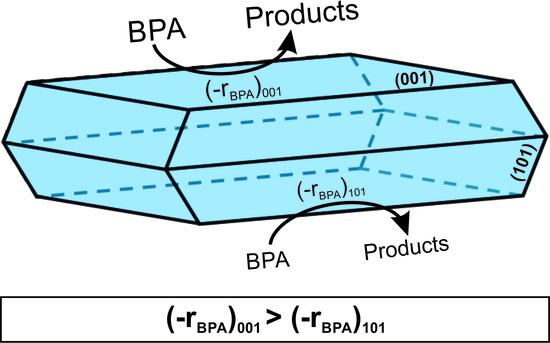
Effect of Surface Chemistry and Crystallographic Parameters of TiO2 Anatase Nanocrystals on Photocatalytic Degradation of Bisphenol A
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
2.1.1. TiO2 Texture and Crystal Shape
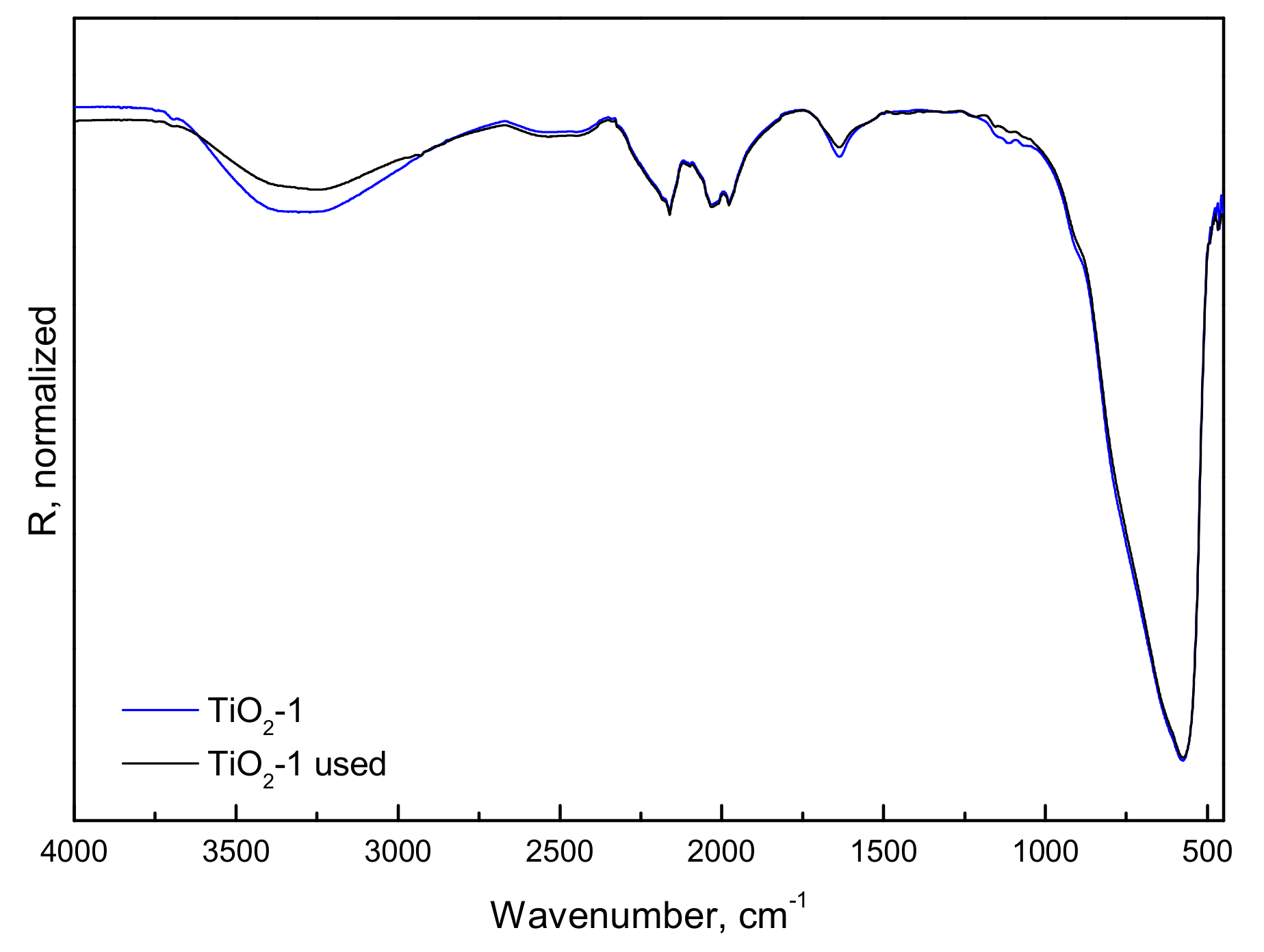
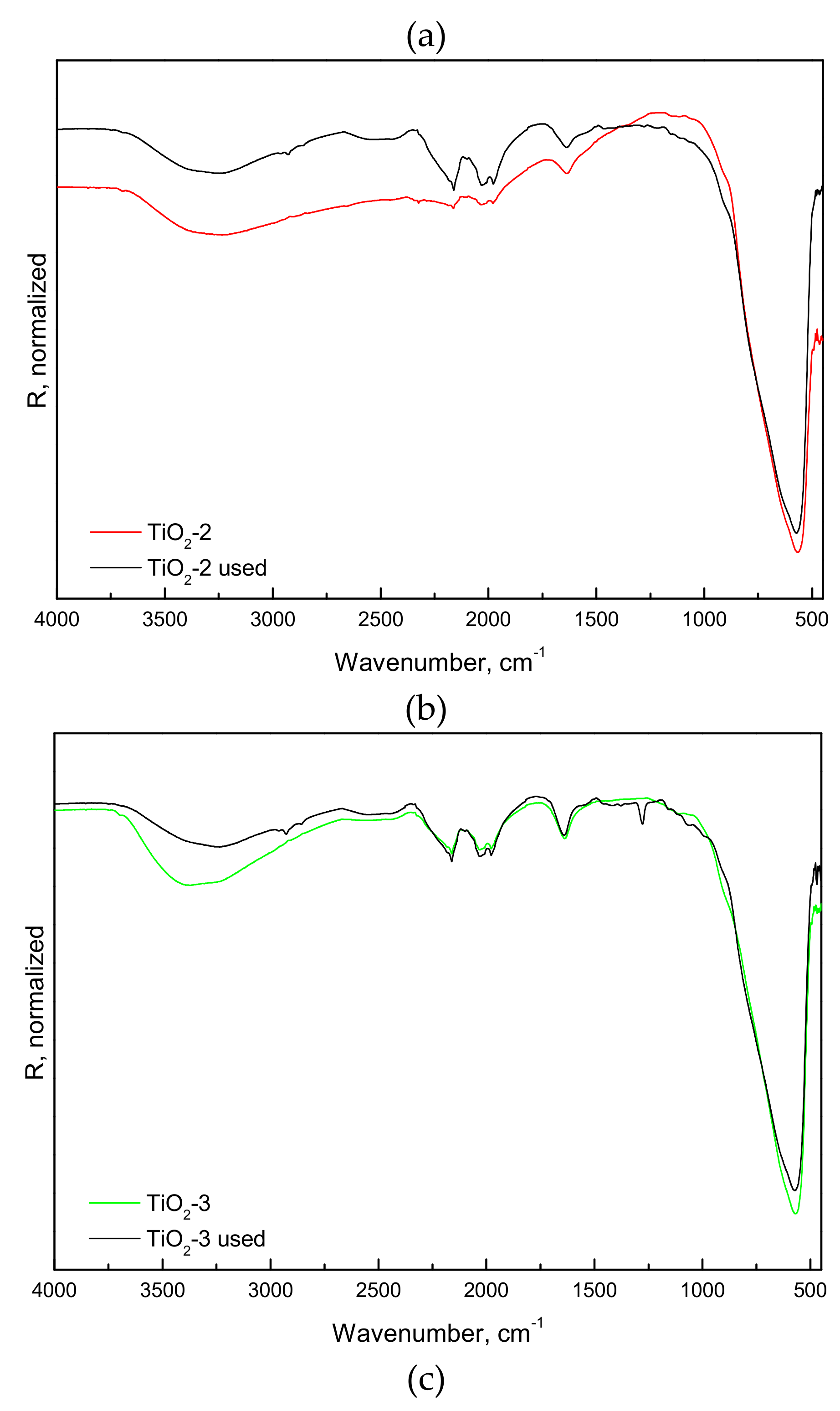
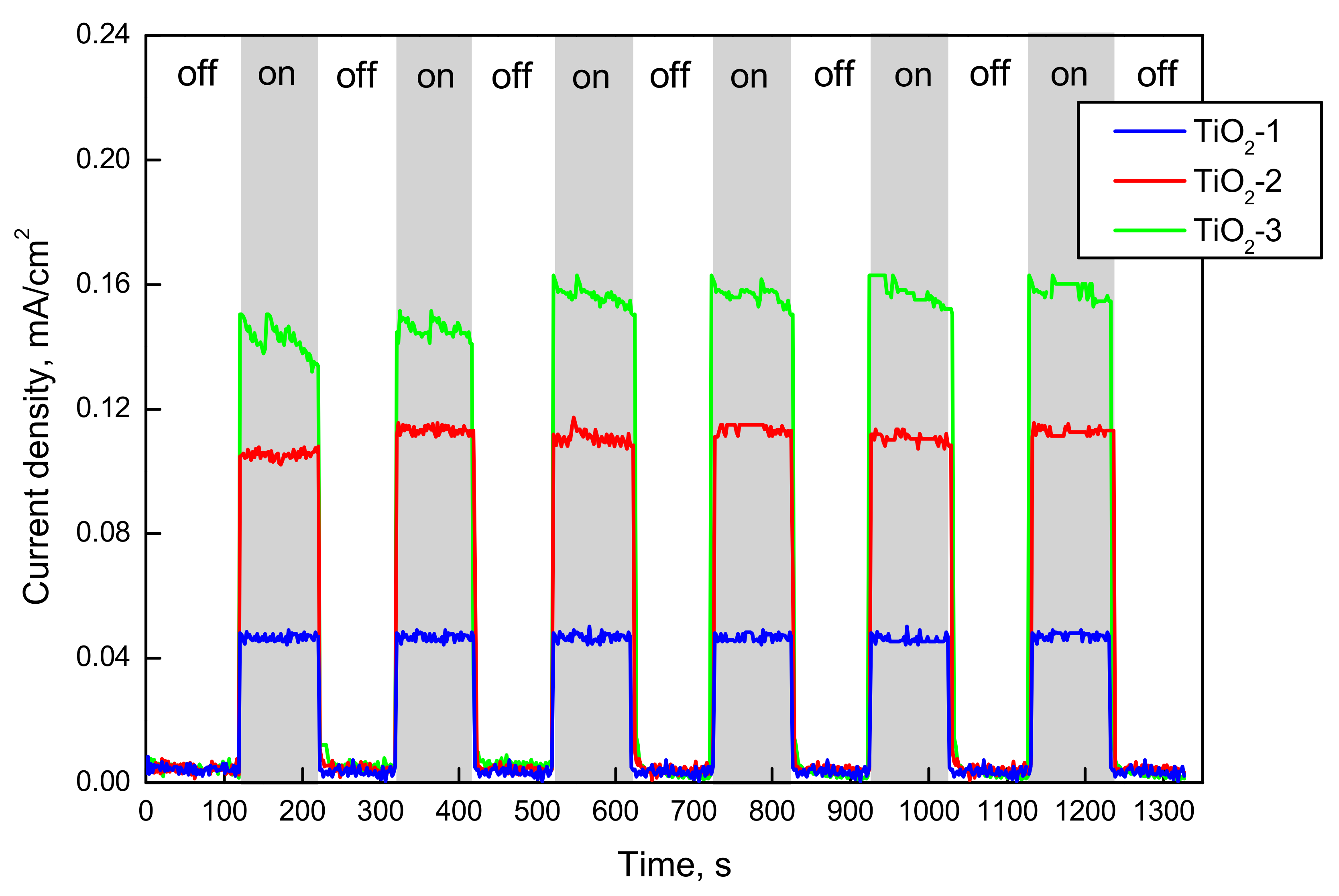
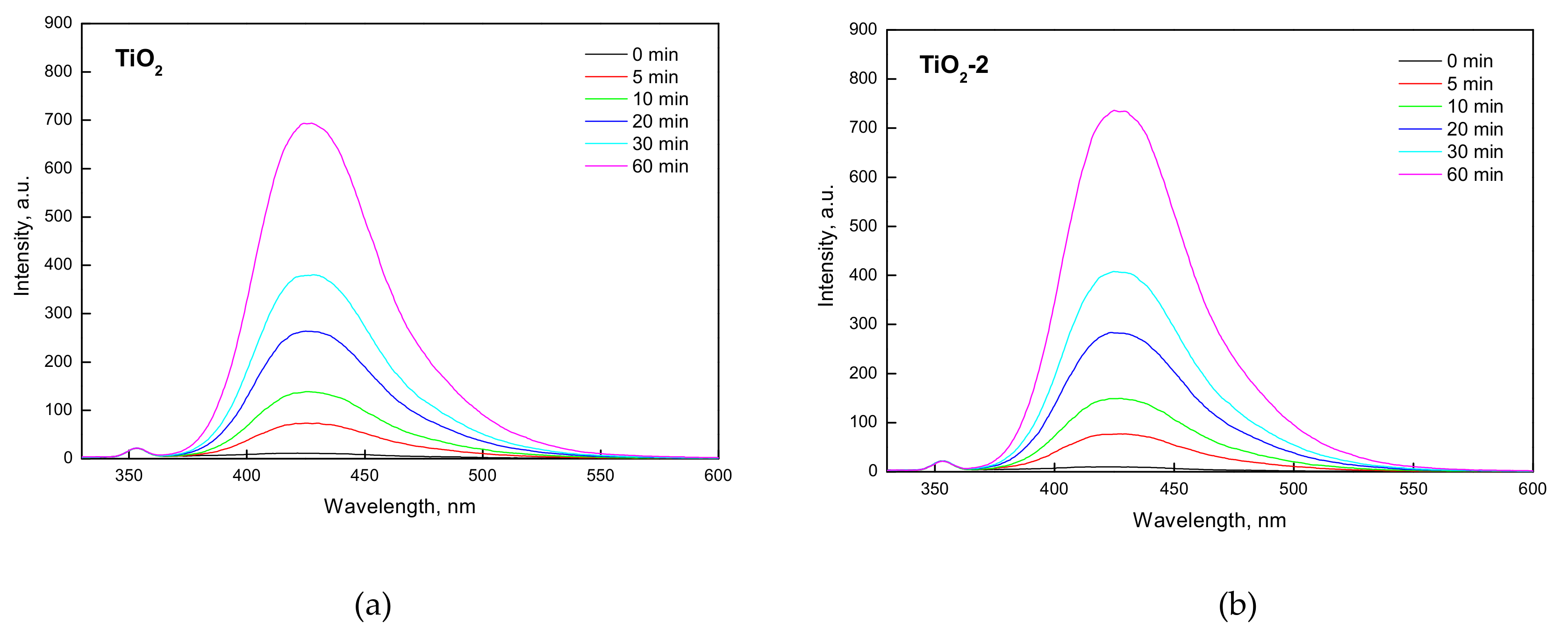
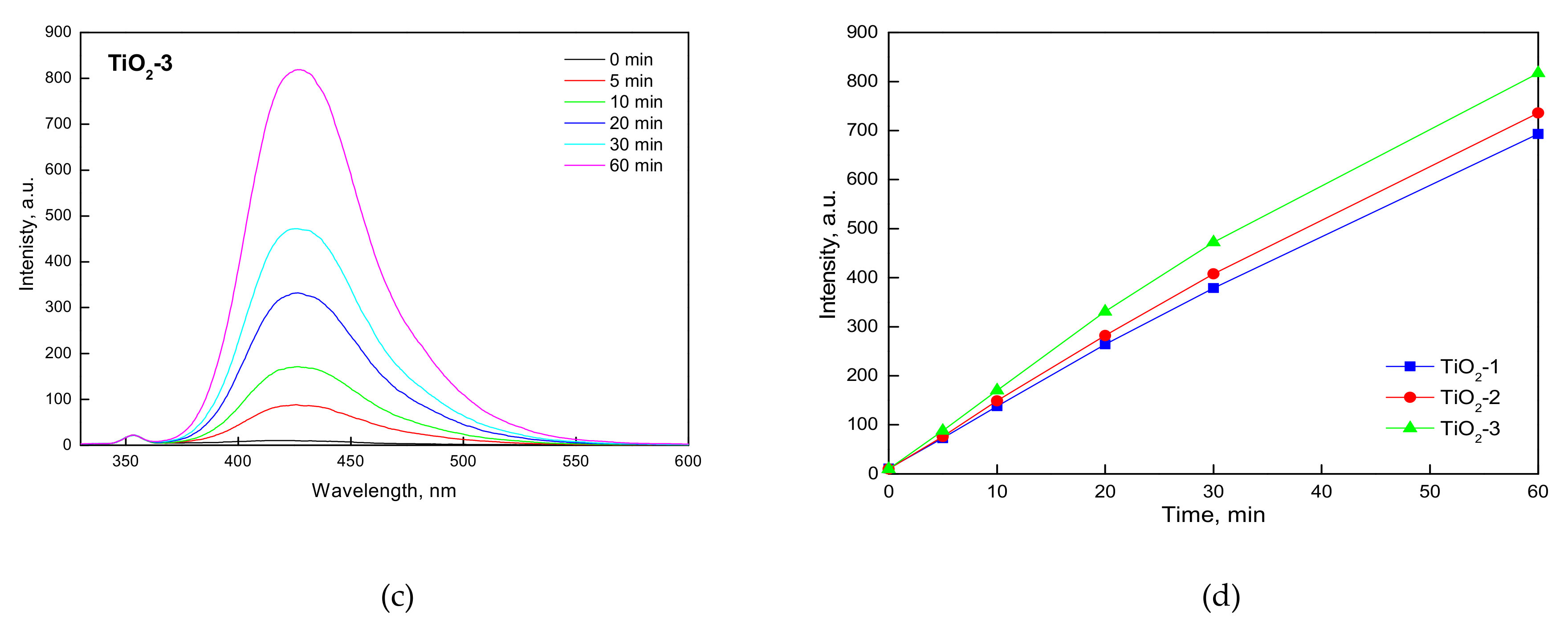
2.1.2. FTIR-ATR, Photo-Electrochemical, and Photo-Luminescence Measurements
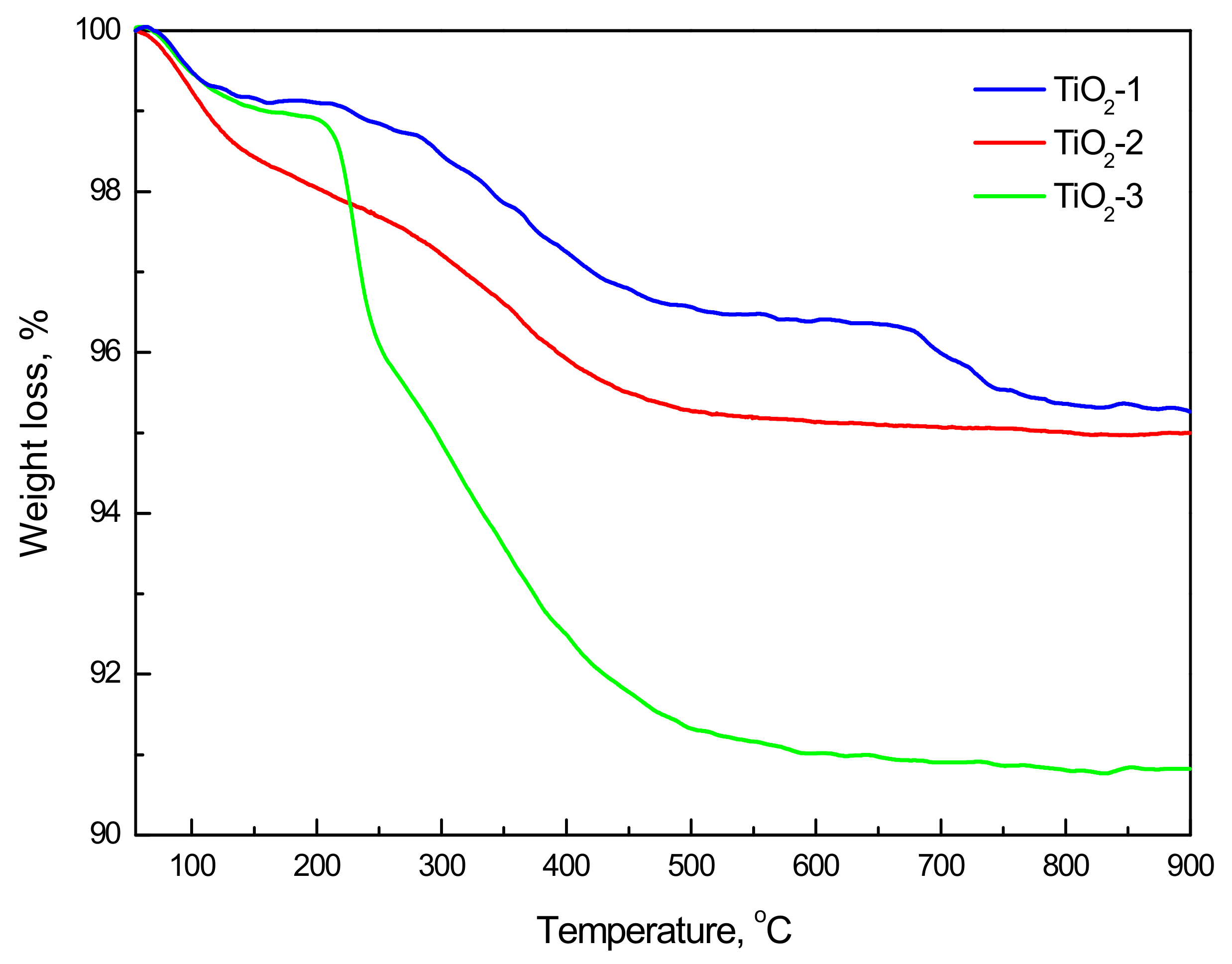
2.1.3. TPD Analysis
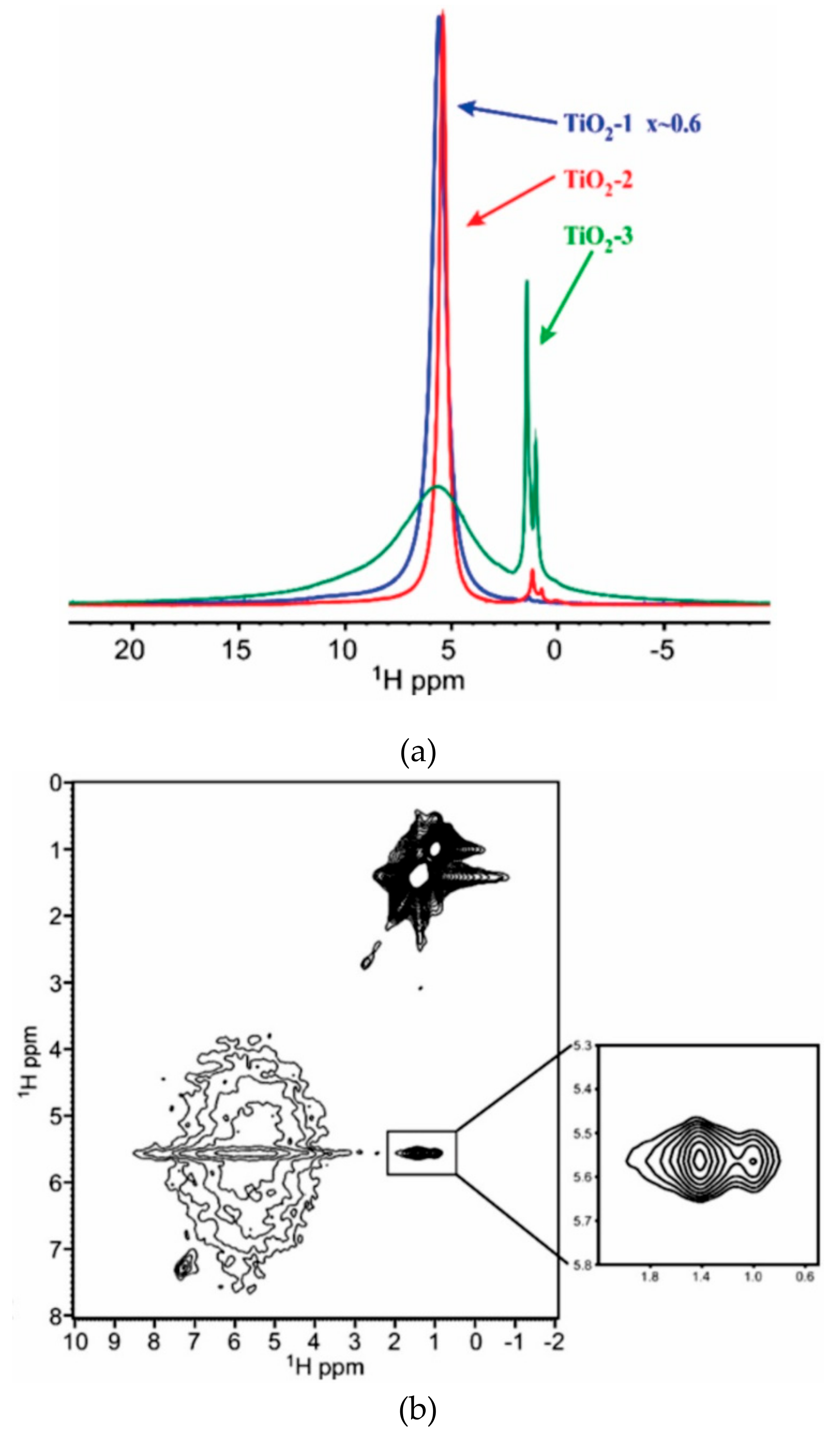
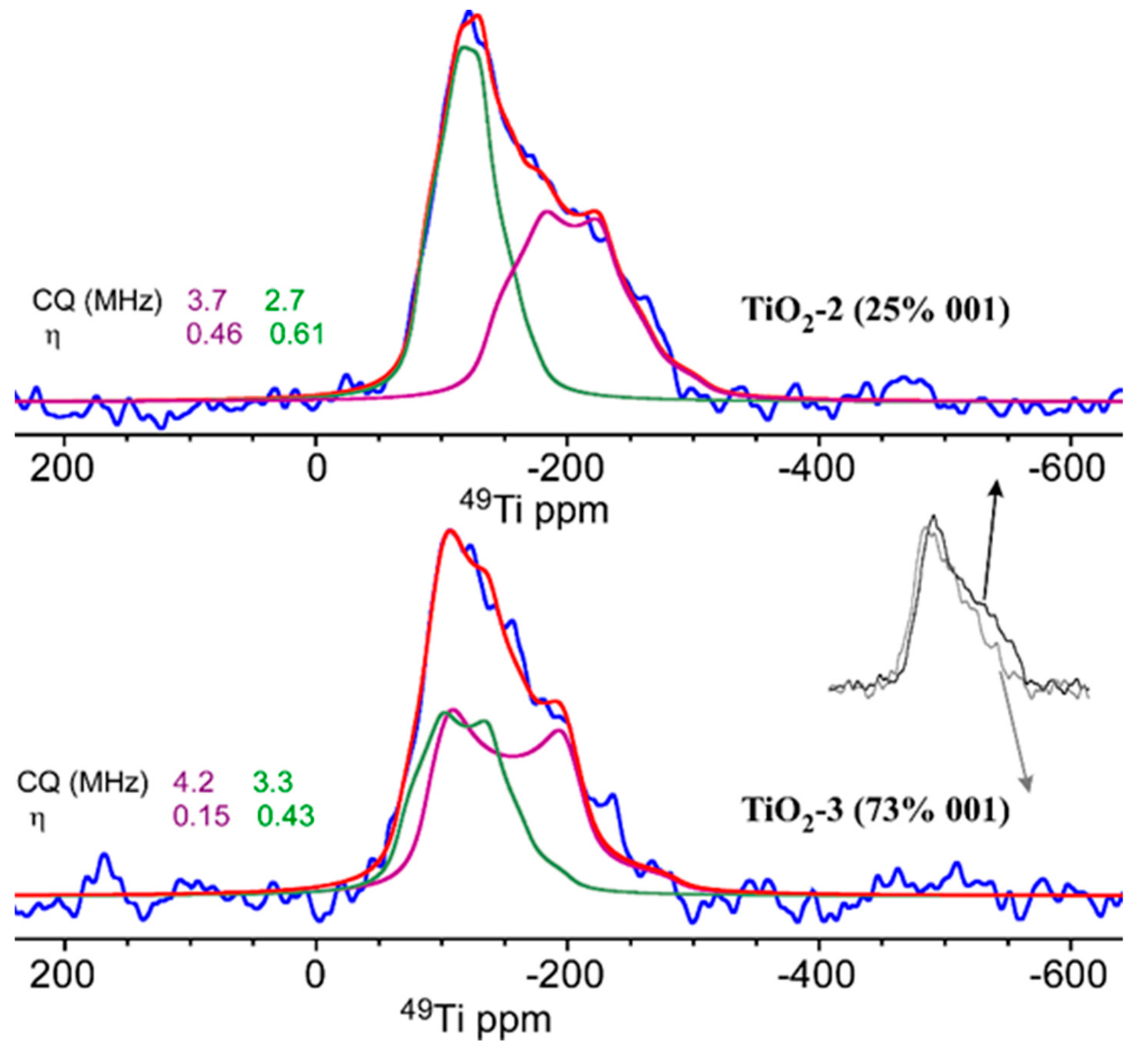
2.1.4. NMR Examination
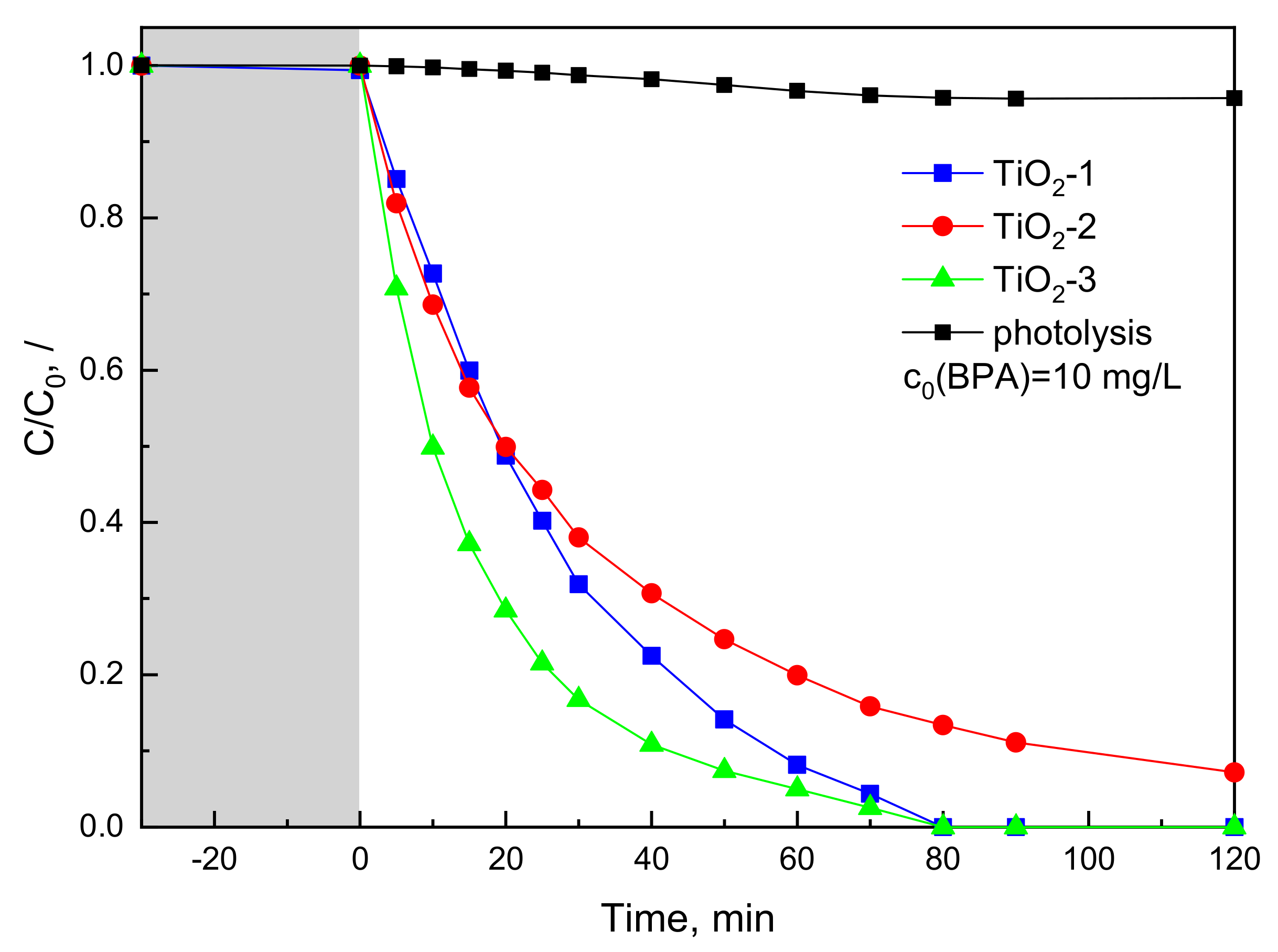
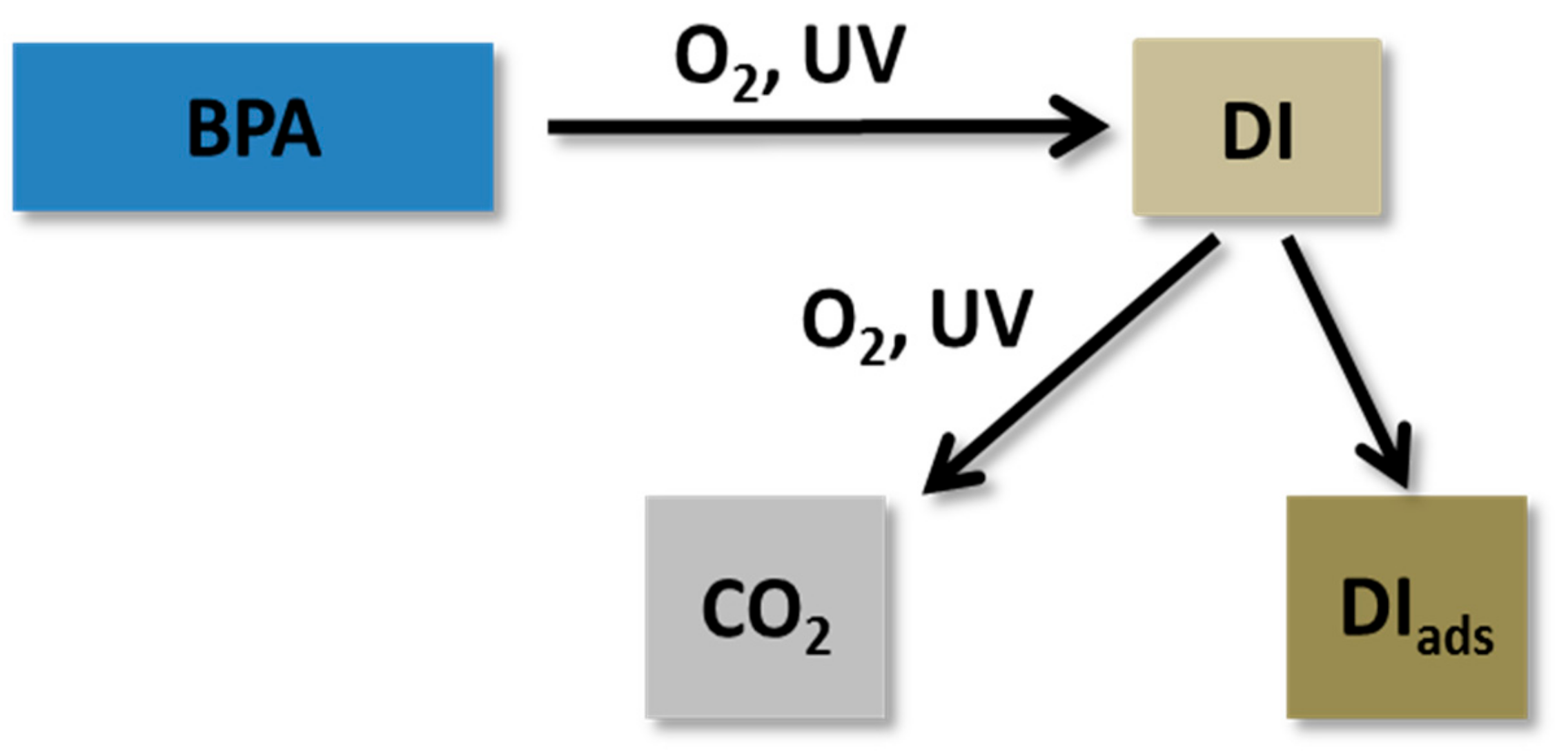
2.2. Photocatalytic BPA Oxidation
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Photocatalytic Oxidation Experiments
3.4. Analysis of End-Product Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 12, 8269–8285. [Google Scholar] [CrossRef]
- Ge, M.; Cao, C.; Huang, J.; Li, S.; Chen, Z.; Zhang, K.-Q.; Al-Deyab, S.S.; Lai, Y. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A 2016, 4, 6772–6801. [Google Scholar] [CrossRef]
- Lee, A.; Libera, J.A.; Waldman, R.Z.; Ahmed, A.; Avila, J.R.; Elam, J.W.; Darling, S.B. Conformal nitrogen-doped TiO2 photocatalytic coatings for sunlight-activated membranes. Adv. Sustain. Syst. 2017, 1, 160041. [Google Scholar] [CrossRef]
- Žerjav, G.; Djinović, P.; Pintar, A. TiO2-Bi2O3/(BiO)2CO3-reduced graphene oxide composite as an effective visible light photocatalyst for degradation of aqueous bisphenol A solution. Catal. Today 2018, 315, 237–246. [Google Scholar] [CrossRef]
- Žerjav, G.; Arshad, M.S.; Djinović, P.; Junkar, I.; Kovač, J.; Zavašnik, J.; Pintar, A. Improved electron-hole separation and migration in anatase TiO2 nanorod/reduced graphene oxide composites and their influence on photocatalytic performance. Nanoscale 2017, 9, 4578–4592. [Google Scholar] [CrossRef] [PubMed]
- Žerjav, G.; Arshad, M.S.; Djinović, P.; Zavašnik, J.; Pintar, A. Electron trapping energy states of TiO2-WO3 composites and their influence on photocatalytic degradation of bisphenol A. Appl. Catal. B 2017, 209, 273–284. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by coexposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, J.; Jaroniec, M. Anatase TiO2 with dominant high-energy {001} facets: Synthesis, properties, and applications. Chem. Mater. 2011, 23, 4085–4093. [Google Scholar] [CrossRef]
- Grabowska, E.; Dial, M.; Marchelek, M.; Zaleska, A. Decahedral TiO2 with exposed facets: Synthesis, properties, photoactivity and applications. Appl. Catal. B 2014, 156–157, 213–235. [Google Scholar] [CrossRef]
- Selloni, A. Crystal growth: Anatase shows its reactive side. Nat. Mater. 2008, 7, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Liu, G.; Qiao, S.Z.; Sun, C.H.; Jin, Y.G.; Campbell Smith, S.; Zou, J.; Cheng, H.M.; Lu, G.Q.M. Solvothermal synthesis and photoreactivity of anatase TiO2 nanosheets with dominant {001} facets. J. Am. Chem. Soc. 2009, 131, 4078–4083. [Google Scholar] [CrossRef]
- Zhang, D.; Li, G.; Yang, X.; Yu, J.C. A micrometer-size TiO2 single-crystal photocatalyst with remarkable 80% level of reactive facets. Chem. Commun. 2009, 45, 4381–4383. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Tunable photocatalytic selectivity of TiO2 films consisted of flower-like microspheres with exposed {001} facets. Chem. Commun. 2011, 47, 4532–4534. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, H.; Zeng, Y.; Huang, T. Anatase TiO2 single crystals with dominant {0 0 1} facets: Facile fabrication from Ti powders and enhanced photocatalytical activity. Appl. Surf. Sci. 2013, 274, 117–123. [Google Scholar] [CrossRef]
- He, Z.; Wen, L.; Wang, D.; Xue, Y.; Lu, Q.; Wu, C.; Chen, J.; Song, S. Photocatalytic reduction of CO2 in aqueous solution on surface-fluorinated anatase TiO2 nanosheets with exposed {001} facets. Energy Fuels 2014, 28, 3982–3993. [Google Scholar] [CrossRef]
- Liu, G.; Sun, C.; Yang, H.G.; Smith, S.C.; Wang, L.; Lu, G.Q.M.; Cheng, H.-M. Nanosized anatase TiO2 single crystals for enhanced photocatalytic activity. Chem. Commun. 2010, 46, 755–757. [Google Scholar] [CrossRef]
- Gordon, T.R.; Cargnello, M.; Paik, T.; Mangolini, F.; Weber, R.T.; Fornasiero, P.; Murray, C.B. Nonaqueous synthesis of TiO2 nanocrystals using TiF4 to engineer morphology, oxygen vacancy concentration, and photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 6751–6761. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y.; Jiang, M.; Wang, P.; Whangbo, M.-H. Highly efficient photocatalyst: TiO2 microspheres produced from TiO2 nanosheets with a high percentage of reactive {001} facets. Chem. Eur. J. 2009, 15, 12576–12579. [Google Scholar] [CrossRef]
- Luan, Y.; Jing, L.; Xie, Y.; Sun, X.; Feng, Y.; Fu, H. Exceptional photocatalytic activity of 001-facet-exposed TiO2 mainly depending on enhanced adsorbed oxygen by residual hydrogen fluoride. ACS Catal. 2013, 3, 1378–1385. [Google Scholar] [CrossRef]
- Roy, N.; Sohn, Y.; Pradhan, D. Synergy of low-energy {101} and high-energy {001} TiO2 crystal facets for enhanced photocatalysis. ACS Nano 2013, 7, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Adán-Más, A.; Wei, D. Photoelectrochemical properties of graphene and its derivatives. Nanomaterials 2013, 3, 325–356. [Google Scholar] [CrossRef]
- Nakabayashi, H.; Kakuta, N.; Ueno, A. Strong acid sites created on small-sized anatase. Bull. Chem. Soc. Jpn. 1991, 64, 2428–2432. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Klissurski, D.G. Surface chemistry of titania (anatase) and titania-supported catalysts. Chem. Soc. Rev. 1996, 25, 61–69. [Google Scholar] [CrossRef]
- Staples, C.A.; Dome, P.B.; Klecka, G.M.; Oblock, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Hatef, A.; Alavi, S.M.H.; Abdulfatah, A.; Fontaine, P.; Rodina, M.; Linhart, O. Adverse effects of bisphenol A on reproductive physiology in male goldfish at environmentally relevant concentrations. Ecotoxicol. Environ. Saf. 2012, 76, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Herath, C.B.; Jin, W.Z.; Watanabe, G.; Arai, K.; Suzuki, A.K.; Taya, K. Adverse effects of environmental toxicants, octylphenol and bisphenol A, on male reproductive functions in pubertal rats. Endocrine 2004, 25, 163–172. [Google Scholar] [CrossRef]
- Braun, J.M.; Hauser, R. Bisphenol A and children’s health. Curr. Opin. Pediatr. 2011, 23, 233–239. [Google Scholar] [CrossRef]
- Wolstenholme, J.T.; Rissman, E.F.; Connelly, J.J. The role of Bisphenol A in shaping the brain, epigenome and behavior. Horm. Behav. 2011, 59, 296–305. [Google Scholar] [CrossRef]
- Kang, J.H.; Kondo, F.; Katayama, Y. Human exposure to bisphenol A. Toxicology 2006, 226, 79–89. [Google Scholar] [CrossRef]
- Doong, R.A.; Chang, S.M.; Tsai, C.W. Enhanced photoactivity of Cu-deposited titanate nanotubes for removal of bisphenol A. Appl. Catal. B 2013, 129, 48–55. [Google Scholar] [CrossRef]
- Doong, R.A.; Liao, C.Y. Enhanced visible-light-responsive photodegradation of bisphenol A by Cu, N-codoped titanate nanotubes prepared by microwave-assisted hydrothermal method. J. Hazard. Mater. 2017, 322, 254–262. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Duong, R.A. Heterostructured ZnFe2O4/TiO2 nanocomposites with a highly recyclable visible-light-response for bisphenol A degradation. RSC Adv. 2017, 7, 50006–50016. [Google Scholar] [CrossRef]
- Zhang, A.-Y.; Long, L.-L.; Li, W.-W.; Wang, W.-K.; Yu, H.-Q. Hexagonal microrods of anatase tetragonal TiO2: Self-directed growth and superior photocatalytic performance. Chem. Commun. 2013, 54, 6075–6077. [Google Scholar] [CrossRef]
- Guo, C.; Ge, M.; Liu, L.; Gao, G.; Feng, Y.; Wang, Y. Directed synthesis of mesoporous TiO2 microspheres: Catalysts and their photocatalysis for bisphenol A degradation. Environ. Sci. Technol. 2010, 44, 419–425. [Google Scholar] [CrossRef]
- Tang, Y.; Wee, P.; Lai, Y.; Wang, X.; Gong, D.; Kanhere, P.D.; Lim, T.-T.; Dong, Z.; Chen, Z. Hierarchical TiO2 nanoflakes and nanoparticles hybrid structure for improved photocatalytic activity. J. Phys. Chem. C 2012, 116, 2772–2780. [Google Scholar] [CrossRef]
- Lazzeri, M.; Vittadini, A.; Selloni, A. Structure and energetics of stoichiometric TiO2 anatase surfaces. Phys. Rev. B 2001, 63, 1555091–1555099. [Google Scholar] [CrossRef]
- Ferentz, M.; Landau, M.V.; Herskowitz, M. Relationship of crystals shape, aggregation mode and surface purity in catalytic wet peroxide oxidation of phenol in dark with titania anatase nanocrystals. Catal. Lett. 2018, 148, 3524–3533. [Google Scholar] [CrossRef]
- Landau, M.; Vidruk, R.; Vingurt, D.; Fuks, D.; Herskowitz, M. Catalytic wet peroxide oxidation of phenol in dark with nanostructured titania catalysts: Effect of exposure of anatase 001 plane. Rev. Chem. Eng. 2014, 30, 379–401. [Google Scholar]
- Erjavec, B.; Kaplan, R.; Pintar, A. Effects of heat and peroxide treatment on photocatalytic activity of titanate nanotubes. Catal. Today 2015, 241, 15–24. [Google Scholar] [CrossRef]
- Dong, P.; Wang, Y.; Guo, L.; Liu, B.; Xin, S.; Zhang, J.; Shi, Y.; Zeng, W.; Yin, S. A facile one-step solvothermal synthesis of graphene/rod-shaped TiO2 nanocomposite and its improved photocatalytic activity. Nanoscale 2012, 4, 4641–4649. [Google Scholar] [CrossRef]
- Morterra, C.; Ghiotti, G.; Garrone, E.; Ficicaro, E. Spectroscopic study of anatase properties. Part 3.-Surface acidity. J. Chem. Soc. Faraday Trans. I 1980, 76, 2102–2113. [Google Scholar] [CrossRef]
- Herrman, M.; Boehm, N.H.P. Über die Chemie der Oberfläche des Titandioxids. II. Saure Hydroxylgruppen auf der Oberfläche. Zeitschrift für Anorganische und Allgemeine Chemie 1969, 368, 73–86. [Google Scholar] [CrossRef]
- Sumita, M.; Hu, C.; Tateyama, Y. Interface water on TiO2 anatase (101) and (001) surfaces: First-principles study with TiO2 slabs dipped in bulk water. J. Phys. Chem. C 2010, 114, 18529–18537. [Google Scholar] [CrossRef]
- Raju, M.; Kim, S.-Y.; van Duin, A.C.T.; Fichthorn, K.A. ReaxFF reactive force field study of the dissociation of water on titania surfaces. J. Phys. Chem. C 2013, 117, 10558–10572. [Google Scholar] [CrossRef]
- Chen, Y.-W.; Chu, I.-H.; Wang, Y.; Cheng, H.-P. Water thin film-silica interaction on α-quartz (0001) surfaces. Phys. Rev. B 2011, 84, 155444–155454. [Google Scholar] [CrossRef]
- Crocker, M.; Herold, R.H.M.; Wilson, A.E.; Mackay, M.; Emeis, C.A.; Hoogendoorn, A.M. 1H NMR spectroscopy of titania. Chemical shift assignments for hydroxy groups in crystalline and amorphous forms of TiO2. Chem. Soc. Faraday Trans. 1996, 92, 2791–2798. [Google Scholar] [CrossRef]
- Mastikhin, V.M.; Mudrakovsky, I.L.; Nosov, A.V. 1H N M R magic angle spinning (MAS) studies of heterogeneous catalysis. Prog. NMR Spectrosc. 1991, 23, 259–299. [Google Scholar] [CrossRef]
- Mogylevsky, G.; Chen, Q.; Kulkarni, H.; Kleinhammes, A.; Mullins, W.Q.M.; Wu, Y. Layered nanostructures of delaminated anatase: Nanosheets and nanotubes. J. Phys. Chem. C 2008, 112, 3239–3246. [Google Scholar] [CrossRef]
- Dec, S.F.; Davis, M.F.; Maciel, G.E.; Bronnimann, C.E.; Fitzgerald, J.J.; Han, S.S. Solid-state multinuclear NMR studies of ferroelectric, piezoelectric, and related materials. Inorg. Chem. 1993, 32, 955–959. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Wang, K.; Yao, F.-Z.; Sun, W.; Zhou, Z.; Zhang, M.-H.; Li, X.; Luo, H.; Lee, J.-S.; Li, J.-F. Identifying phase transition behavior in Bi½Na½TiO3-BaTiO3 single crystals by piezoresponse force microscopy. J. Appl. Phys. 2017, 121, 174103–174113. [Google Scholar] [CrossRef]
- Bräuniger, T.; Madhu, P.K.; Pampel, A.; Reichert, D. Application of fast amplitude-modulated pulse trains for signal enhancement in static and magic-angle-spinning 47,49Ti-NMR spectra. Solid State Nucl. Magn. Reson. 2004, 26, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Kwak, H.-T.; Sakellariou, D.; Emsley, L.; Grandinetti, P.J. Sensitivity enhancement of the central transition NMR signal of quadrupolar nuclei under magic-angle spinning. Chem. Phys. Lett. 2000, 327, 85–90. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Calvé, S.; Alonso, B.; Durand, J.-O.; Bujoli, B.; Gan, Z.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
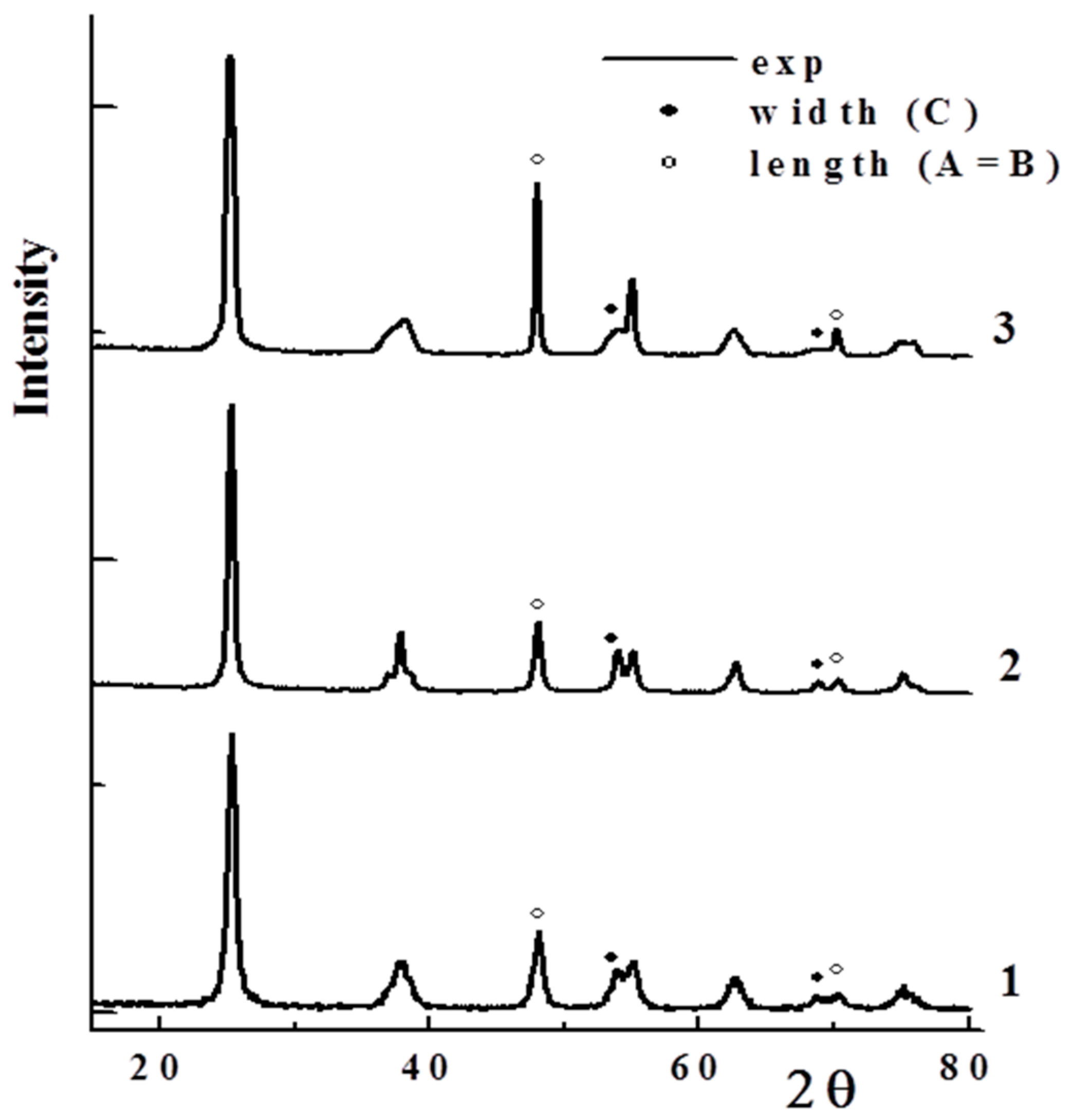
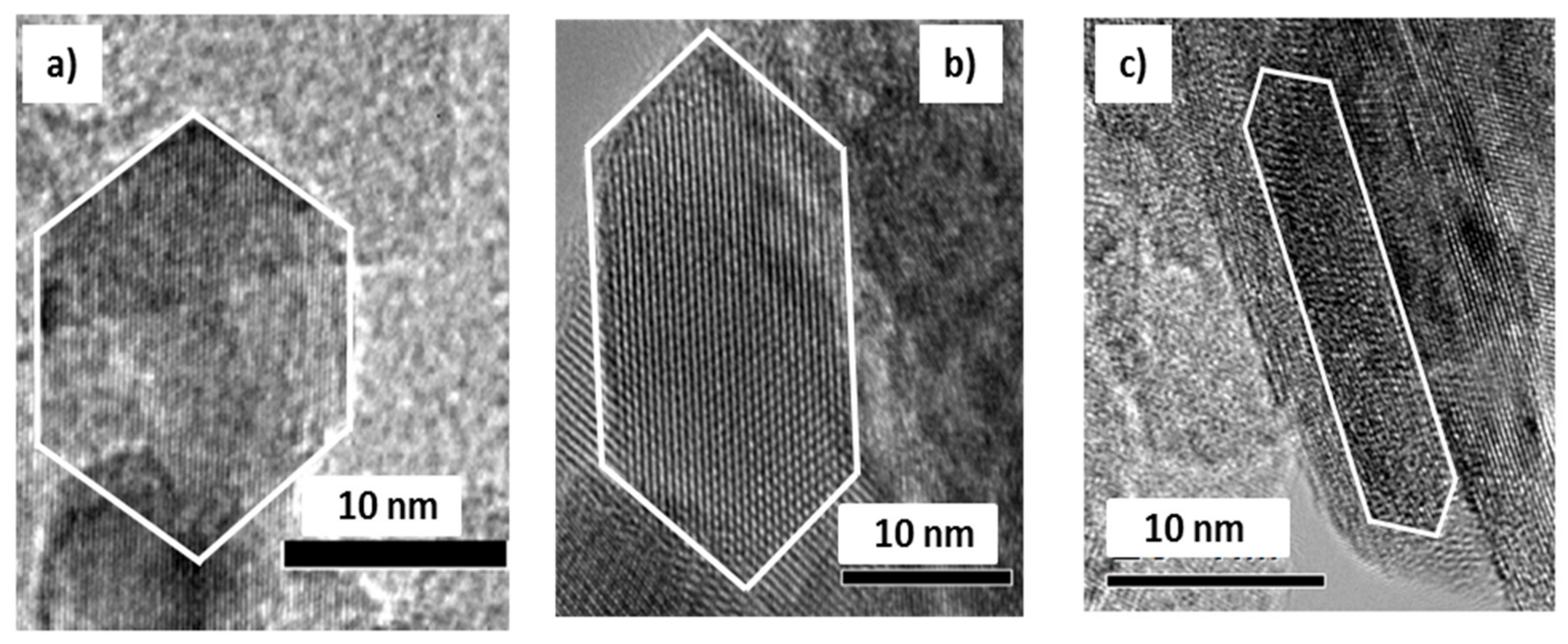
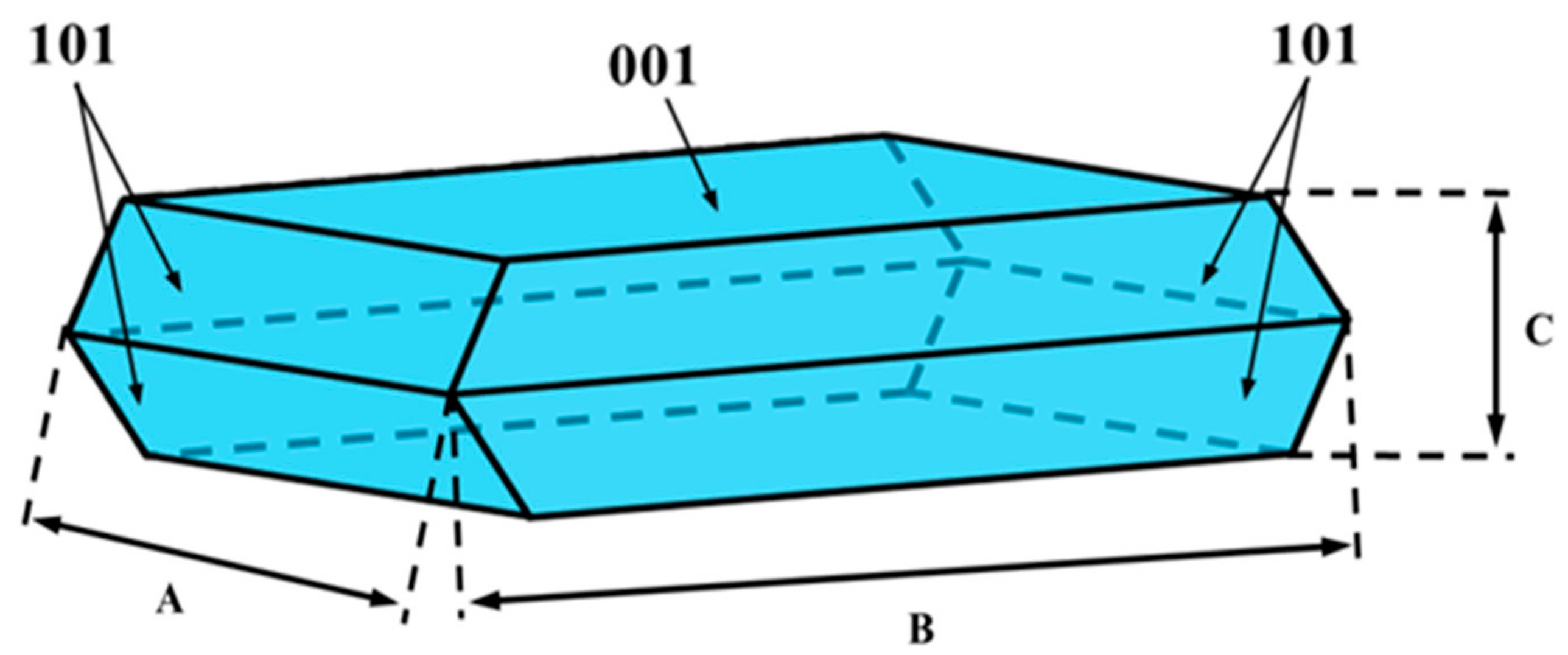

| Sample | Texture Parameters | Mean Crystal Size, nm | (001) Plane Exposure | |||||
|---|---|---|---|---|---|---|---|---|
| Crystals | Aggregates | |||||||
| Surface Area, m2/g | Total Pore Volume, cm3/g | Average Pore Diameter, nm | Length (A = B) | Thickness C | % | % | m2/g | |
| TiO2-1 | 150 ± 15 | 0.43 ± 0.005 | 11 ± 0.1 | 16 ± 0.02 | 15 ± 0.02 | 12 ± 0.01 | 12 ± 0.01 | 18 ± 2 |
| TiO2-2 | 65 ± 7 | 0.15 ± 0.002 | 5 ± 0.05 | 24 ± 0.02 | 12 ± 0.01 | 38 ± 0.04 | 38 ± 0.04 | 25 ± 2 |
| TiO2-3 | 120 ± 12 | 0.46 ± 0.005 | 18 ± 0.2 | 24 ± 0.02 | 5 ± 0.005 | 66 ± 0.07 | 63 ± 0.07 | 73 ± 7 |
| Sample | Acidity (NH3-TPD), mmol NH3/gcat | Basicity (CO2-TPD), mmol CO2/gcat | ||
|---|---|---|---|---|
| Total | Strong (>350 °C) | Total | Strong (>450 °C) | |
| TiO2-1 | 3.7 ± 0.4 | 1.1 ± 0.1 | 0.17 ± 0.02 | 0.06 ± 0.006 |
| TiO2-2 | 3.8 ± 0.4 | 1.3 ± 0.1 | 0.14 ± 0.01 | 0.08 ± 0.008 |
| TiO2-3 | 5.9 ± 0.6 | 2.1 ± 0.2 | 0.16 ± 0.02 | 0.02 ± 0.002 |
| Sample | TOCR | TCfresh | TCused | TCused − TCfresh | TOCm | TOCads | TOCResid |
|---|---|---|---|---|---|---|---|
| (%) | (wt. %) | (%) | |||||
| TiO2-1 | 89 | 0.28 | 0.99 | 0.71 | 77 | 12 | 11 |
| TiO2-2 | 65 | 0.28 | 1.63 | 1.35 | 43 | 22 | 35 |
| TiO2-3 | 72 | 0.18 | 3.09 | 2.91 | 24 | 48 | 28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žerjav, G.; Pintar, A.; Ferentz, M.; Landau, M.; Haimovich, A.; Goldbourt, A.; Herskowitz, M. Effect of Surface Chemistry and Crystallographic Parameters of TiO2 Anatase Nanocrystals on Photocatalytic Degradation of Bisphenol A. Catalysts 2019, 9, 447. https://doi.org/10.3390/catal9050447
Žerjav G, Pintar A, Ferentz M, Landau M, Haimovich A, Goldbourt A, Herskowitz M. Effect of Surface Chemistry and Crystallographic Parameters of TiO2 Anatase Nanocrystals on Photocatalytic Degradation of Bisphenol A. Catalysts. 2019; 9(5):447. https://doi.org/10.3390/catal9050447
Chicago/Turabian StyleŽerjav, Gregor, Albin Pintar, Michael Ferentz, Miron Landau, Anat Haimovich, Amir Goldbourt, and Moti Herskowitz. 2019. "Effect of Surface Chemistry and Crystallographic Parameters of TiO2 Anatase Nanocrystals on Photocatalytic Degradation of Bisphenol A" Catalysts 9, no. 5: 447. https://doi.org/10.3390/catal9050447
APA StyleŽerjav, G., Pintar, A., Ferentz, M., Landau, M., Haimovich, A., Goldbourt, A., & Herskowitz, M. (2019). Effect of Surface Chemistry and Crystallographic Parameters of TiO2 Anatase Nanocrystals on Photocatalytic Degradation of Bisphenol A. Catalysts, 9(5), 447. https://doi.org/10.3390/catal9050447