Fast Degradation of Monochloroacetic Acid by BiOI-Enhanced UV/S(IV) Process: Efficiency and Mechanism
Abstract
:1. Introduction
2. Results and Discussion
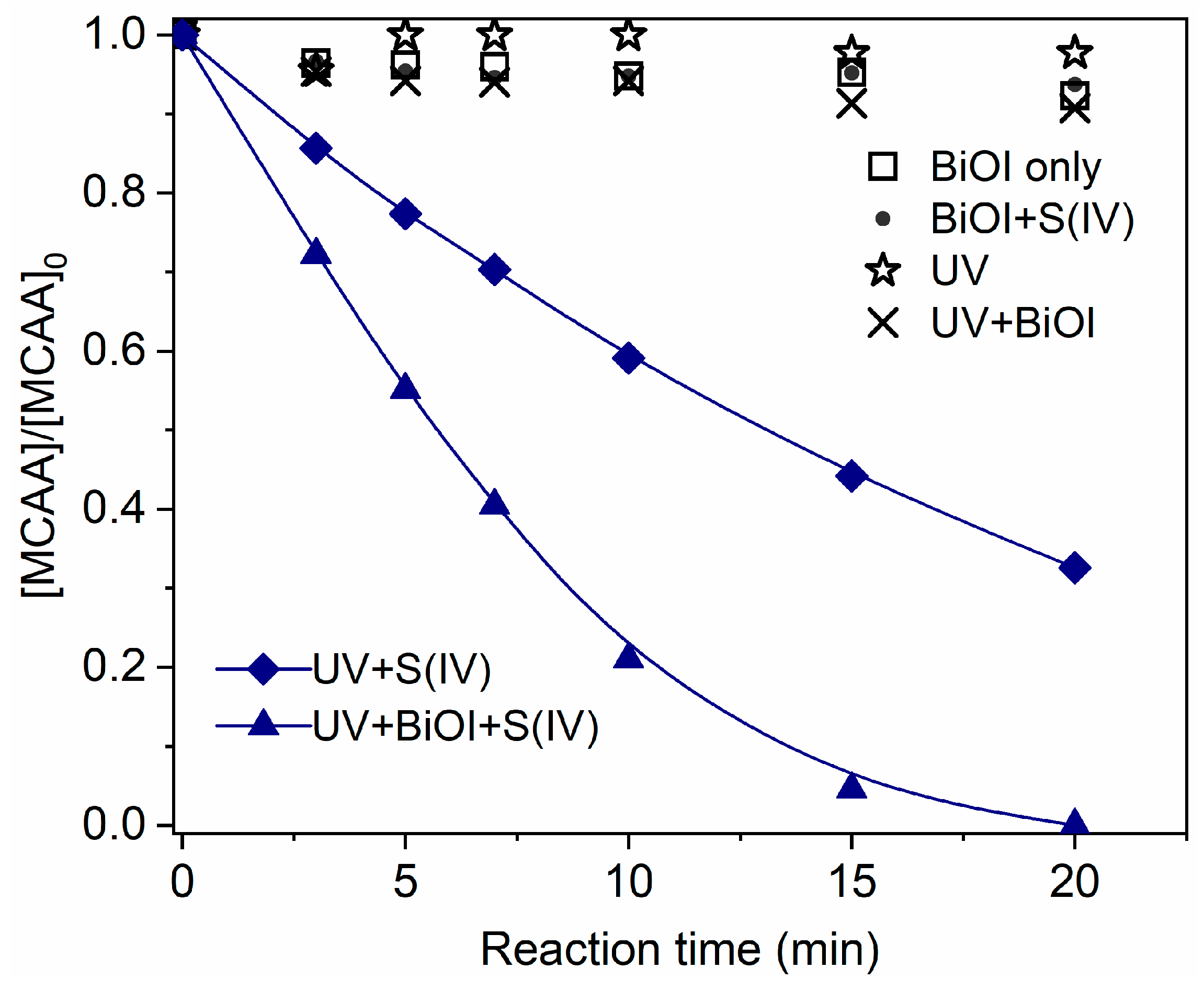
2.1. Degradation of MCAA in the UV/S(IV)/BiOI Process
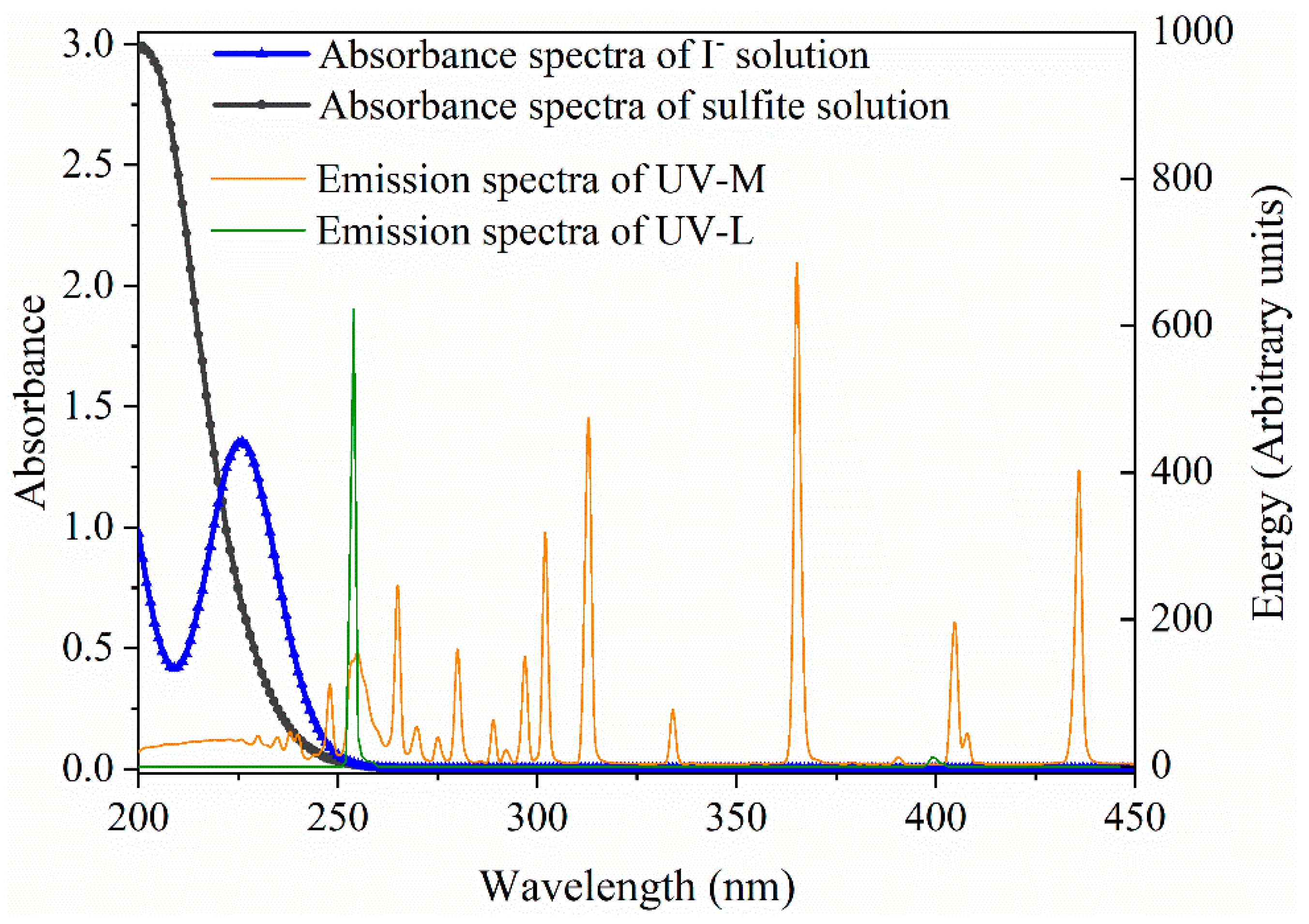
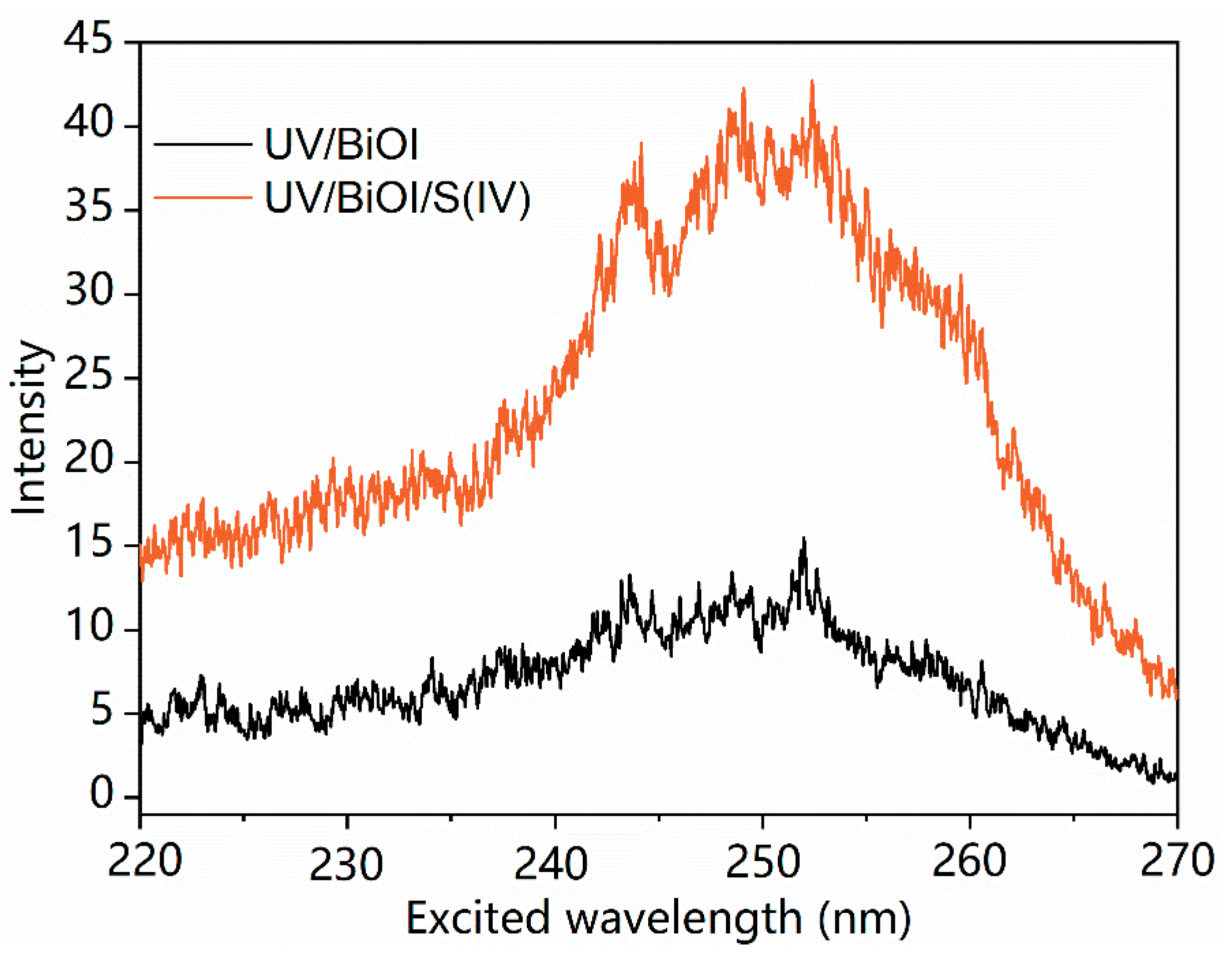
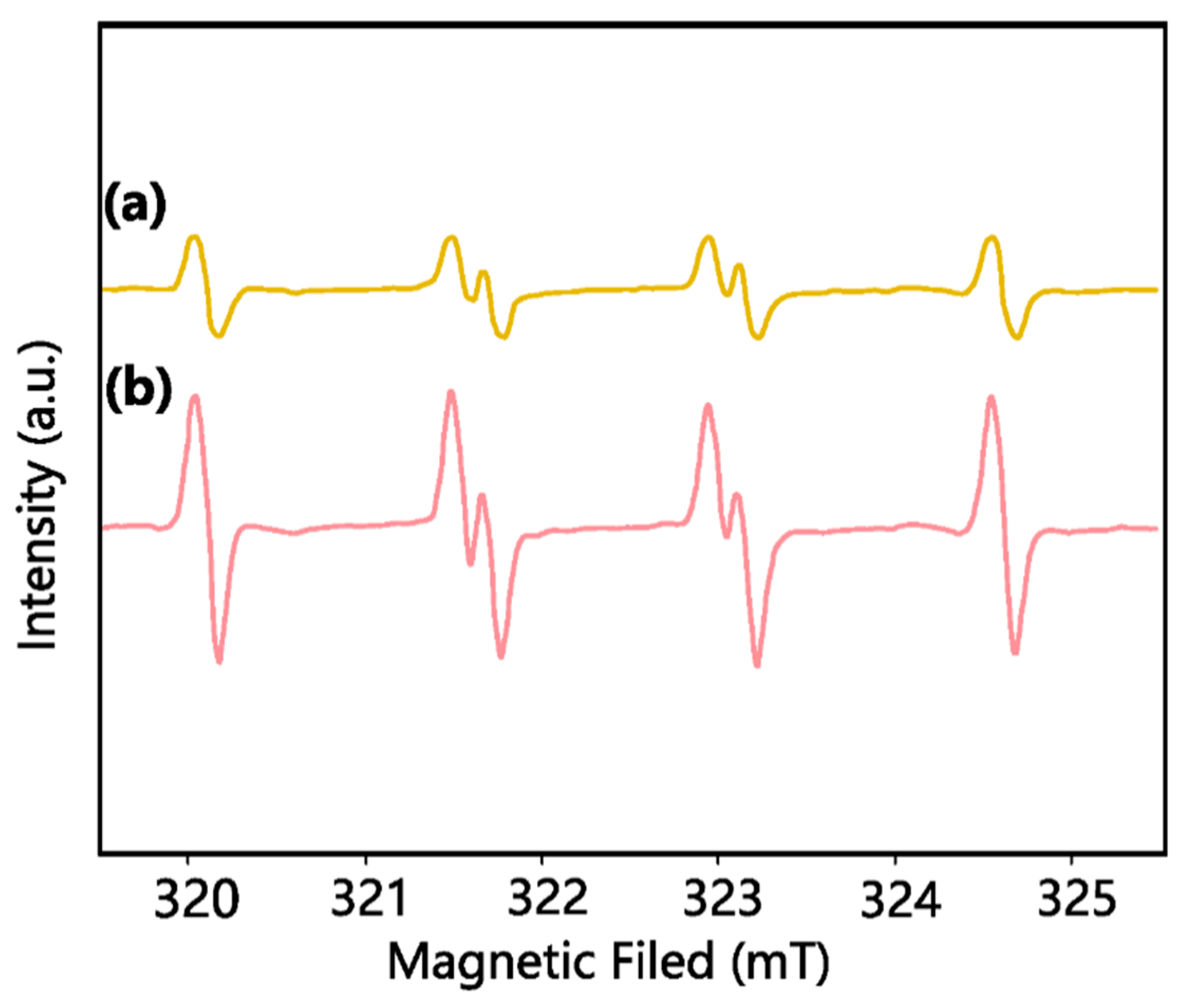
2.2. Identification of Main Reactive Species for MCAA Degradation by UV/S(IV)/BiOI
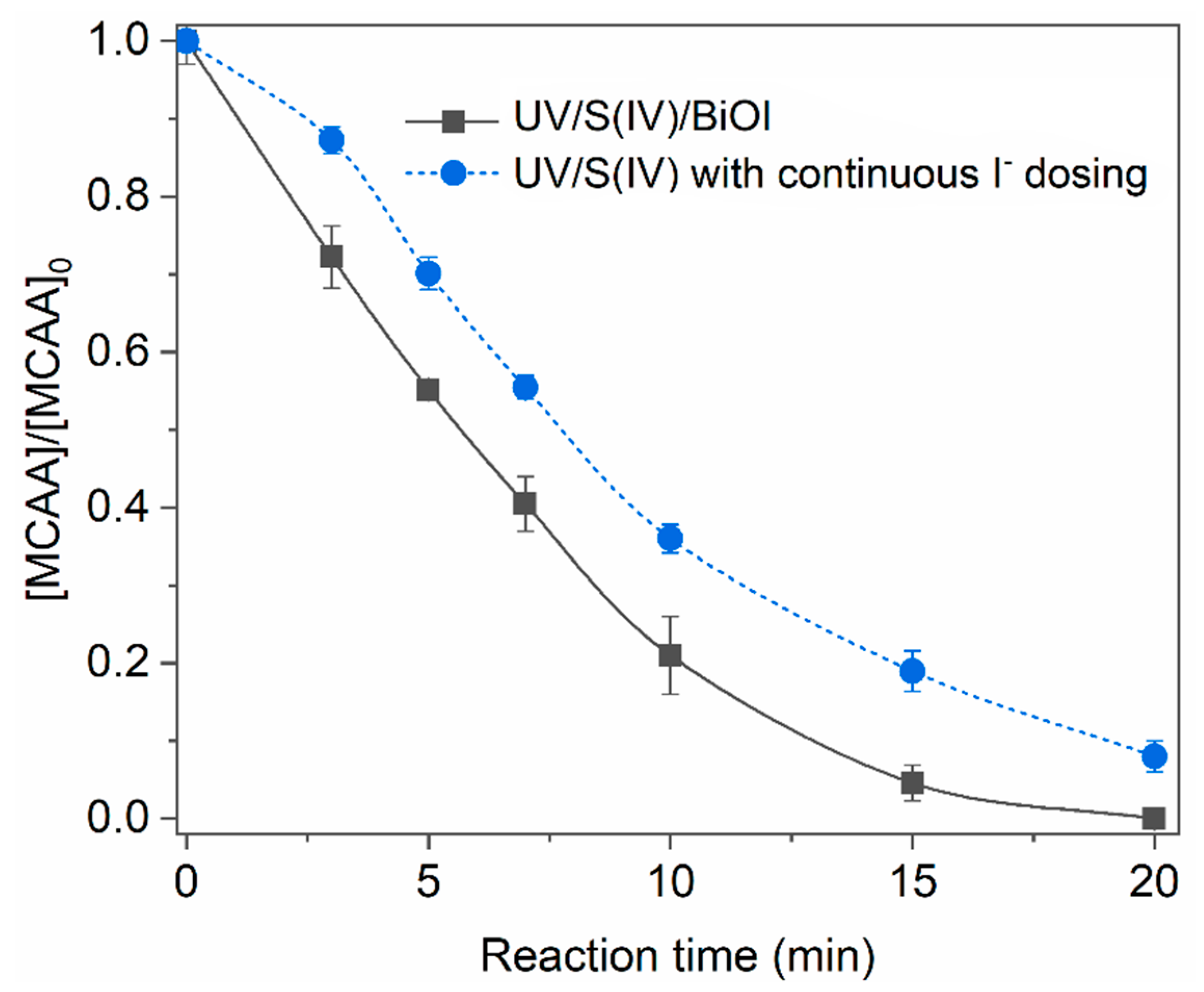
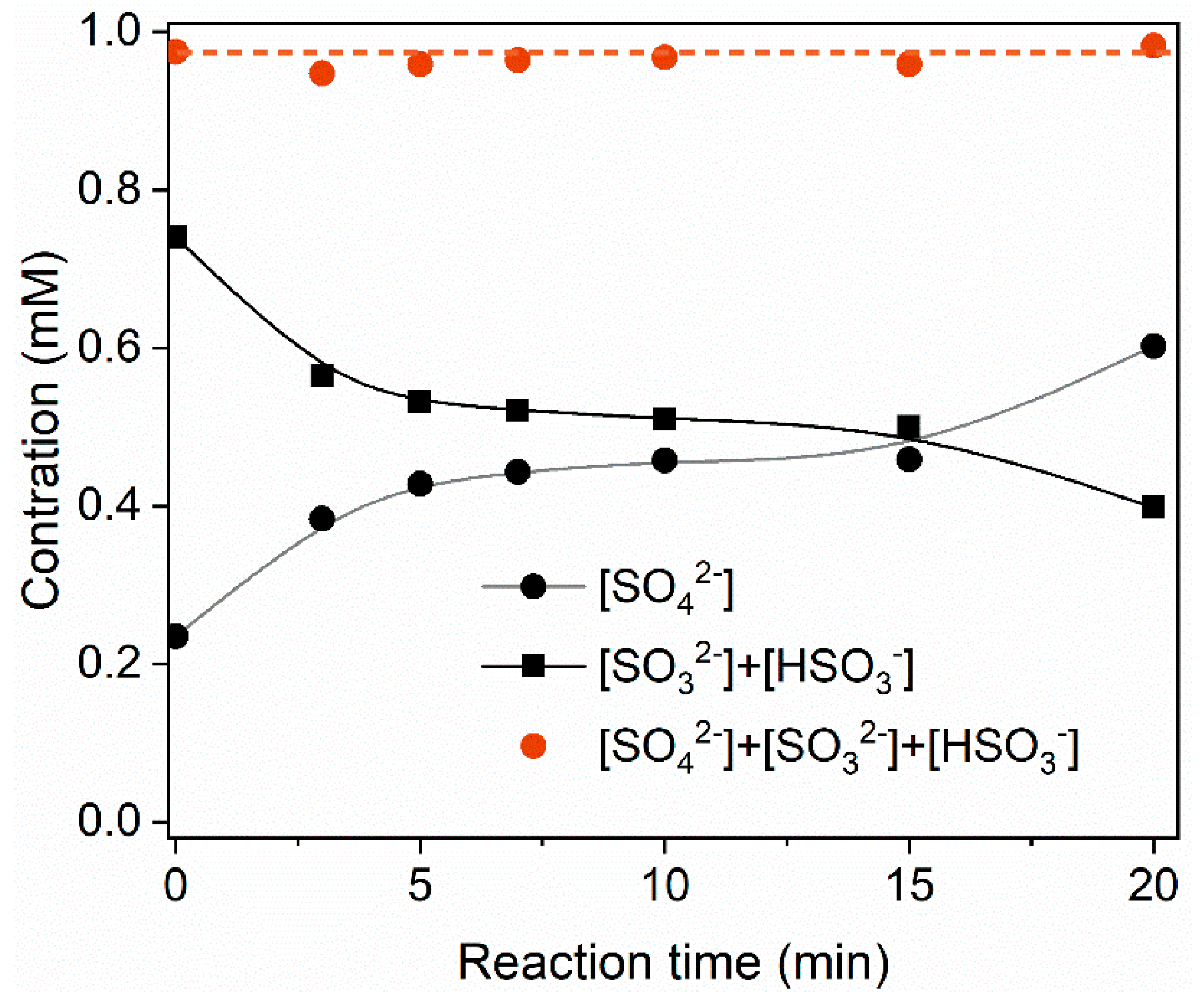
2.3. Role of BiOI in the UV/S(IV)/BiOI System
2.4. Degradation Products of MCAA and Biotoxicity of Treated Water
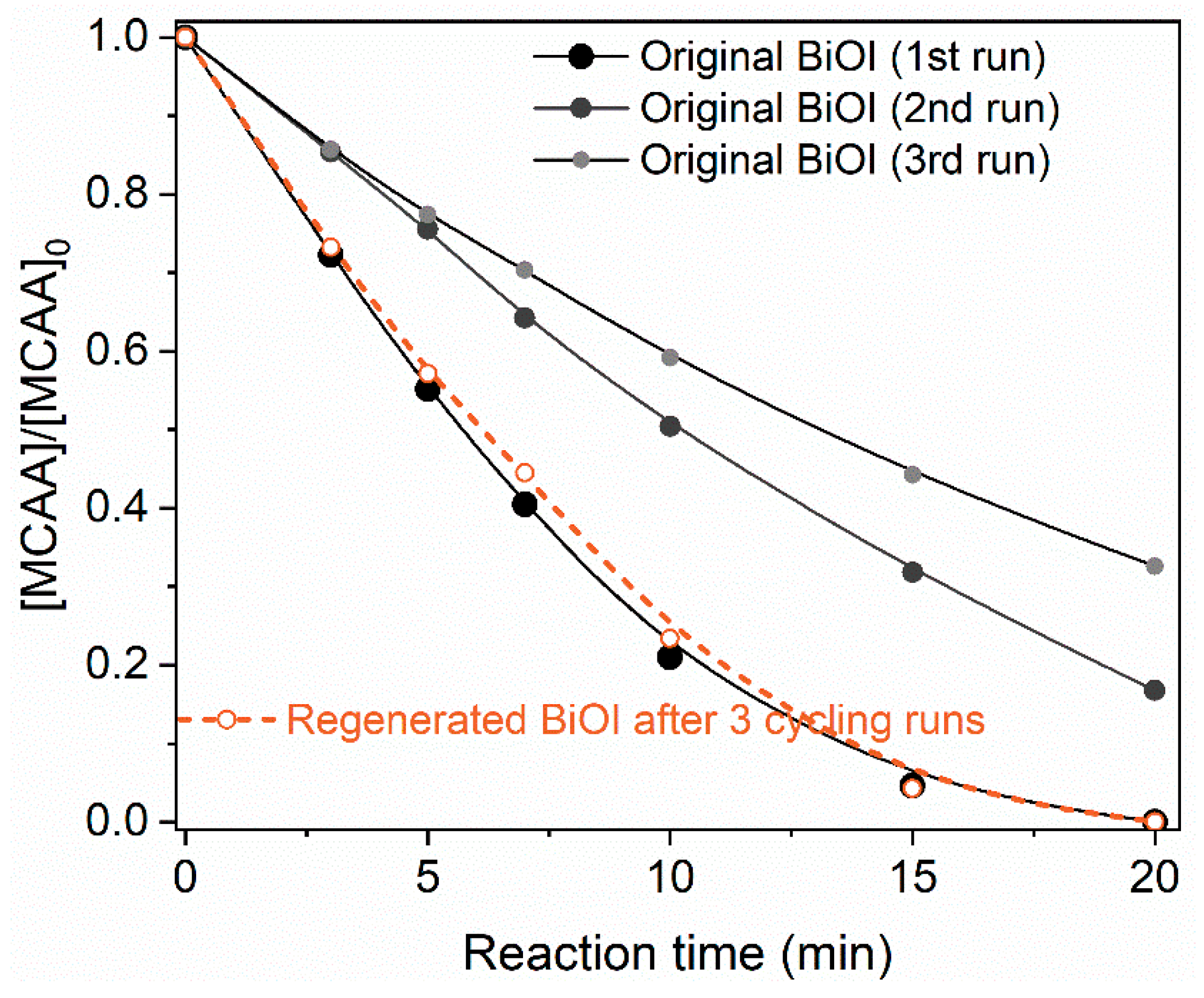
2.5. Regeneration of BiOI
3. Materials and Methods
3.1. Materials and Chemicals
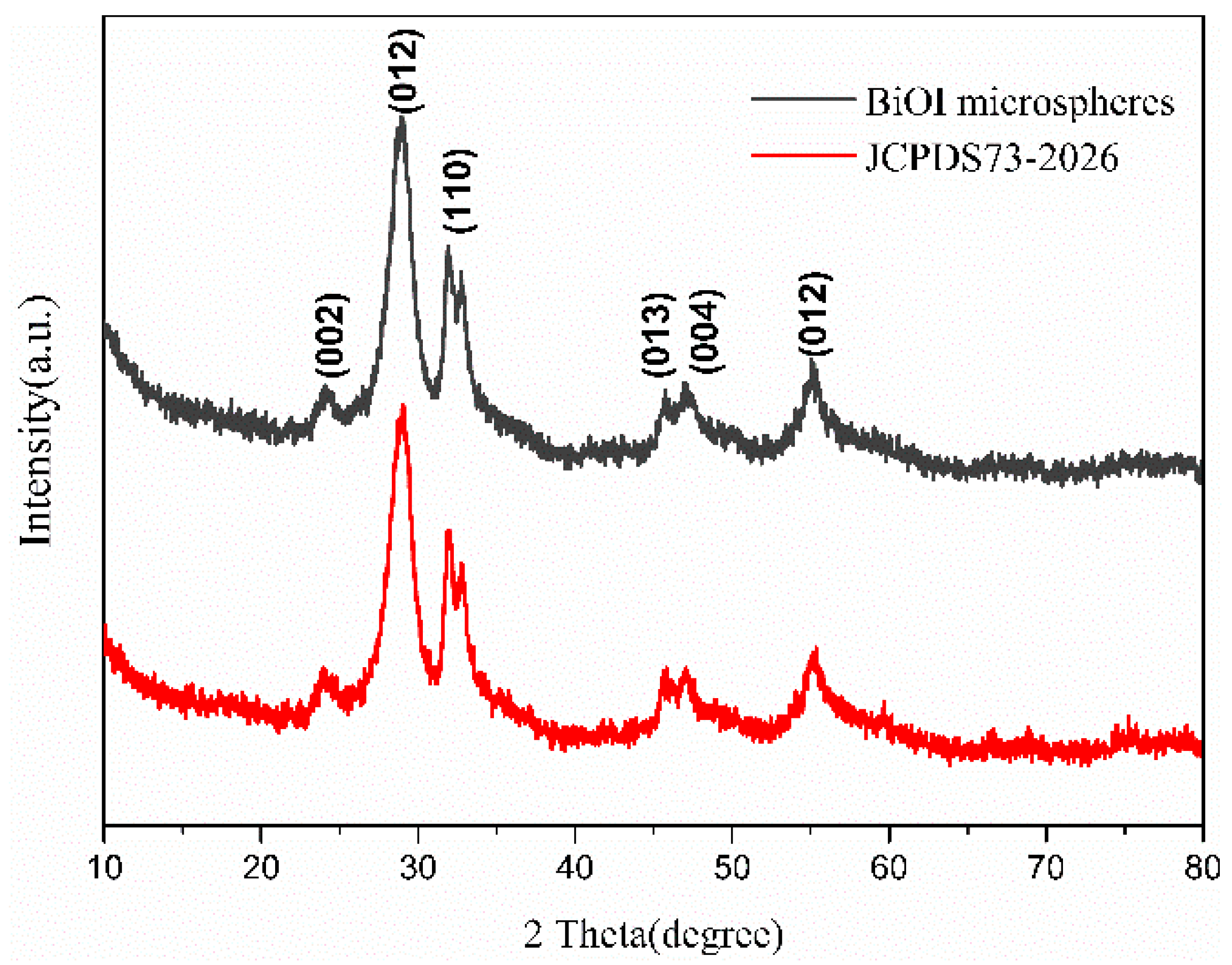
3.2. Synthesis and Characterization of BiOX Microstructures
3.3. Experimental Procedures
3.4. Analytical Methods
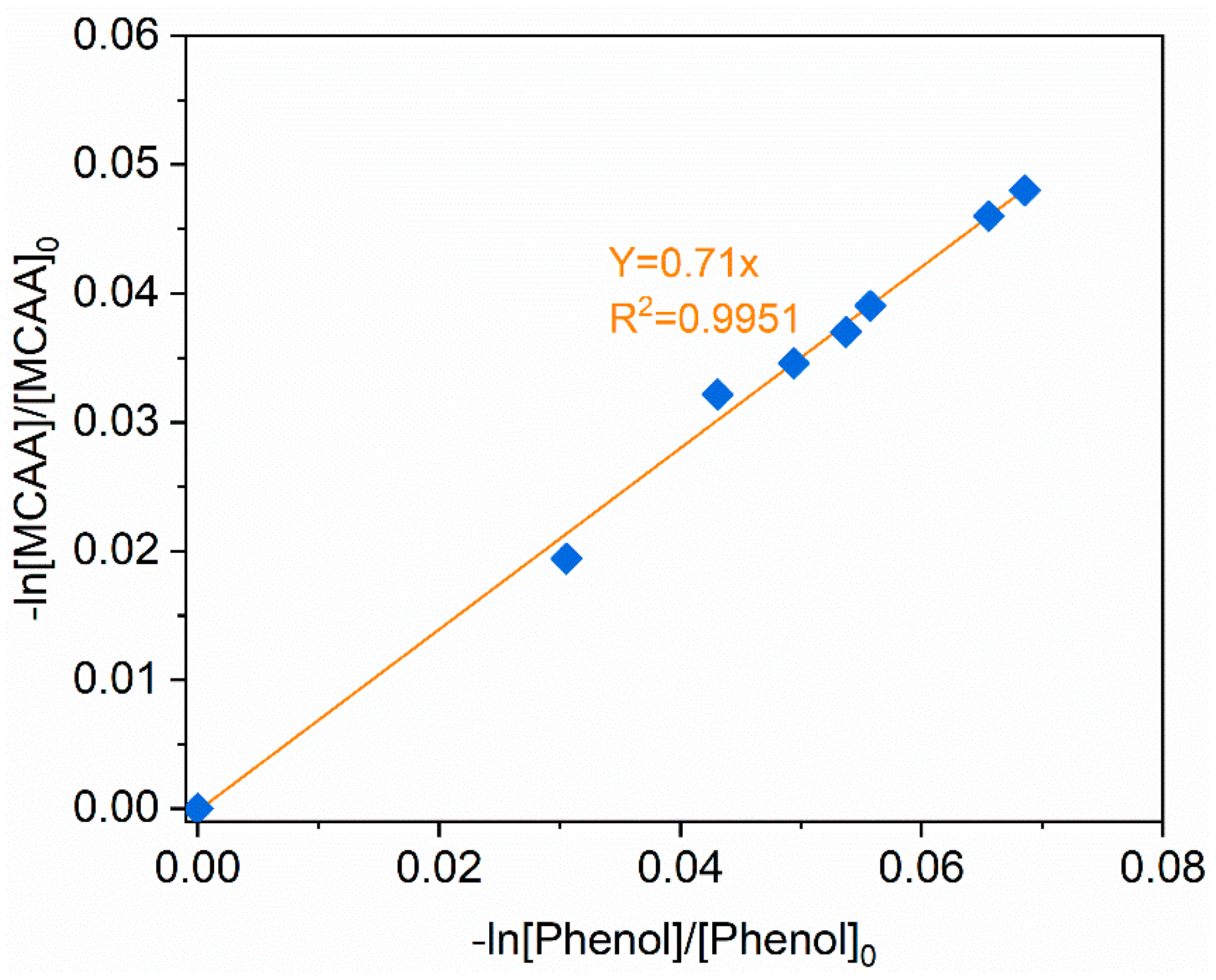
3.5. Determination of Reaction Rate Constant of with MCAA
3.6. Test of BiOI Regeneration
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
References
- Yu, K.; Li, X.; Chen, L.; Fang, J.; Chen, H.; Li, Q.; Chi, N.; Ma, J. Mechanism and efficiency of contaminant reduction by hydrated electron in the sulfite/iodide/UV process. Water Res. 2017, 129, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Yu, S.; Li, L.; Wang, T.; Liao, X.; Ye, Y. An overview of advanced reduction processes for bromate removal from drinking water: Reducing agents, activation methods, applications and mechanisms. J. Hazard. Mater. 2017, 324, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Dong, W.; Luo, C.; Liu, T. Efficient reductive decomposition of perfluorooctanesulfonate in a high photon flux UV/sulfite system. Environ. Sci. Technol. 2016, 50, 10554–10561. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Zhang, T.Q.; Wang, L.L.; Shao, Y.; Fang, L. Hydrated electron-based degradation of atenolol in aqueous solution. Chem. Eng. J. 2015, 260, 740–748. [Google Scholar] [CrossRef]
- Bensalah, N.; Liu, X.; Abdel-Wahab, A. Bromate reduction by ultraviolet light irradiation using medium pressure lamp. Int. J. Environ. Stud. 2013, 70, 566–582. [Google Scholar] [CrossRef]
- Bolton, J.R. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. J. Environ. Eng. 2003, 129, 209–215. [Google Scholar] [CrossRef]
- Zhang, T.Q.; Wang, J.J.; Yan, D.Y.; Wang, L.L.; Liu, X.W. Efficient reduction of bromate by iodide-assisted UV/sulfite process. Catalysts 2018, 8, 652. [Google Scholar] [CrossRef]
- Zhang, T.; Chu, S.; Li, J.; Wang, L.; Chen, R.; Shao, Y.; Liu, X.; Ye, M. Efficient degradation of aqueous carbamazepine by bismuth oxybromide-activated peroxide oxidation. Catalysts 2017, 7, 315. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Z.; Jia, F.; Zhang, L. Generalized one-pot synthesis, characterization, and photocatalytic activity of hierarchical BiOX (X = Cl, Br, I) nanoplate microspheres. J. Phys. Chem. C 2008, 112, 747–753. [Google Scholar] [CrossRef]
- Wei, P.; Yang, Q.; Guo, L. Bismuth Oxyhalide Compounds as Photocatalysts. Prog. Chem. 2009, 1734–1741, WOS:000270373000003. [Google Scholar]
- Li, X.; Ma, J.; Liu, G.; Fang, J.; Yue, S.; Guan, Y.; Chen, L.; Liu, X. Efficient reductive dechlorination of monochloroacetic acid by sulfite/UV process. Environ. Sci. Technol. 2012, 46, 7342–7349. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical-review of rate constants for reactions of hydrated electrons, hydrogen-atoms and hydroxyl radicals (•OH/•O−) in aqueous-solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Yazdanbakhsh, A.; Eslami, A.; Moussavi, G.; Rafiee, M.; Sheikhmohammadi, A. Photo-assisted degradation of 2, 4, 6-trichlorophenol by an advanced reduction process based on sulfite anion radical: Degradation, dechlorination and mineralization. Chemosphere 2018, 191, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Zhao, H.; Pan, F.; Feng, X.; Jung, B.; Abdel-Wahab, A.; Batchelor, B.; Li, Y. Visible-light-driven photocatalytic degradation of organic water pollutants promoted by sulfite addition. Environ. Sci. Technol. 2017, 51, 13372–13379. [Google Scholar] [CrossRef]
- Cao, J.; Nie, W.; Huang, L.; Ding, Y.; Lv, K.; Tang, H. Photocatalytic activation of sulfite by nitrogen vacancy modified graphitic carbon nitride for efficient degradation of carbamazepine. Appl. Catal. B-Environ. 2019, 241, 18–27. [Google Scholar] [CrossRef]
- Erben-Russ, M.; Bors, W.; Winter, R.; Saran, M. The reaction of sulfite anion radical (SO3•−) with polyunsaturated fatty acids. Int. J. Rad. App. Instr. C. Radiat. Phys. Chem. 1986, 27, 419–424. [Google Scholar] [CrossRef]
- Chemseddine, A.; Boehm, H.P. A study of the primary step in the photochemical degradation of acetic acid and chloroacetic acids on a TiO2 photocatalyst. J. Mol. Catal. 1990, 60, 295–311. [Google Scholar] [CrossRef]
- Liang, J.; Liu, F.; Li, M.; Liu, W.; Tong, M. Facile synthesis of magnetic Fe3O4@BiOI@AgI for water decontamination with visible light irradiation: Different mechanisms for different organic pollutants degradation and bacterial disinfection. Water Res. 2018, 137, 120–129. [Google Scholar] [CrossRef]
- Xie, P.; Zhang, L.; Chen, J.; Ding, J.; Wan, Y.; Wang, S.; Wang, Z.; Zhou, A.; Ma, J. Enhanced degradation of organic contaminants by zero-valent iron/sulfite process under simulated sunlight irradiation. Water Res. 2019, 149, 169–178. [Google Scholar] [CrossRef]
- Jones, W.; Martin, D.J.; Caravaca, A.; Beale, A.M.; Bowker, M.; Maschmeyer, T.; Hartley, G.; Masters, A. A comparison of photocatalytic reforming reactions of methanol and triethanolamine with Pd supported on titania and graphitic carbon nitride. App. Catal B-Environ. 2019, 240, 373–379. [Google Scholar] [CrossRef]
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications, 1st ed.; IWA Publishing: London, UK, 2017; pp. 125–127. [Google Scholar] [CrossRef]
- Huie, R.E.; Neta, P. Rate constants for some oxidations of S(IV) by radicals in aqueous-solutions. Atmos. Environ. 1987, 21, 1743–1747. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yao, H.; Dang, L.; Li, Z. Efficient decomposition of organic compounds and reaction mechanism with BiOI photocatalyst under visible light irradiation. J. Mol. Catal. A-Chem 2011, 334, 116–122. [Google Scholar] [CrossRef]
- Li, J.; Sun, S.; Chen, R.; Zhang, T.; Ren, B.; Dionysiou, D.D.; Wu, Z.; Liu, X.; Ye, M. Adsorption behavior and mechanism of ibuprofen onto biocl microspheres with exposed {001} facets. Environ. Sci. Pollut. R. 2017, 24, 9556–9565. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, D.; Wang, Y.; Chang, S. Oxidation of aqueous sulfite ion by nitrogen-dioxide. Environ. Sci. Technol. 1993, 27, 2162–2167. [Google Scholar] [CrossRef]
- Bard, A.J.; Parsons, R.; Jordan, J. Standard Potentials in Aqueous Solution, 1st ed.; Marcel Dekker: New York, NY, USA, 1985; pp. 127–413. ISBN 0-8247-7291-1. [Google Scholar]
- Wang, A.; Li, X.; Zhao, Y.; Wu, W.; Chen, J.; Meng, H. Preparation and characterizations of Cu2O/reduced graphene oxide nanocomposites with high photo-catalytic performances. Powder Technol. 2014, 261, 42–48. [Google Scholar] [CrossRef]
- Marrani, A.G.; Bonomo, M.; Dini, D. Adsorption dynamics of redox active species onto polarized surfaces of sensitized nio. ACS Omega 2019, 4, 1690–1699. [Google Scholar] [CrossRef]
- Treinin, A.; Hayon, E. Laser photolysis of iodide ions: Spectroscopic evidence for iodine atoms and kinetics of their reaction with iodide in various solvents. Int. J. Radiat. Phys. Ch. 1975, 7, 387–393. [Google Scholar] [CrossRef]
- Ranguelova, K.; Rice, A.B.; Khajo, A.; Triquigneaux, M.; Garantziotis, S.; Magliozzo, R.S.; Mason, R.P. Formation of reactive sulfite-derived free radicals by the activation of human neutrophils: An ESR study. Free Radical Bio. Med. 2012, 52, 1264–1271. [Google Scholar] [CrossRef]
- Erben-Russ, M.; Michel, C.; Bors, W.; Saran, M. Determination of sulfite radical (SO3•−) reaction rate constants by means of competition kinetics. Radiat. Environ. Bioph. 1987, 26, 289–294. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liu, X. Fast Degradation of Monochloroacetic Acid by BiOI-Enhanced UV/S(IV) Process: Efficiency and Mechanism. Catalysts 2019, 9, 460. https://doi.org/10.3390/catal9050460
Wang L, Liu X. Fast Degradation of Monochloroacetic Acid by BiOI-Enhanced UV/S(IV) Process: Efficiency and Mechanism. Catalysts. 2019; 9(5):460. https://doi.org/10.3390/catal9050460
Chicago/Turabian StyleWang, Lili, and Xiaowei Liu. 2019. "Fast Degradation of Monochloroacetic Acid by BiOI-Enhanced UV/S(IV) Process: Efficiency and Mechanism" Catalysts 9, no. 5: 460. https://doi.org/10.3390/catal9050460
APA StyleWang, L., & Liu, X. (2019). Fast Degradation of Monochloroacetic Acid by BiOI-Enhanced UV/S(IV) Process: Efficiency and Mechanism. Catalysts, 9(5), 460. https://doi.org/10.3390/catal9050460





