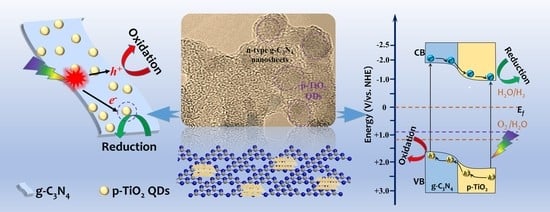
Controllable Fabrication of Heterogeneous p-TiO2 QDs@g-C3N4 p-n Junction for Efficient Photocatalysis
Abstract
:1. Introduction
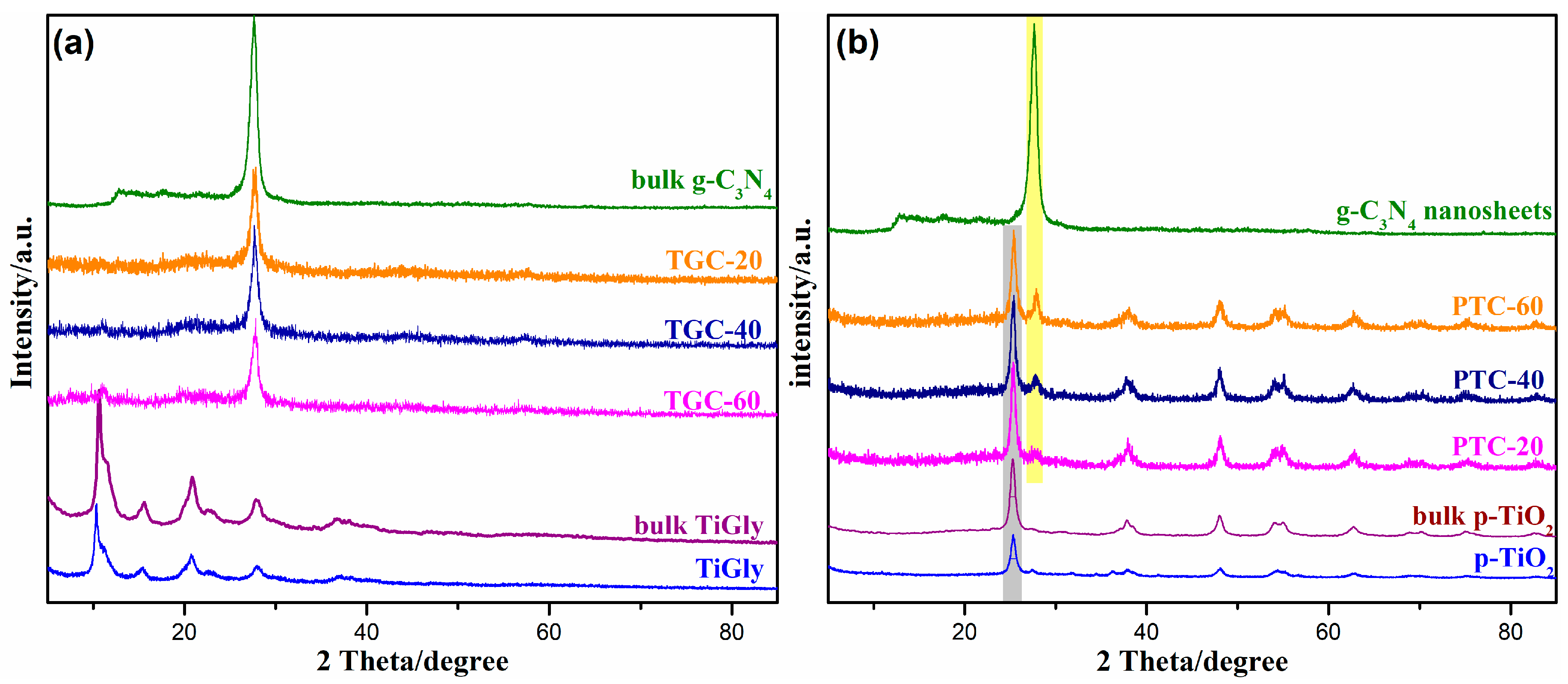
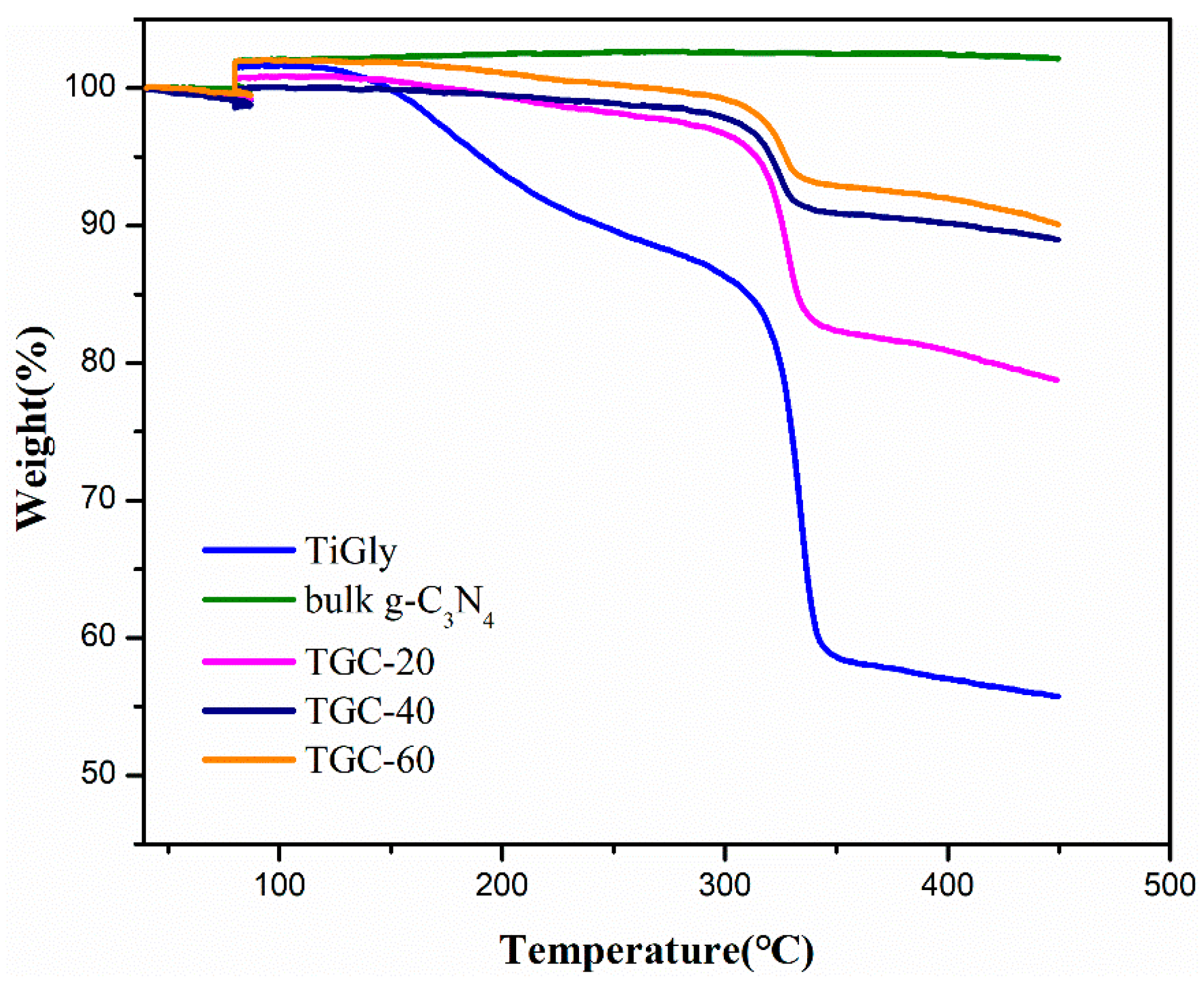
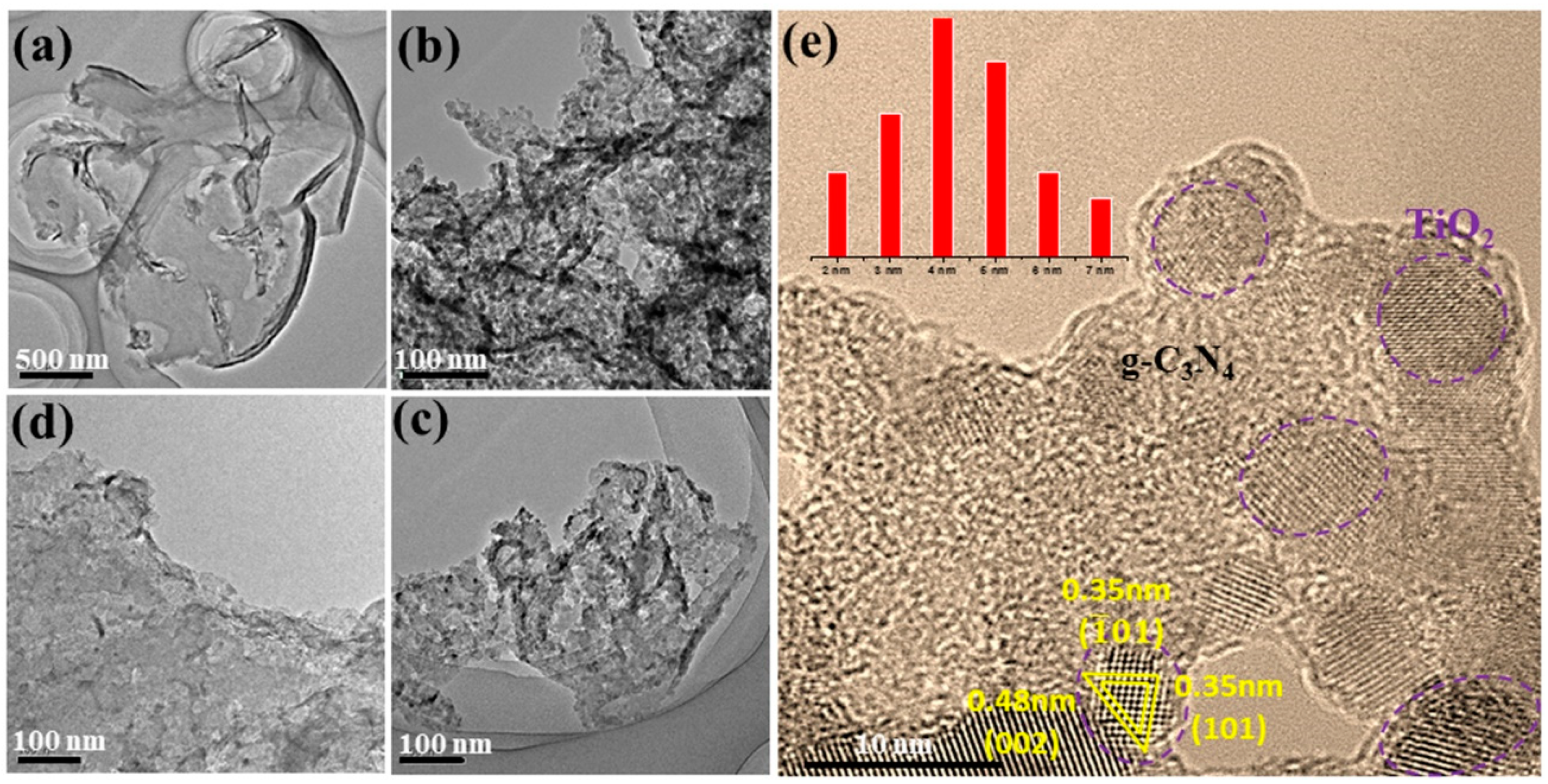
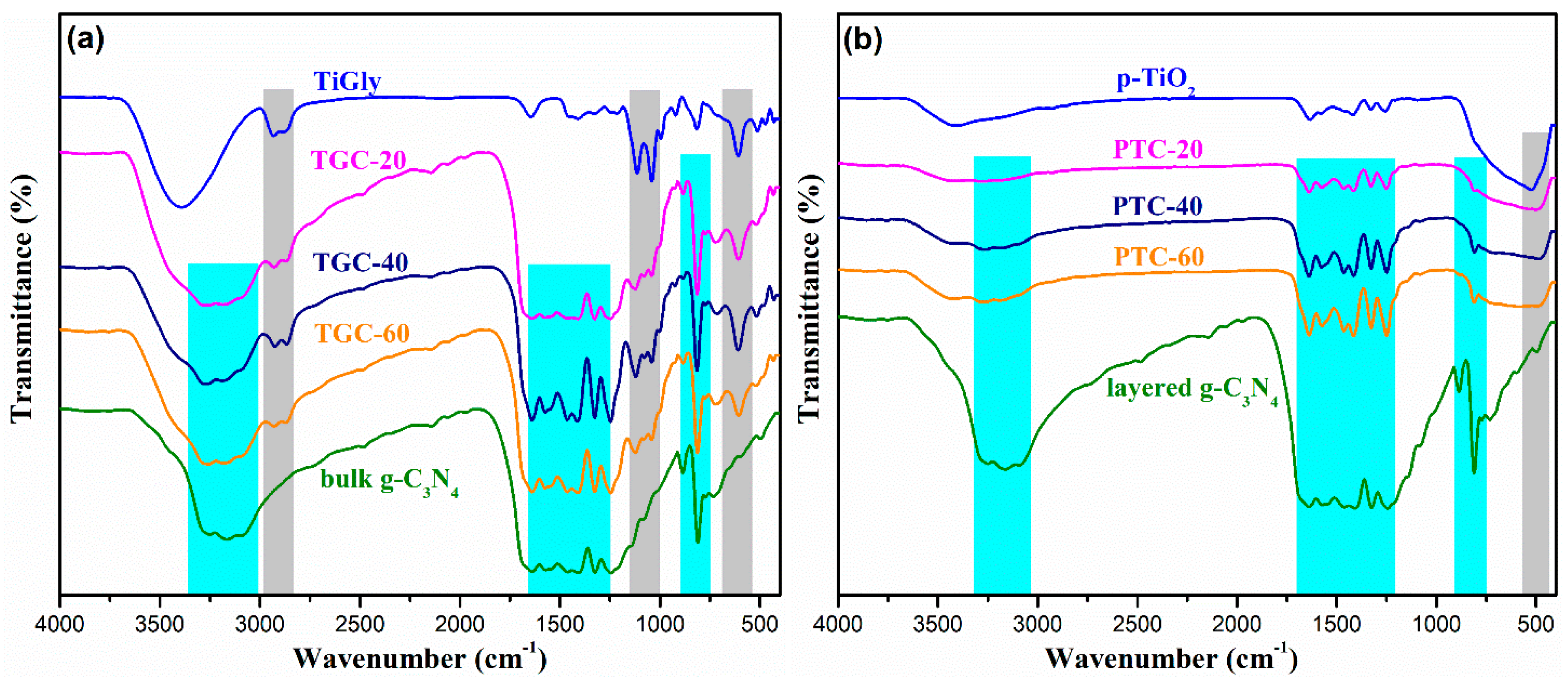
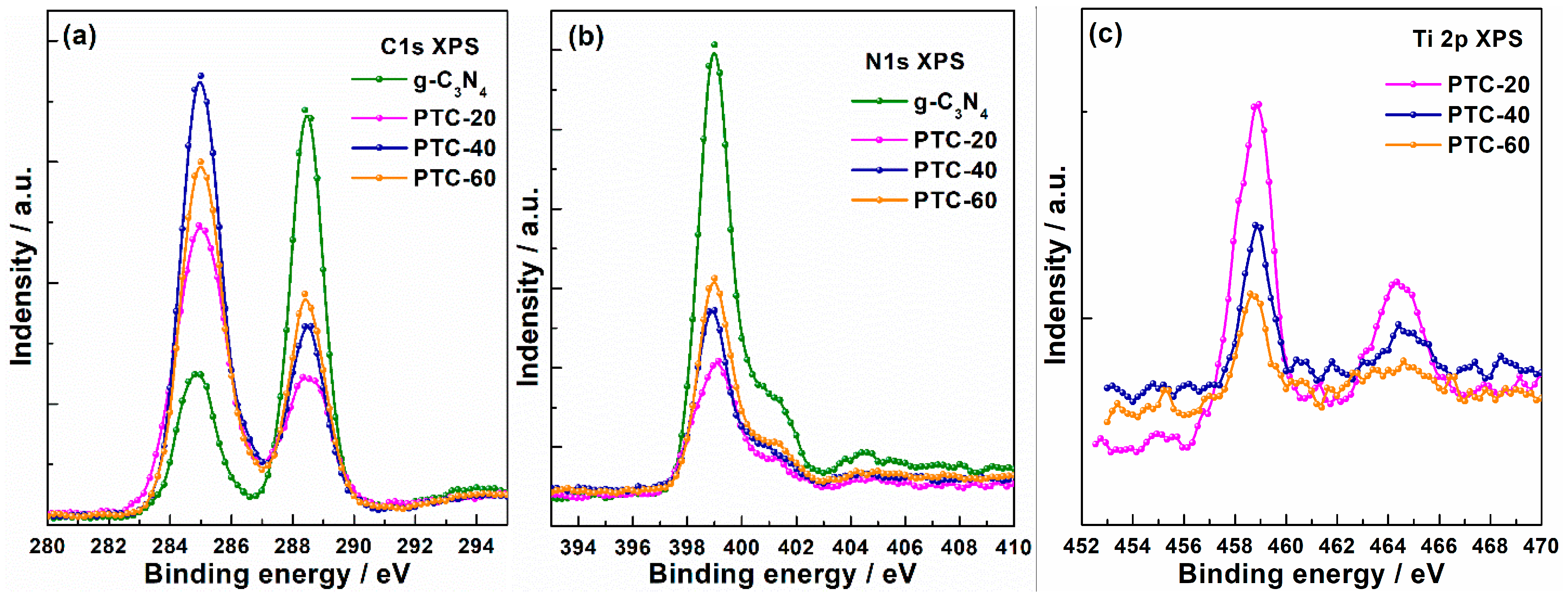
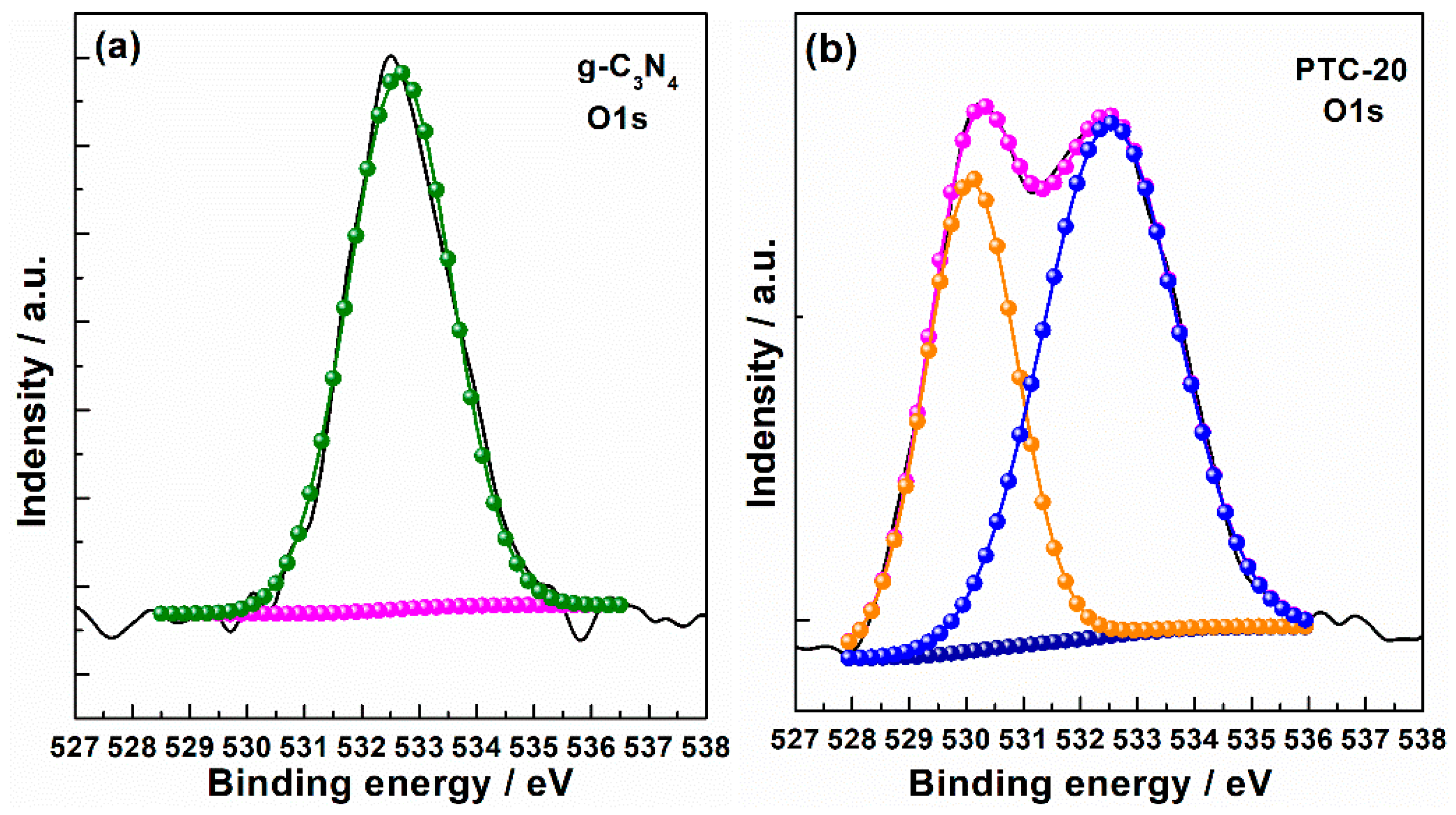
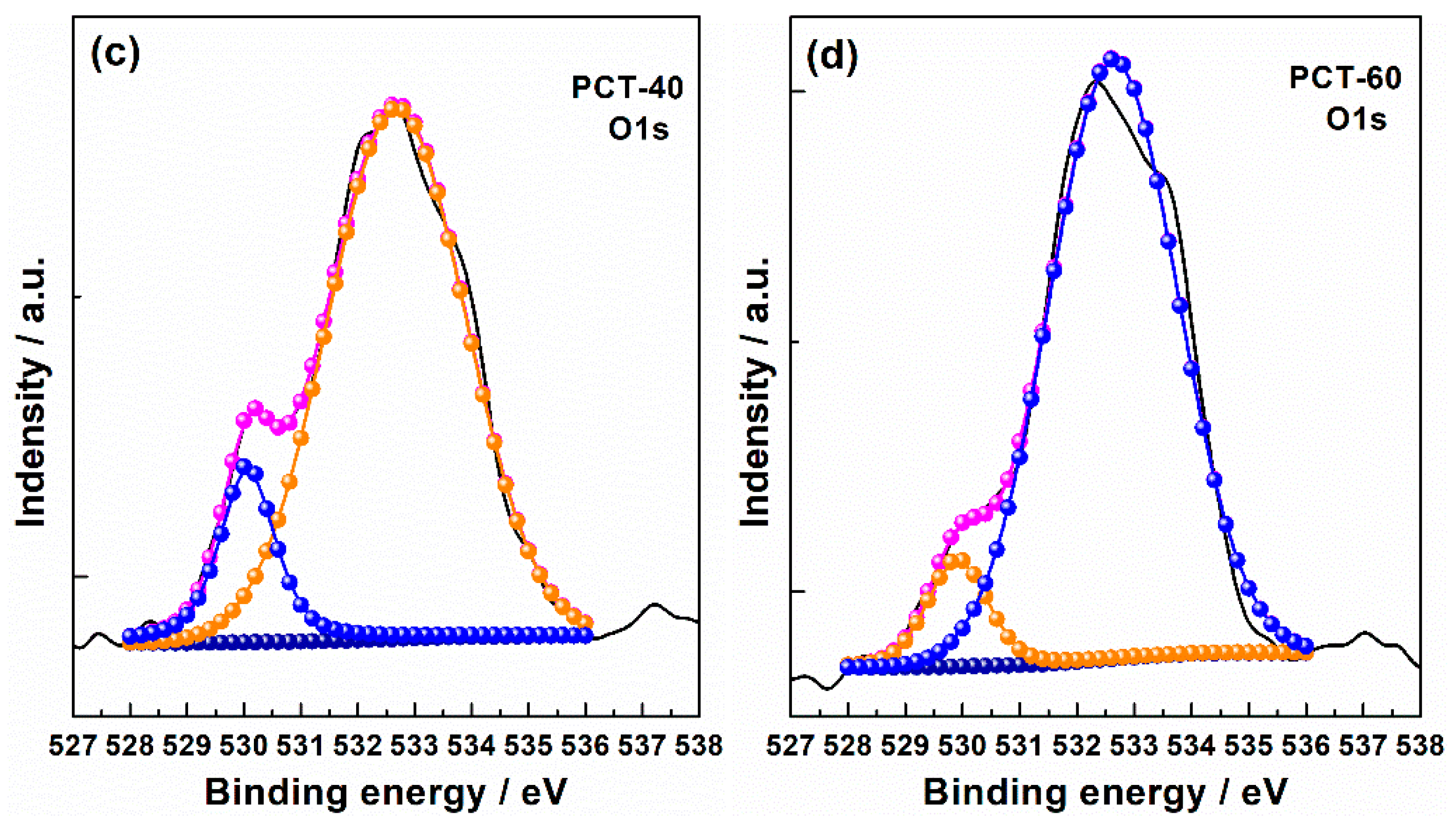
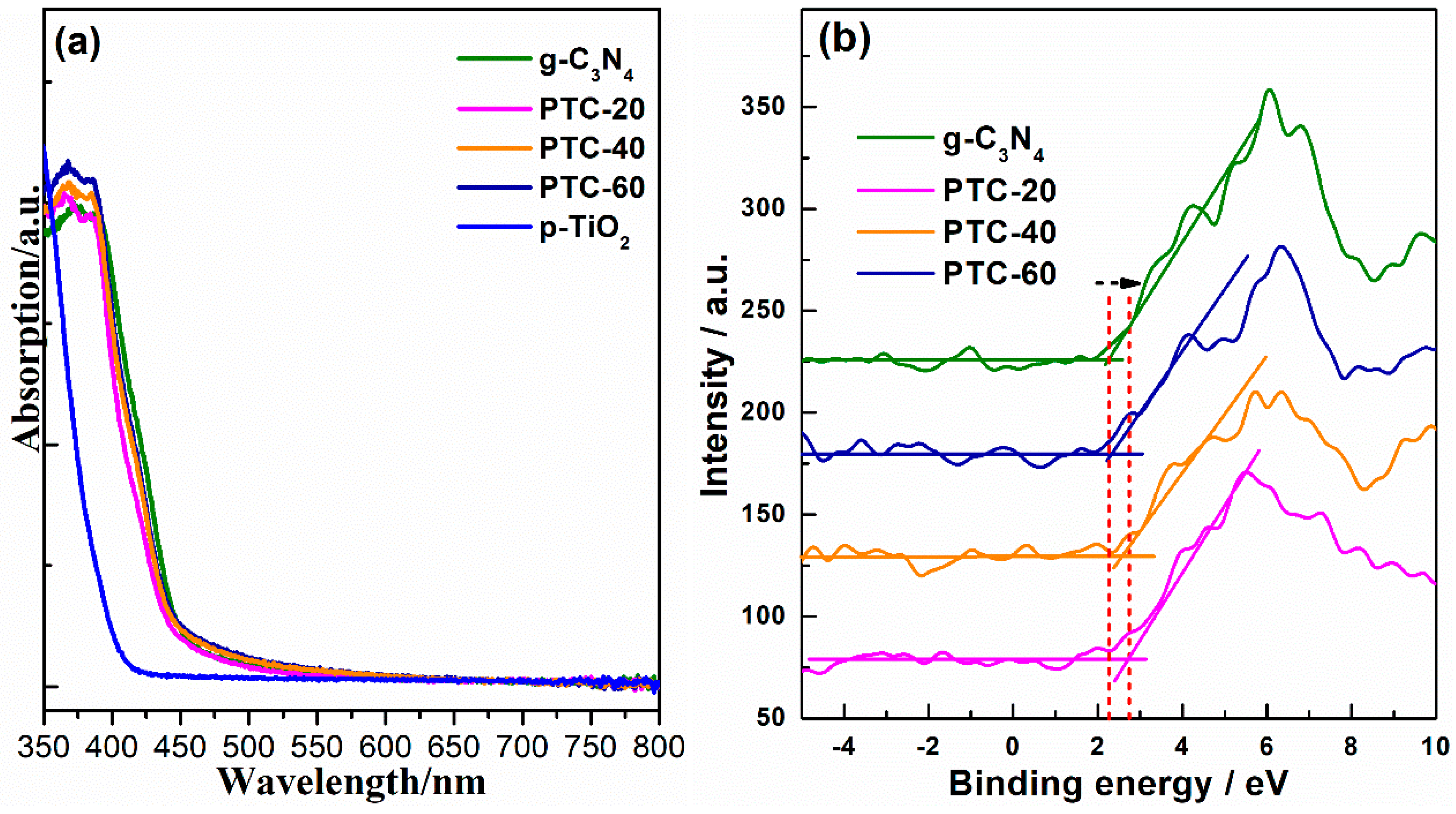
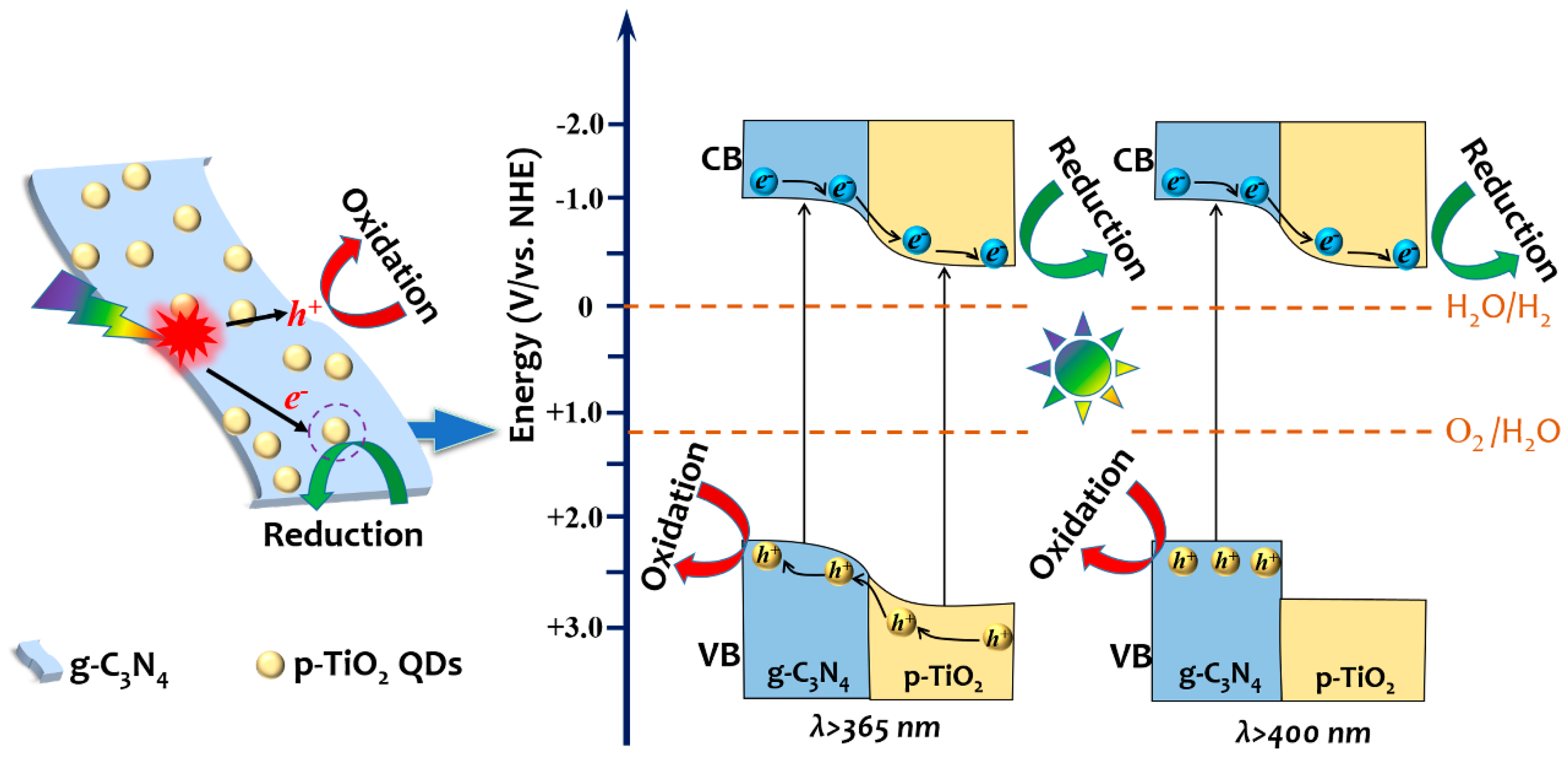
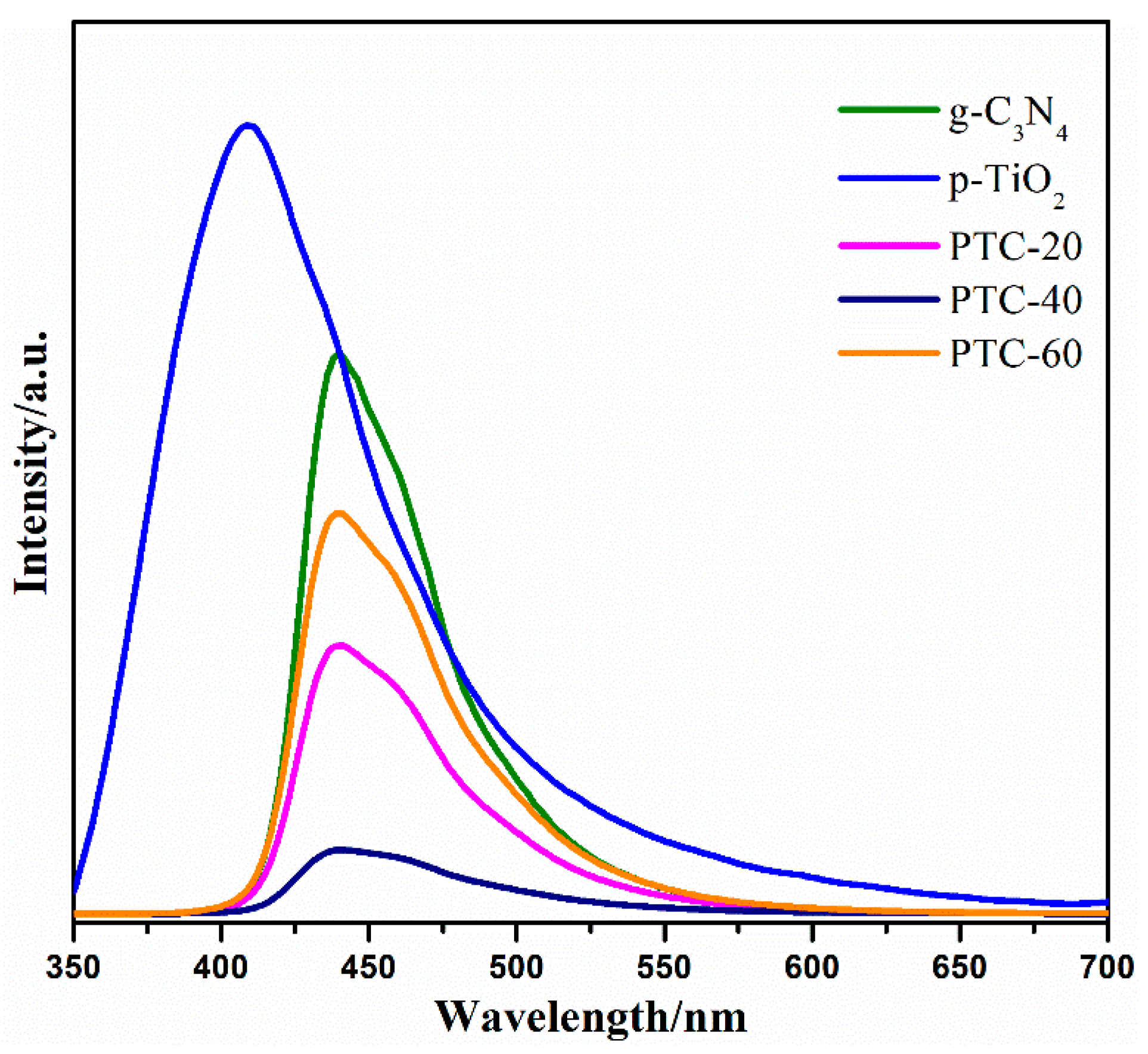
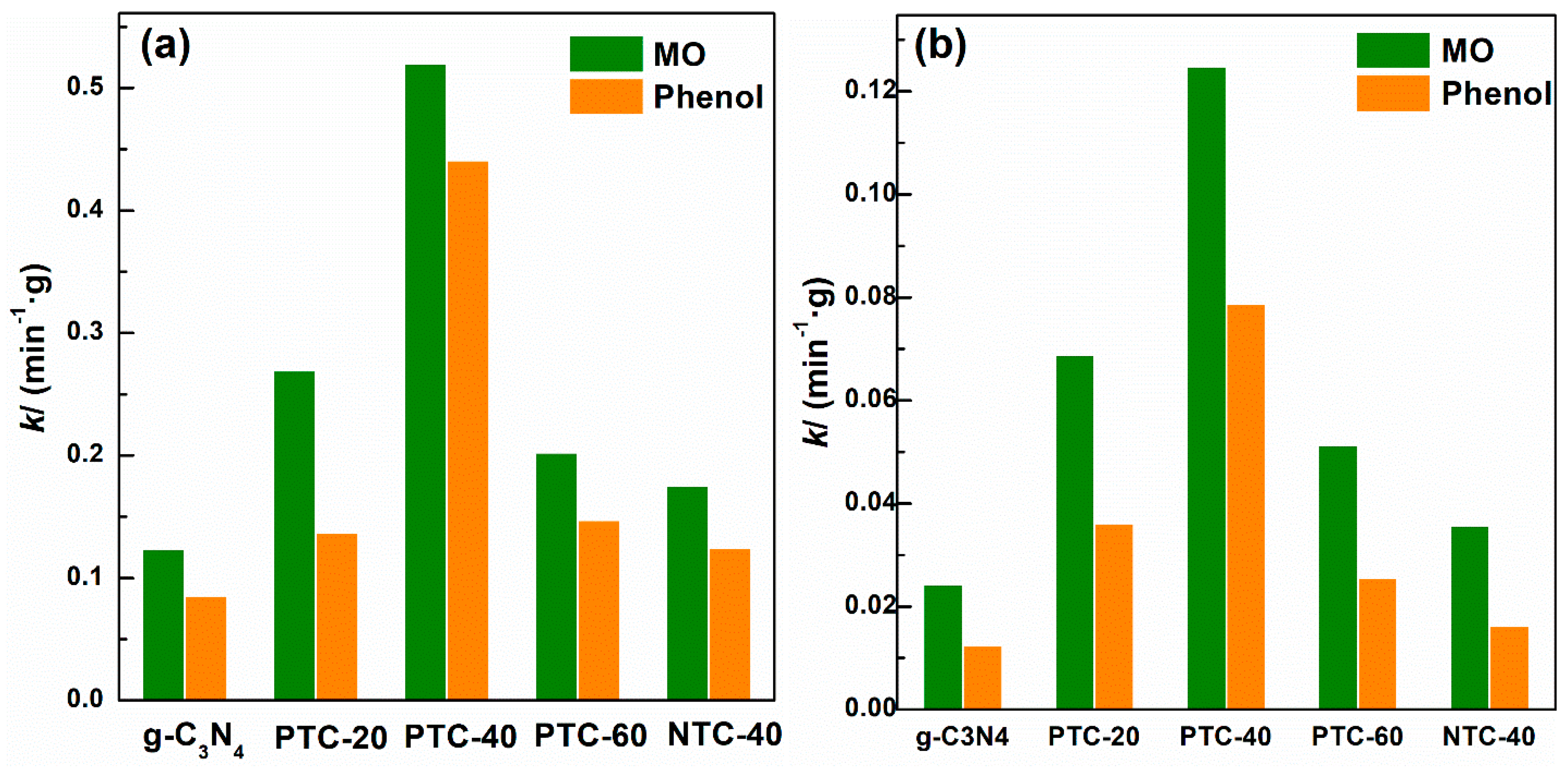
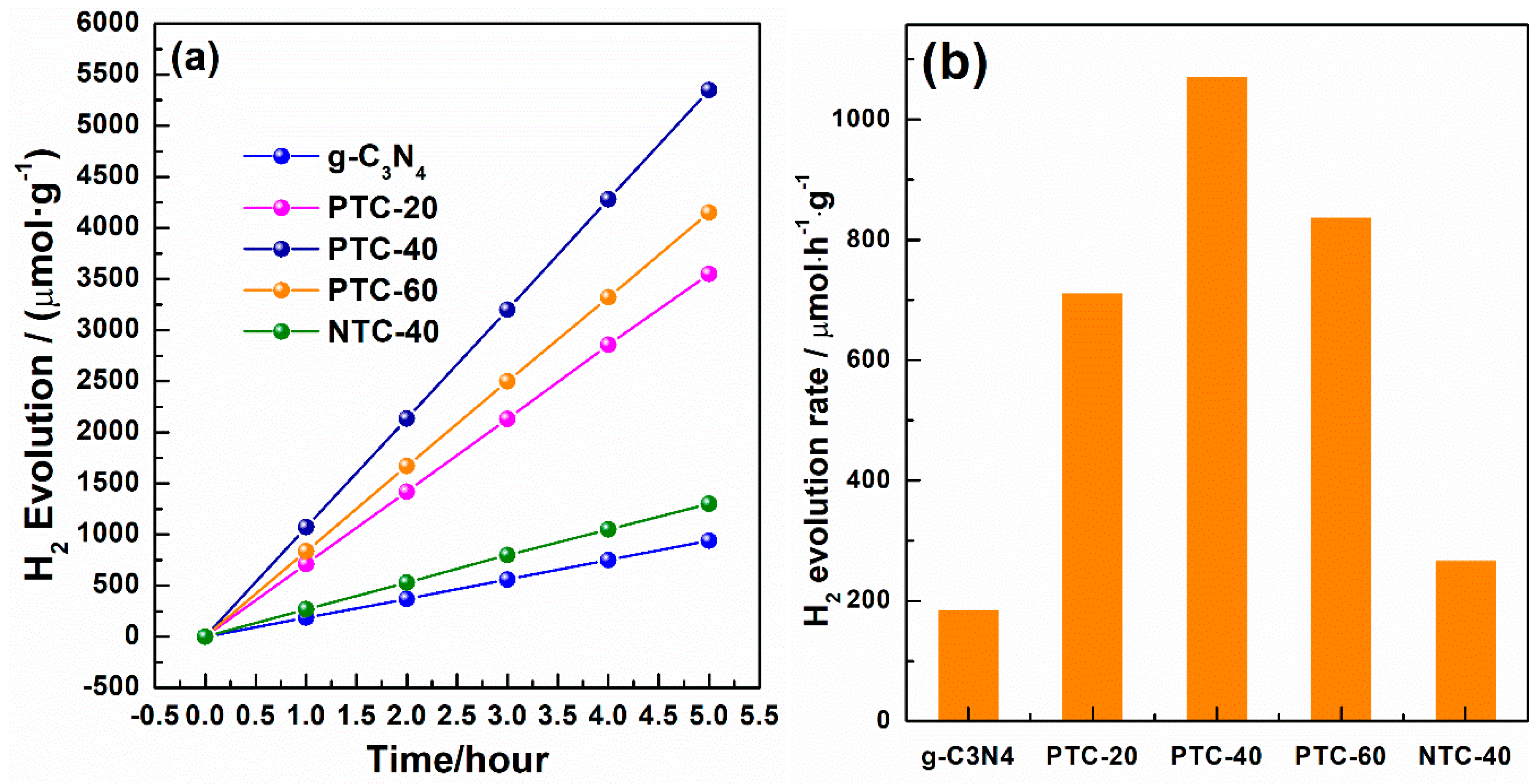
2. Results and Discussion
3. Experimental
3.1. Materials
3.2. Preparation of Bulk g-C3N4
3.3. Preparation of TiO2 QDs@g-C3N4 p-n Heterojunction
3.4. Characterization of Photocatalysts
3.5. Photocatalytic Degradation and Hydrogen Evolution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Wu, S.-M.; Liu, X.-L.; Lian, X.-L.; Tian, G.; Janiak, C.; Zhang, Y.-X.; Lu, Y.; Yu, H.-Z.; Hu, J.; Wei, H.; et al. Homojunction of Oxygen and Titanium Vacancies and its Interfacial n–p Effect. Adv. Mater. 2018, 30, 1802173. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.-Q.; Liu, S.; Sun, Y.; Xu, Y.-J. Waltzing with the Versatile Platform of Graphene to Synthesize Composite Photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef] [PubMed]
- Wondraczek, L.; Tyystjärvi, E.; Méndez-Ramos, J.; Müller, F.A.; Zhang, Q. Shifting the Sun: Solar Spectral Conversion and Extrinsic Sensitization in Natural and Artificial Photosynthesis. Adv. Sci. 2015, 2, 1500218. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, G.; Shi, L.; Liu, H.; Wang, T.; Ye, J. Engineering coordination polymers for photocatalysis. Nano Energy 2016, 22, 149–168. [Google Scholar] [CrossRef]
- Kapilashrami, M.; Zhang, Y.; Liu, Y.-S.; Hagfeldt, A.; Guo, J. Probing the Optical Property and Electronic Structure of TiO2 Nanomaterials for Renewable Energy Applications. Chem. Rev. 2014, 114, 9662–9707. [Google Scholar] [CrossRef] [PubMed]
- Fresno, F.; Portela, R.; Suárez, S.; Coronado, J.M. Photocatalytic materials: recent achievements and near future trends. J. Mater. Chem. A 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638. [Google Scholar] [CrossRef]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 2015, 6, 5881. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.; Cheng, H.-M. Titanium Dioxide Crystals with Tailored Facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef] [PubMed]
- Di Valentin, C.; Pacchioni, G.; Selloni, A. Reduced and n-Type Doped TiO2: Nature of Ti3+ Species. J. Phys. Chem. C 2009, 113, 20543–20552. [Google Scholar] [CrossRef]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; Di Valentin, C.; Pacchioni, G. Origin of Photoactivity of Nitrogen-Doped Titanium Dioxide under Visible Light. J. Am. Chem. Soc. 2006, 128, 15666–15671. [Google Scholar] [CrossRef]
- Li, J.-G.; Büchel, R.; Isobe, M.; Mori, T.; Ishigaki, T. Cobalt-Doped TiO2 Nanocrystallites: Radio-Frequency Thermal Plasma Processing, Phase Structure, and Magnetic Properties. J. Phys. Chem. C 2009, 113, 8009–8015. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, Y.; Liu, L.; Ye, J. A p-type Cr-doped TiO2 photo-electrode for photo-reduction. Chem. Commun. 2013, 49, 3440–3442. [Google Scholar] [CrossRef]
- Wang, Y.; Han, P.; Lv, X.; Zhang, L.; Zheng, G. Defect and Interface Engineering for Aqueous Electrocatalytic CO2 Reduction. Joule 2018, 2, 2551–2582. [Google Scholar] [CrossRef]
- Wang, T.; Liu, L.; Ge, G.; Liu, M.; Zhou, W.; Chang, K.; Yang, F.; Wang, D.; Ye, J. Two-dimensional titanium oxide nanosheets rich in titanium vacancies as an efficient cocatalyst for photocatalytic water oxidation. J. Catal. 2018, 367, 296–305. [Google Scholar] [CrossRef]
- Nowotny, M.K.; Bogdanoff, P.; Dittrich, T.; Fiechter, S.; Fujishima, A.; Tributsch, H. Observations of p-type semiconductivity in titanium dioxide at room temperature. Mater. Lett. 2010, 64, 928–930. [Google Scholar] [CrossRef]
- Wu, Q.; Huang, F.; Zhao, M.; Xu, J.; Zhou, J.; Wang, Y. Ultra-small yellow defective TiO2 nanoparticles for co-catalyst free photocatalytic hydrogen production. Nano Energy 2016, 24, 63–71. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, Y.; Tu, W.; Ye, J.; Zou, Z. State-of-the-Art Progress in Diverse Heterostructured Photocatalysts toward Promoting Photocatalytic Performance. Adv. Funct. Mater. 2015, 25, 998–1013. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Xiao, T.; Tian, Y.; Jiang, Z. Biomimetic fabrication of g-C3N4/TiO2 nanosheets with enhanced photocatalytic activity toward organic pollutant degradation. Chem. Eng. J. 2015, 260, 117–125. [Google Scholar] [CrossRef]
- Wang, S.; Huang, C.-Y.; Pan, L.; Chen, Y.; Zhang, X.; Fazale, A.; Zou, J.-J. Controllable fabrication of homogeneous ZnO p-n junction with enhanced charge separation for efficient photocatalysis. Catal. Today 2018. [Google Scholar] [CrossRef]
- Wang, M.; Sun, L.; Lin, Z.; Cai, J.; Xie, K.; Lin, C. p–n Heterojunction photoelectrodes composed of Cu2O-loaded TiO2 nanotube arrays with enhanced photoelectrochemical and photoelectrocatalytic activities. Energy Environ. Sci. 2013, 6, 1211–1220. [Google Scholar] [CrossRef]
- Wang, S.; Pan, L.; Song, J.-J.; Mi, W.; Zou, J.-J.; Wang, L.; Zhang, X. Titanium-Defected Undoped Anatase TiO2 with p-Type Conductivity, Room-Temperature Ferromagnetism, and Remarkable Photocatalytic Performance. J. Am. Chem. Soc. 2015, 137, 2975–2983. [Google Scholar] [CrossRef]
- Pan, L.; Wang, S.; Xie, J.; Wang, L.; Zhang, X.; Zou, J.-J. Constructing TiO2 p-n homojunction for photoelectrochemical and photocatalytic hydrogen generation. Nano Energy 2016, 28, 296–303. [Google Scholar] [CrossRef]
- Ma, L.; Han, H.; Pan, L.; Tahir, M.; Wang, L.; Zhang, X.; Zou, J.-J. Fabrication of TiO2 nanosheets via Ti3+ doping and Ag3PO4 QD sensitization for highly efficient visible-light photocatalysis. RSC Adv. 2016, 6, 63984–63990. [Google Scholar] [CrossRef]
- Pan, L.; Zou, J.-J.; Wang, S.; Huang, Z.-F.; Yu, A.; Wang, L.; Zhang, X. Quantum dot self-decorated TiO2 nanosheets. Chem. Commun. 2013, 49, 6593–6595. [Google Scholar] [CrossRef]
- Anpo, M.; Kawamura, T.; Kodama, S.; Maruya, K.; Onishi, T. Photocatalysis on titanium-aluminum binary metal oxides: enhancement of the photocatalytic activity of titania species. J. Phys. Chem. 1988, 92, 438–440. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. g-C3N4-Based Photocatalysts for Hydrogen Generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric Graphitic Carbon Nitride for Heterogeneous Photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Low, J.; Cao, S.; Yu, J.; Wageh, S. Two-dimensional layered composite photocatalysts. Chem. Commun. 2014, 50, 10768–10777. [Google Scholar] [CrossRef]
- Butchosa, C.; Guiglion, P.; Zwijnenburg, M.A. Carbon Nitride Photocatalysts for Water Splitting: A Computational Perspective. J. Phys. Chem. C 2014, 118, 24833–24842. [Google Scholar] [CrossRef]
- Zhang, H.; Zuo, X.; Tang, H.; Li, G.; Zhou, Z. Origin of photoactivity in graphitic carbon nitride and strategies for enhancement of photocatalytic efficiency: insights from first-principles computations. Phys. Chem. Chem. Phys. 2015, 17, 6280–6288. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Pan, L.; Wang, Z.; Zhang, X.; Zou, J.-J.; Mi, W.; Zhang, X.; Wang, L. Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution. Nano Energy 2015, 12, 646–656. [Google Scholar] [CrossRef]
- Zhang, J.-W.; Gong, S.; Mahmood, N.; Pan, L.; Zhang, X.; Zou, J.-J. Oxygen-doped nanoporous carbon nitride via water-based homogeneous supramolecular assembly for photocatalytic hydrogen evolution. Appl. Catal. B-Environ. 2018, 221, 9–16. [Google Scholar] [CrossRef]
- Ye, C.; Li, J.-X.; Li, Z.-J.; Li, X.-B.; Fan, X.-B.; Zhang, L.-P.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Enhanced Driving Force and Charge Separation Efficiency of Protonated g-C3N4 for Photocatalytic O2 Evolution. ACS Catal. 2015, 5, 6973–6979. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Wang, X.; Huang, Y.; Zeng, M.; Xu, J. Rationally designed 1D Ag@AgVO3 nanowire/graphene/protonated g-C3N4 nanosheet heterojunctions for enhanced photocatalysis via electrostatic self-assembly and photochemical reduction methods. J. Mater. Chem. A 2015, 3, 10119–10126. [Google Scholar] [CrossRef]
- Yang, S.B.; Gong, Y.J.; Zhang, J.S.; Zhan, L.; Ma, L.L.; Fang, Z.Y.; Vajtai, R.; Wang, X.C.; Ajayan, P.M. Exfoliated Graphitic Carbon Nitride Nanosheets as Efficient Catalysts for Hydrogen Evolution Under Visible Light. Adv. Mater. 2013, 25, 2452–2456. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.L.; Liu, G.; Cheng, H.M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Xu, H.; Yan, J.; She, X.; Xu, L.; Xia, J.; Xu, Y.; Song, Y.; Huang, L.; Li, H. Graphene-analogue carbon nitride: novel exfoliation synthesis and its application in photocatalysis and photoelectrochemical selective detection of trace amount of Cu2+. Nanoscale 2014, 6, 1406–1415. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.-C.; Kang, X.; Liu, G.; Cheng, H.-M. An Amorphous Carbon Nitride Photocatalyst with Greatly Extended Visible-Light-Responsive Range for Photocatalytic Hydrogen Generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef]
- Jun, Y.-S.; Lee, E.Z.; Wang, X.; Hong, W.H.; Stucky, G.D.; Thomas, A. From Melamine-Cyanuric Acid Supramolecular Aggregates to Carbon Nitride Hollow Spheres. Adv. Funct. Mater. 2013, 23, 3661–3667. [Google Scholar] [CrossRef]
- Liang, Q.; Li, Z.; Yu, X.; Huang, Z.-H.; Kang, F.; Yang, Q.-H. Macroscopic 3D Porous Graphitic Carbon Nitride Monolith for Enhanced Photocatalytic Hydrogen Evolution. Adv. Mater. 2015, 27, 4634–4639. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Lin, L.; Wang, X. Sol Processing of Conjugated Carbon Nitride Powders for Thin-Film Fabrication. Angew. Chem. Int. Edit. 2015, 54, 6297–6301. [Google Scholar] [CrossRef]
- Das, J.; Freitas, F.S.; Evans, I.R.; Nogueira, A.F.; Khushalani, D. A facile nonaqueous route for fabricating titania nanorods and their viability in quasi-solid-state dye-sensitized solar cells. J. Mater. Chem. 2010, 20, 4425–4431. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, F.; Su, Z.; Wang, X.; Han, Y.; Zhang, L.; Xiang, J.; Du, W.; Tang, N. Controllable Fabrication of Heterogeneous p-TiO2 QDs@g-C3N4 p-n Junction for Efficient Photocatalysis. Catalysts 2019, 9, 439. https://doi.org/10.3390/catal9050439
Wang S, Wang F, Su Z, Wang X, Han Y, Zhang L, Xiang J, Du W, Tang N. Controllable Fabrication of Heterogeneous p-TiO2 QDs@g-C3N4 p-n Junction for Efficient Photocatalysis. Catalysts. 2019; 9(5):439. https://doi.org/10.3390/catal9050439
Chicago/Turabian StyleWang, Songbo, Feifan Wang, Zhiming Su, Xiaoning Wang, Yicheng Han, Lei Zhang, Jun Xiang, Wei Du, and Na Tang. 2019. "Controllable Fabrication of Heterogeneous p-TiO2 QDs@g-C3N4 p-n Junction for Efficient Photocatalysis" Catalysts 9, no. 5: 439. https://doi.org/10.3390/catal9050439
APA StyleWang, S., Wang, F., Su, Z., Wang, X., Han, Y., Zhang, L., Xiang, J., Du, W., & Tang, N. (2019). Controllable Fabrication of Heterogeneous p-TiO2 QDs@g-C3N4 p-n Junction for Efficient Photocatalysis. Catalysts, 9(5), 439. https://doi.org/10.3390/catal9050439