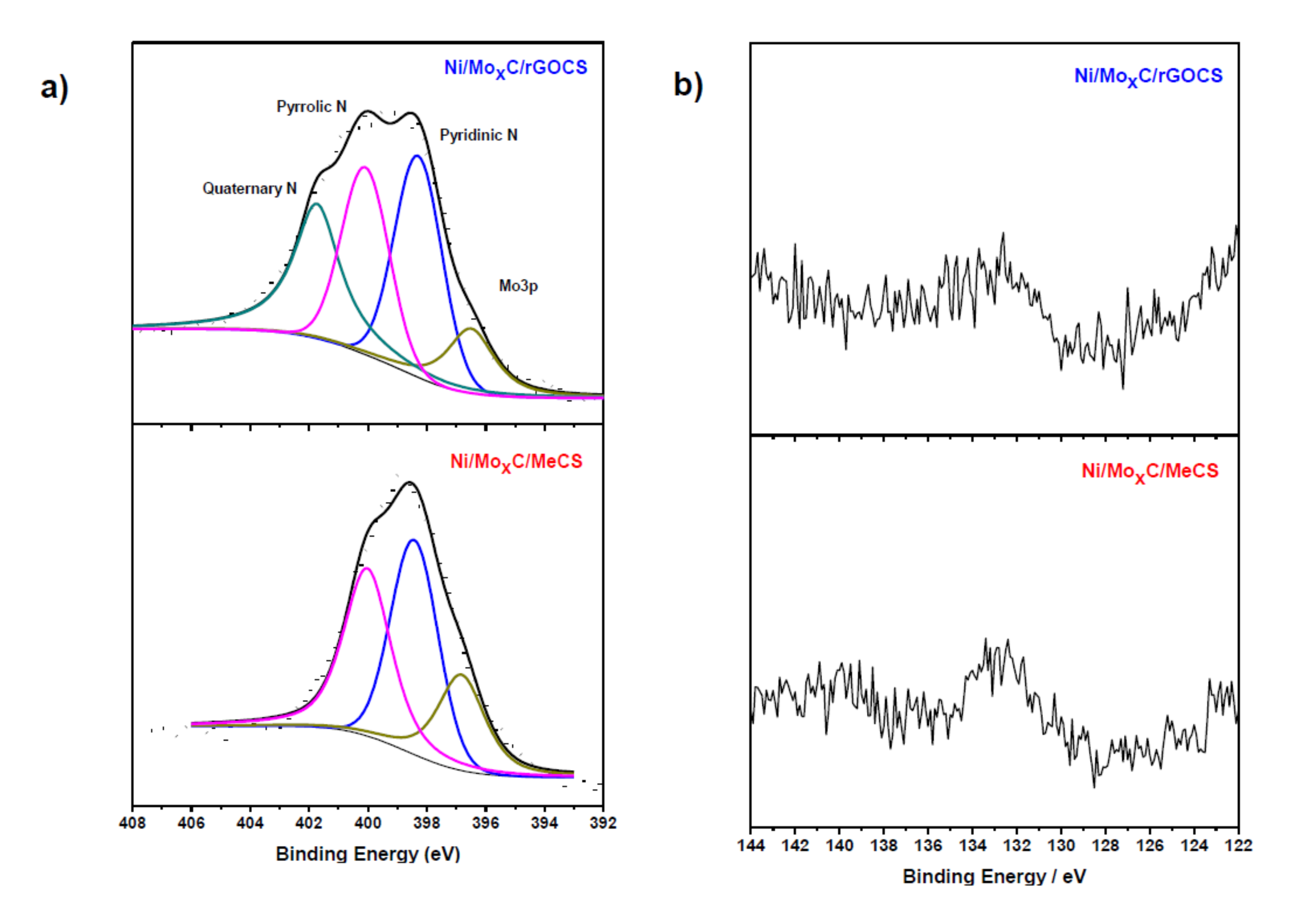
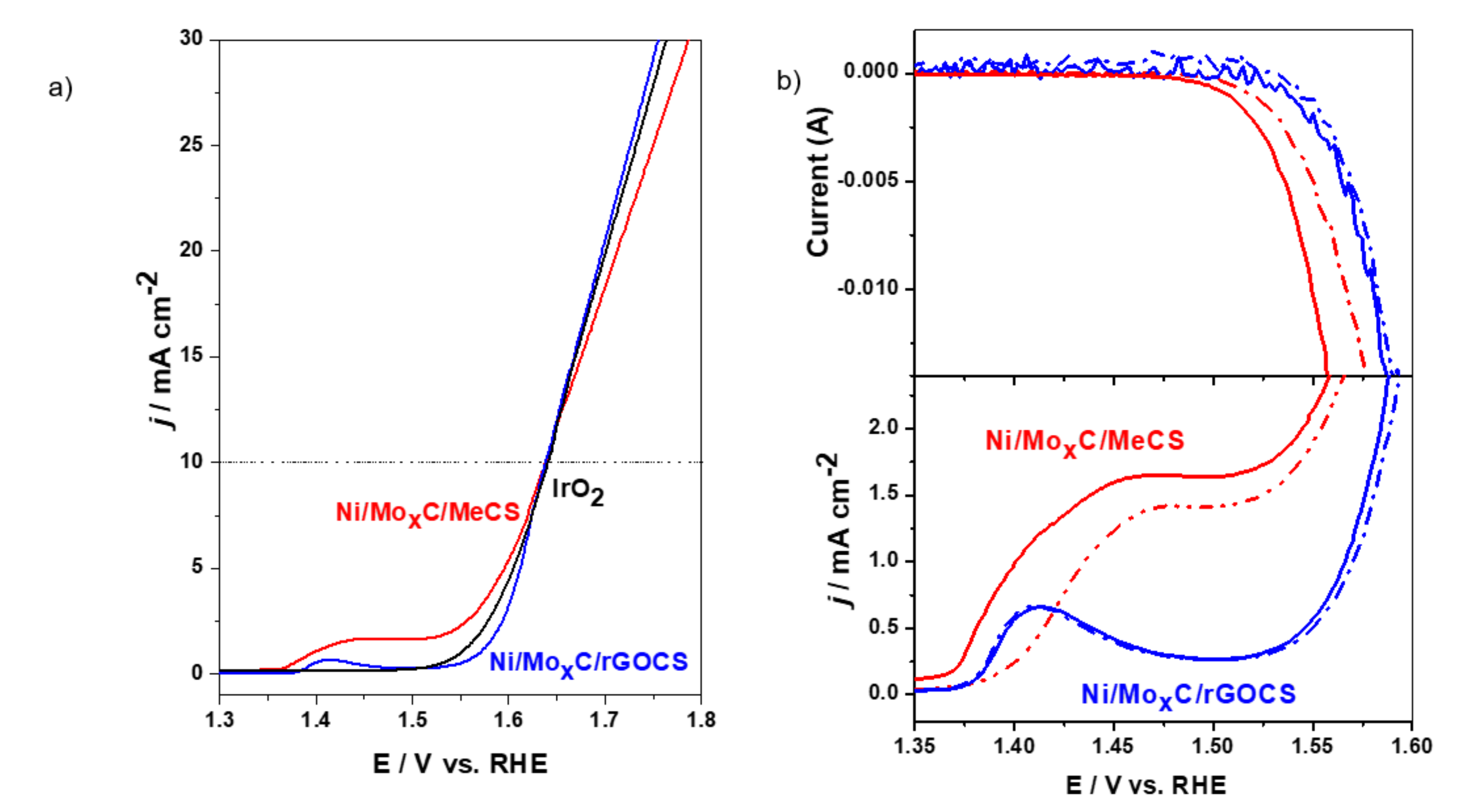
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Sol. Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Wurster, R.; Schindler, J. Solar and wind energy coupled with electrolysis and fuel cells. Handb. Fuel Cells 2003, 3, 62–77. [Google Scholar]
- Yang, Y.; Zhou, K.; Ma, L.; Liang, Y.; Yang, X.; Cui, Z.; Zhu, S.; Li, Z. Free-standing ternary NiWP film for efficient water oxidation reaction. Appl. Surf. Sci. 2018, 434, 871–878. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Fabbri, E.; Schmidt, T.J. Oxygen Evolution Reaction—The Enigma in Water Electrolysis. ACS Catal. 2018, 8, 9765–9774. [Google Scholar] [CrossRef]
- Man, I.C.; Su, H.-Y.; Hansen, H.A.; Martinez, J.I.; Inoglu, N.G.; Kitchin, J.; Jaramillo, T.F.; Nørskov, J.K.; Rossmeisl, J.; Calle-Vallejo, F.; et al. Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces. ChemCatChem 2011, 3, 1159–1165. [Google Scholar] [CrossRef]
- Seong, K.J.; Byunghoon, K.; Hyunah, K.; Kisuk, K. Recent Progress on Multimetal Oxide Catalysts for the Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1702774. [Google Scholar] [CrossRef]
- Suen, N.-T.; Hung, S.-F.; Quan, Q.; Zhang, N.; Xu, Y.-J.; Chen, H.M. Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives. Chem. Soc. Rev. 2017, 46, 337–365. [Google Scholar]
- Slavcheva, E.; Radev, I.; Bliznakov, S.; Topalov, G.; Andreev, P.; Budevski, E. Sputtered iridium oxide films as electrocatalysts for water splitting via PEM electrolysis. Electrochim. Acta 2007, 52, 3889–3894. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Q.; Long, S.; Huang, X. Cobalt-molybdenum nanosheet arrays as highly efficient and stable earth-abundant electrocatalysts for overall water splitting. Nano Energy 2018, 45, 448–455. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, Q.; Xiao, W.; Wang, J.; Wang, L. Design of highly efficient Ni-based water-electrolysis catalysts by a third transition metal addition into Ni 3 Mo. Intermetallics 2018, 94, 99–105. [Google Scholar] [CrossRef]
- Kim, B.; Oh, A.; Kabiraz, M.K.; Hong, Y.; Joo, J.; Baik, H.; Choi, S.-I.; Lee, K. NiOOH Exfoliation-Free Nickel Octahedra as Highly Active and Durable Electrocatalysts Toward the Oxygen Evolution Reaction in an Alkaline Electrolyte. ACS Appl. Mater. Interfaces 2018, 10, 10115–10122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, L.; Han, Y.; Xu, M.; Dong, S. Nitrogen-doped carbon encapsulating γ-MoC/Ni heterostructures for efficient oxygen evolution electrocatalysts. Nanoscale 2017, 9, 5583–5588. [Google Scholar] [CrossRef]
- Jahangir, M.; Polydoros-Chrysovalantis, I.; Nikolaos, L.; Panayotis, K.; Manashi, N. A Molecular Ni-complex Containing Tetrahedral Nickel Selenide Core as Highly Efficient Electrocatalyst for Water Oxidation. ChemSusChem 2016, 9, 3128–3132. [Google Scholar] [CrossRef]
- Xiong, X.; You, C.; Liu, Z.; Asiri, A.M.; Sun, X. Co-Doped CuO Nanoarray: An Efficient Oxygen Evolution Reaction Electrocatalyst with Enhanced Activity. ACS Sustain. Chem. Eng. 2018, 6, 2883–2887. [Google Scholar] [CrossRef]
- Gao, M.; Sheng, W.; Zhuang, Z.; Fang, Q.; Gu, S.; Jiang, J.; Yan, Y. Efficient Water Oxidation Using Nanostructured α-Nickel-Hydroxide as an Electrocatalyst. J. Am. Chem. Soc. 2014, 136, 7077–7084. [Google Scholar] [CrossRef] [PubMed]
- Louie, M.W.; Bell, A.T. An Investigation of Thin-Film Ni–Fe Oxide Catalysts for the Electrochemical Evolution of Oxygen. J. Am. Chem. Soc. 2013, 135, 12329–12337. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-Y.; Duan, Y.; Gao, M.-R.; Lang, C.-C.; Zheng, Y.-R.; Yu, S.-H. A one-dimensional porous carbon-supported Ni/Mo2C dual catalyst for efficient water splitting. Chem. Sci. 2017, 8, 968–973. [Google Scholar] [CrossRef]
- Das, D.; Santra, S.; Nanda, K.K. In Situ Fabrication of a Nickel/Molybdenum Carbide-Anchored N-Doped Graphene/CNT Hybrid: An Efficient (Pre)catalyst for OER and HER. ACS Appl. Mater. Interfaces 2018, 10, 35025–35038. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, H.; Li, J.; Yue, X.; Han, Y.; Shen, P.K.; Cui, Y. Porous MoO 2 Nanosheets as Non-noble Bifunctional Electrocatalysts for Overall Water Splitting. Adv. Mater. 2016, 28, 3785–3790. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, Q.; Zeng, C.; Ai, L. Cobalt/molybdenum carbide@N-doped carbon as a bifunctional electrocatalyst for hydrogen and oxygen evolution reactions. J. Mater. Chem. A 2017, 5, 16929–16935. [Google Scholar] [CrossRef]
- Anjum, M.A.R.; Lee, M.H.; Lee, J.S. Boron- and Nitrogen-Codoped Molybdenum Carbide Nanoparticles Imbedded in a BCN Network as a Bifunctional Electrocatalyst for Hydrogen and Oxygen Evolution Reactions. ACS Catal. 2018, 8, 8296–8305. [Google Scholar] [CrossRef]
- Qiao, M.; Tang, C.; He, G.; Qiu, K.; Binions, R.; Parkin, I.P.; Zhang, Q.; Guo, Z.X.; Titirici, M. Graphene/nitrogen-doped porous carbon sandwiches for the metal-free oxygen reduction reaction: Conductivity versus active sites. J. Mater. Chem. A 2016, 4, 12658–12666. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Jablonska, M.; Lieder, M. N-doped mesoporous carbon nanosheets obtained by pyrolysis of a chitosan–melamine mixture for the oxygen reduction reaction in alkaline media. RSC Adv. 2015, 5, 44969–44977. [Google Scholar] [CrossRef]
- Xu, Z.; Si, J. Preparation of N-doped Nanoporous Carbon from Crude Biomass and its Electrochemical Activity. NANO 2016, 11, 1650028. [Google Scholar] [CrossRef]
- Aghabarari, B.; Nezafati, N.; Roca-Ayats, M.; Capel-Sánchez, M.C.; Lázaro, M.J.; Martínez-Huerta, M.V. Effect of molybdophosphoric acid in iron and cobalt graphene/chitosan composites for oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 28093–28101. [Google Scholar] [CrossRef]
- Aghabarari, B.; Martínez-Huerta, M.V.; Ghiaci, M.; Fierro, J.L.G.; Peña, M.A. Hybrid chitosan derviative-carbon support for oxygen reduction reactions. RSC Adv. 2013, 3, 5378–5381. [Google Scholar] [CrossRef]
- Selvam, S.; Balamuralitharan, B.; Jegatheeswaran, S.; Kim, M.-Y.; Karthick, S.N.; Raj, J.A.; Boomi, P.; Sundrarajan, M.; Prabakar, K.; Kim, H.-J. Electrolyte-imprinted graphene oxide–chitosan chelate with copper crosslinked composite electrodes for intense cyclic-stable, flexible supercapacitors. J. Mater. Chem. A 2017, 5, 1380–1386. [Google Scholar] [CrossRef]
- Li, R.; Cao, A.; Zhang, Y.; Li, G.; Jiang, F.; Li, S.; Chen, D.; Wang, C.; Ge, J.; Shu, C. Formation of Nitrogen-Doped Mesoporous Graphitic Carbon with the Help of Melamine. ACS Appl. Mater. Interfaces 2014, 6, 20574–20578. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, H.; Yang, Z.; Ji, G.; Yu, B.; Liu, X.; Liu, Z. Mesoporous nitrogen-doped carbons with high nitrogen contents and ultrahigh surface areas: Synthesis and applications in catalysis. Green Chem. 2016, 18, 1976–1982. [Google Scholar] [CrossRef]
- Wang, M.; Dipazir, S.; Lu, P.; Wang, Y.; Yuan, M.; Li, S.; Zhang, G. Synthesis of polyoxometalates derived bifunctional catalyst towards efficient overall water splitting in neutral and alkaline medium. J. Colloid Interface Sci. 2018, 532, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Razmjooei, F.; Singh, K.P.; Bae, E.J.; Yu, J.-S. A new class of electroactive Fe- and P-functionalized graphene for oxygen reduction. J. Mater. Chem. A 2015, 3, 11031–11039. [Google Scholar] [CrossRef]
- Razmjooei, F.; Singh, K.P.; Song, M.Y.; Yu, J.-S. Enhanced electrocatalytic activity due to additional phosphorous doping in nitrogen and sulfur-doped graphene: A comprehensive study. Carbon 2014, 78, 257–267. [Google Scholar] [CrossRef]
- Roca-Ayats, M.; Roca-Moreno, M.; Martínez-Huerta, M. Optimization of alkaline catalytic inks for three-electrode electrochemical half-cell measurements. Int. J. Hydrogen Energy 2016, 41, 19656–19663. [Google Scholar] [CrossRef]
- Juodkazis, K.; Juodkazytė, J.; Vilkauskaitė, R.; Jasulaitienė, V.; Juodkazis, S. Nickel surface anodic oxidation and electrocatalysis of oxygen evolution. J. Solid State Electrochem. 2008, 12, 1469–1479. [Google Scholar] [CrossRef]
- Trotochaud, L.; Ranney, J.K.; Williams, K.N.; Boettcher, S.W. Solution-Cast Metal Oxide Thin Film Electrocatalysts for Oxygen Evolution. J. Am. Chem. Soc. 2012, 134, 17253–17261. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.D.L.; Prévot, M.S.; Fagan, R.D.; Trudel, S.; Berlinguette, C.P. Water Oxidation Catalysis: Electrocatalytic Response to Metal Stoichiometry in Amorphous Metal Oxide Films Containing Iron, Cobalt, and Nickel. J. Am. Chem. Soc. 2013, 135, 11580–11586. [Google Scholar] [CrossRef] [PubMed]

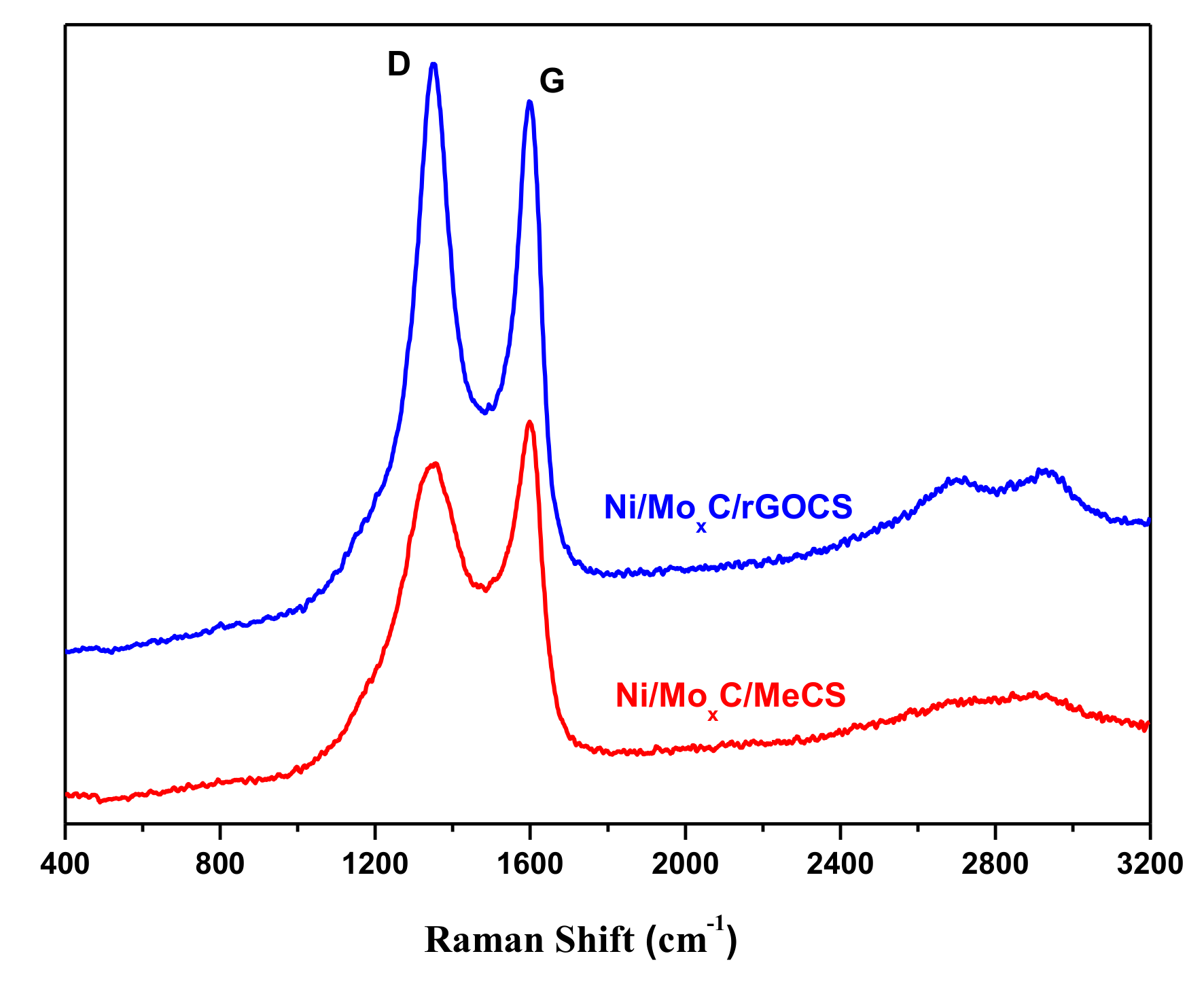

| Catalysts | C | N | P | Ni | Mo | BET (m2/g) | ID/IG |
|---|---|---|---|---|---|---|---|
| Ni/MoxC/rGOCS | 45 | 1.4 | 2.1 | 6.3 | 17 | 132 | 1.09 |
| Ni/MoxC/MeCS | 40 | 1.3 | 0.7 | 8.0 | 22 | 17 | 0.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghabarari, B.; Luque-Centeno, J.M.; Capel-Sánchez, M.; Lázaro Elorri, M.J.; Martínez-Huerta, M.V. Ni-Based Composites from Chitosan Biopolymer a One-Step Synthesis for Oxygen Evolution Reaction. Catalysts 2019, 9, 471. https://doi.org/10.3390/catal9050471
Aghabarari B, Luque-Centeno JM, Capel-Sánchez M, Lázaro Elorri MJ, Martínez-Huerta MV. Ni-Based Composites from Chitosan Biopolymer a One-Step Synthesis for Oxygen Evolution Reaction. Catalysts. 2019; 9(5):471. https://doi.org/10.3390/catal9050471
Chicago/Turabian StyleAghabarari, Behzad, José Manuel Luque-Centeno, Maricarmen Capel-Sánchez, Maria Jesús Lázaro Elorri, and Maria Victoria Martínez-Huerta. 2019. "Ni-Based Composites from Chitosan Biopolymer a One-Step Synthesis for Oxygen Evolution Reaction" Catalysts 9, no. 5: 471. https://doi.org/10.3390/catal9050471
APA StyleAghabarari, B., Luque-Centeno, J. M., Capel-Sánchez, M., Lázaro Elorri, M. J., & Martínez-Huerta, M. V. (2019). Ni-Based Composites from Chitosan Biopolymer a One-Step Synthesis for Oxygen Evolution Reaction. Catalysts, 9(5), 471. https://doi.org/10.3390/catal9050471