Fundamentals of Sulfate Species in Methane Combustion Catalyst Operation and Regeneration—A Simulated Exhaust Gas Study
Abstract
:1. Introduction
2. Results and Discussion
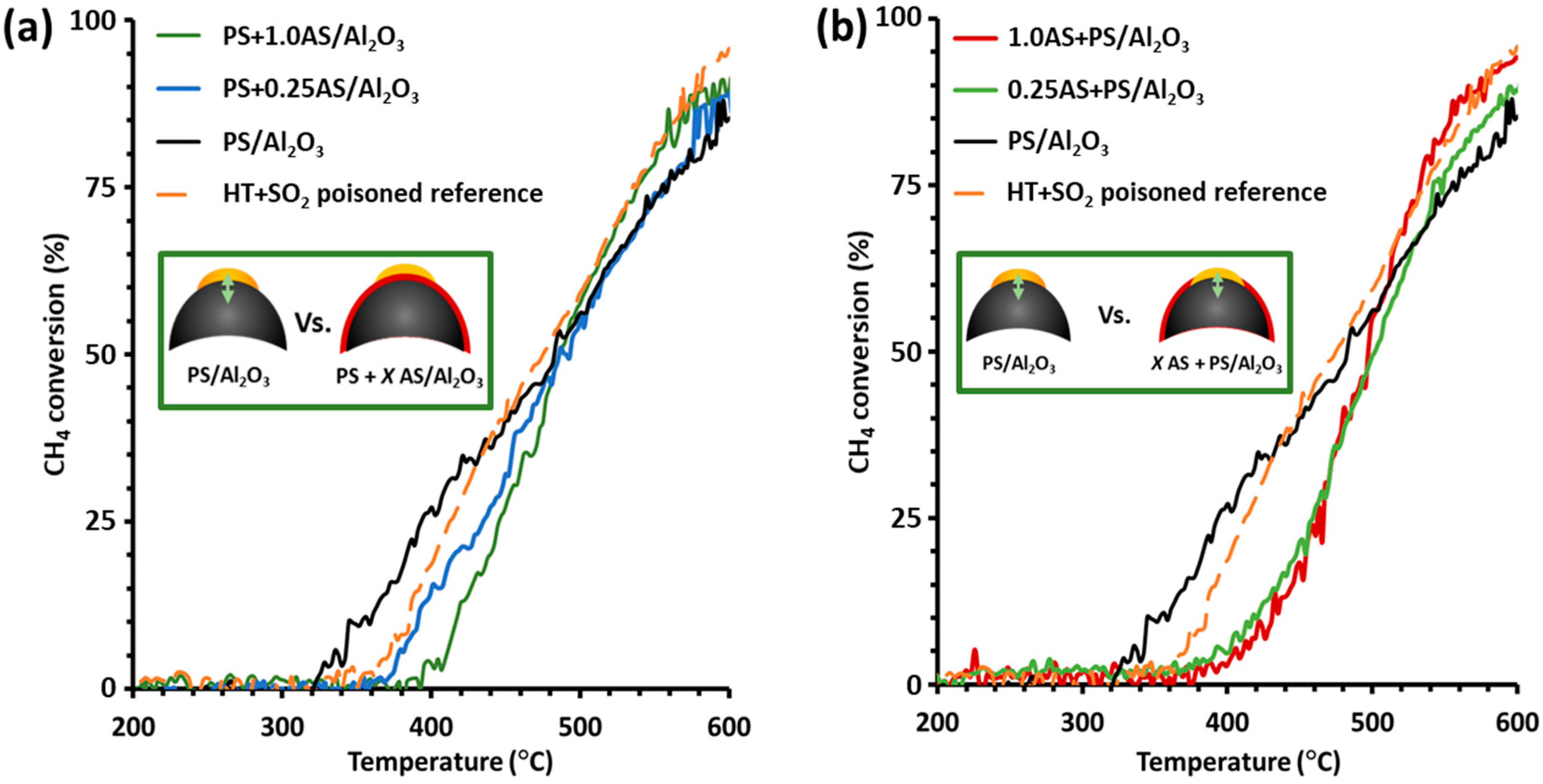
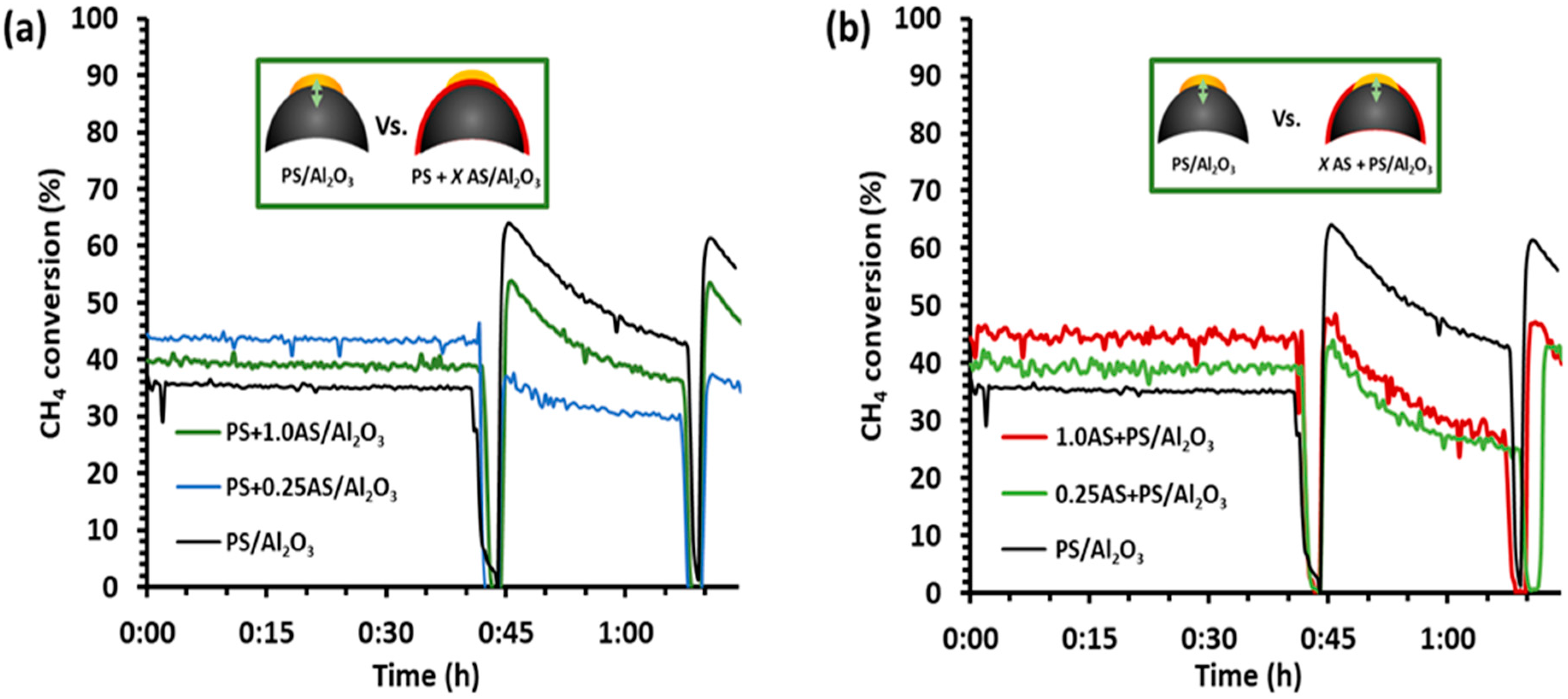
2.1. Methane Conversion Activity and Regeneration of Model Catalysts under Simulated Exhaust Gas
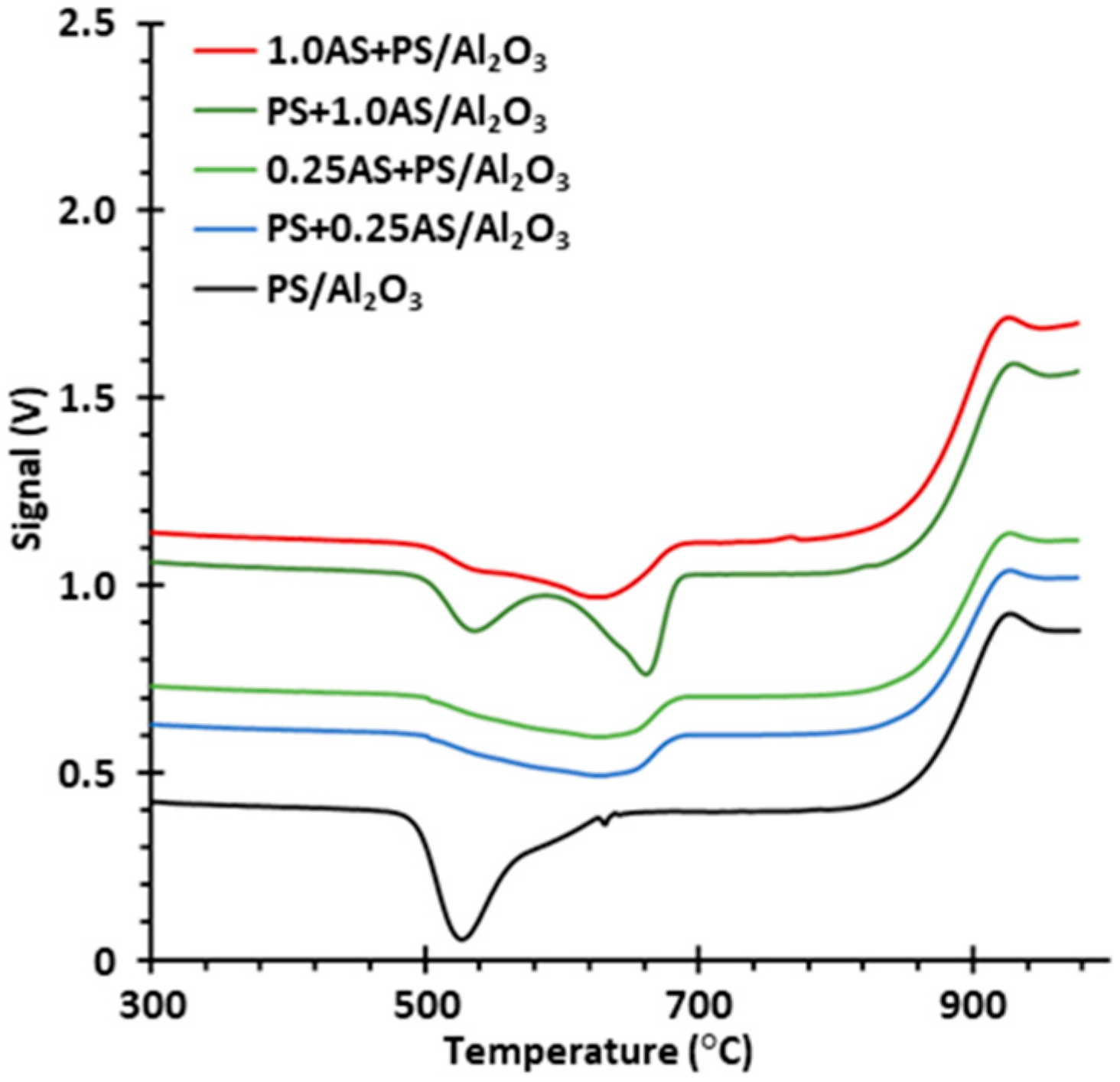
2.2. Palladium State after Regeneration under Simulated Exhaust Gas
3. Materials and Methods
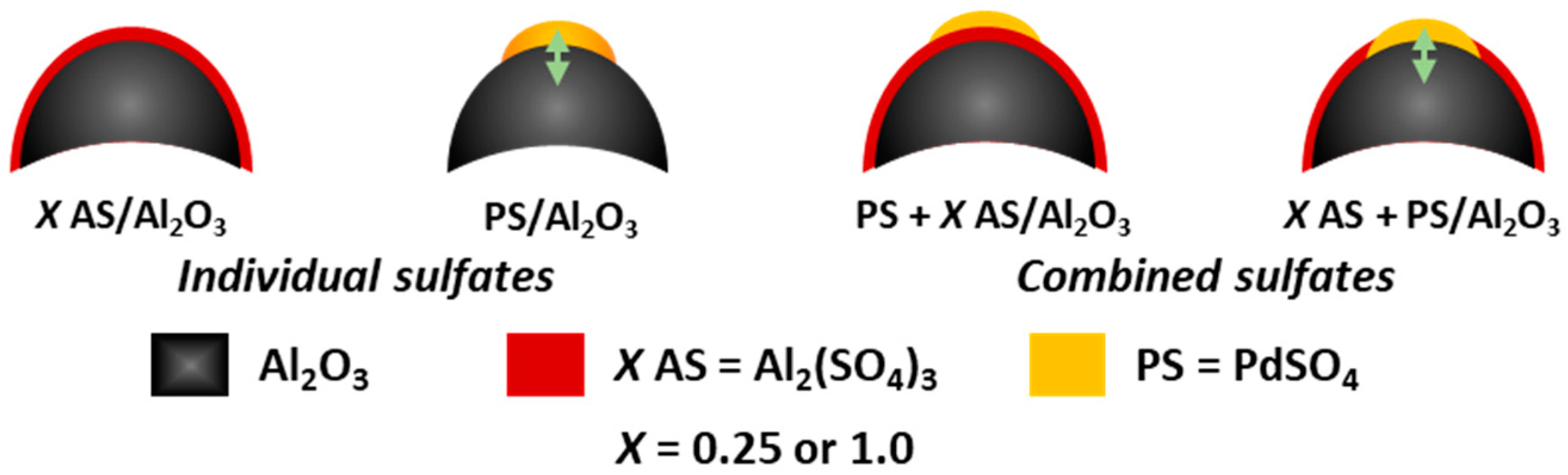
3.1. Catalysts
3.2. Characterization Techniques
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Regulation (EC) No 595/2009 of the European Council. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:188:0001:0013:EN:PDF (accessed on 30 April 2019).
- Gélin, P.; Primet, M. Complete oxidation of methane at low temperature over noble metal based catalysts: A review. Appl. Catal. B Environ. 2002, 39, 1–37. [Google Scholar] [CrossRef]
- Gélin, P.; Urfels, L.; Primet, M.; Tena, E. Complete oxidation of methane at low temperature over Pt and Pd catalysts for the abatement of lean-burn natural gas fuelled vehicles emissions: Influence of water and sulphur containing compounds. Catal. Today 2003, 83, 45–57. [Google Scholar] [CrossRef]
- Antony, A.; Asthagiri, A.; Weaver, J.F. Pathways and kinetics of methane and ethane C–H bond cleavage on PdO(101). J. Chem. Phys. 2013, 139, 104702. [Google Scholar] [CrossRef]
- Ciuparu, D.; Lyubovsky, M.R.; Altman, E.; Pfefferle, L.D.; Datye, A. Catalytic Combustion of Methane over Palladium-Based Catalysts. Catal. Rev. 2002, 44, 593–649. [Google Scholar] [CrossRef]
- Lampert, J.K.; Kazi, M.S.; Farrauto, R.J. Palladium catalyst performance for methane emissions abatement from lean burn natural gas vehicles. Appl. Catal. B Environ. 1997, 14, 211–223. [Google Scholar] [CrossRef]
- Mowery, D.L.; McCormick, R.L. Deactivation of alumina supported and unsupported PdO methane oxidation catalyst: The effect of water on sulfate poisoning. Appl. Catal. B Environ. 2001, 34, 287–297. [Google Scholar] [CrossRef]
- Chenakin, S.P.; Melaet, G.; Szukiewicz, R.; Kruse, N. XPS study of the surface chemical state of a Pd/(SiO2 + TiO2) catalyst after methane oxidation and SO2 treatment. J. Catal. 2014, 312, 1–11. [Google Scholar] [CrossRef]
- Honkanen, M.; Wang, J.; Kärkkäinen, M.; Huuhtanen, M.; Jiang, H.; Kallinen, K.; Keiski, R.L.; Akola, J.; Vippola, M. Regeneration of sulfur-poisoned Pd-based catalyst for natural gas oxidation. J. Catal. 2018, 358, 253–265. [Google Scholar] [CrossRef]
- Escandón, L.S.; Niño, D.; Díaz, E.; Ordóñez, S.; Díez, F. V Effect of hydrothermal ageing on the performance of Ce-promoted PdO/ZrO2 for methane combustion. Catal. Commun. 2008, 9, 2291–2296. [Google Scholar] [CrossRef]
- Kinnunen, N.; Kinnunen, T.; Kallinen, K. Improved Sulfur Resistance of Noble Metal Catalyst for Lean-Burn Natural Gas Applications; SAE Technical Paper 2013-24-0155; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Venezia, A.M.; Di Carlo, G.; Pantaleo, G.; Liotta, L.F.; Melaet, G.; Kruse, N. Oxidation of CH4 over Pd supported on TiO2-doped SiO2: Effect of Ti(IV) loading and influence of SO2. Appl. Catal. B Environ. 2009, 88, 430–437. [Google Scholar] [CrossRef]
- Corro, G.; Cano, C.; Fierro, J.L.G. A study of Pt–Pd/γ-Al2O3 catalysts for methane oxidation resistant to deactivation by sulfur poisoning. J. Mol. Catal. A Chem. 2010, 315, 35–42. [Google Scholar] [CrossRef]
- Wilburn, M.S.; Epling, W.S. Sulfur deactivation and regeneration of mono- and bimetallic Pd-Pt methane oxidation catalysts. Appl. Catal. B Environ. 2017, 206, 589–598. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Chesalov, Y.A.; Ishchenko, A.V.; Kaichev, V.V.; Ismagilov, Z.R. Effect of Pt addition on sulfur dioxide and water vapor tolerance of Pd-Mn-hexaaluminate catalysts for high-temperature oxidation of methane. Appl. Catal. B Environ. 2017, 204, 89–106. [Google Scholar] [CrossRef]
- Honkanen, M.; Kärkkäinen, M.; Kolli, T.; Heikkinen, O.; Viitanen, V.; Zeng, L.; Jiang, H.; Kallinen, K.; Huuhtanen, M.; Keiski, R.L.; et al. Accelerated deactivation studies of the natural-gas oxidation catalyst—Verifying the role of sulfur and elevated temperature in catalyst aging. Appl. Catal. B Environ. 2016, 182, 439–448. [Google Scholar] [CrossRef]
- Hoyos, L.J.; Praliaud, H.; Primet, M. Catalytic combustion of methane over palladium supported on alumina and silica in presence of hydrogen sulfide. Appl. Catal. A Gen. 1993, 98, 125–138. [Google Scholar] [CrossRef]
- Ordóñez, S.; Hurtado, P.; Sastre, H.; Diez, F.V. Methane catalytic combustion over Pd/Al2O3 in presence of sulphur dioxide: Development of a deactivation model. Appl. Catal. A Gen. 2004, 259, 41–48. [Google Scholar] [CrossRef]
- Jones, J.M.; Dupont, V.A.; Brydson, R.; Fullerton, D.J.; Nasri, N.S.; Ross, A.B.; Westwood, A.V.K. Sulphur poisoning and regeneration of precious metal catalysed methane combustion. Catal. Today 2003, 81, 589–601. [Google Scholar] [CrossRef]
- Kinnunen, N.M.; Hirvi, J.T.; Kallinen, K.; Maunula, T.; Keenan, M.; Suvanto, M. Case study of a modern lean-burn methane combustion catalyst for automotive applications: What are the deactivation and regeneration mechanisms? Appl. Catal. B Environ. 2017, 207, 114–119. [Google Scholar] [CrossRef]
- Keenan, M.; Pickett, R.; Tronconi, E.; Nova, I.; Kinnunen, N.; Suvanto, M.; Maunula, T.; Kallinen, K.; Baert, R. The Catalytic Challenges of Implementing a Euro VI Heavy Duty Emissions Control System for a Dedicated Lean Operating Natural Gas Engine. Top. Catal. 2018, 62, 273–281. [Google Scholar] [CrossRef]
- Maunula, T.; Kallinen, K.; Kinnunen, N.; Keenan, M.; Wolff, T. Methane Abatement and Catalyst Durability in Heterogeneous Lean-Rich and Dual-Fuel Conditions. Top. Catal. 2019, 62, 315–323. [Google Scholar] [CrossRef]
- Maunula, T.; Kallinen, K.; Savimäki, A.; Wolff, T. Durability Evaluations and Rapid Ageing Methods in Commercial Emission Catalyst Development for Diesel, Natural Gas and Gasoline Applications. Top. Catal. 2016, 59, 1049–1053. [Google Scholar] [CrossRef]
- Chen, J.; Arandiyan, H.; Gao, X.; Li, J. Recent Advances in Catalysts for Methane Combustion. Catal. Surv. Asia 2015, 19, 140–171. [Google Scholar] [CrossRef]
- Arosio, F.; Colussi, S.; Groppi, G.; Trovarelli, A. Regeneration of S-poisoned Pd/Al2O3 catalysts for the combustion of methane. Catal. Today 2006, 117, 569–576. [Google Scholar] [CrossRef]
- Ordóñez, S.; Hurtado, P.; Diez, F.V. Methane catalytic combustion over Pd/Al2O3 in presence of sulphur dioxide: Development of a regeneration procedure. Catal. Lett. 2005, 100, 27–34. [Google Scholar] [CrossRef]
- Yu, T.-C.; Shaw, H. The effect of sulfur poisoning on methane oxidation over palladium supported on γ-alumina catalysts. Appl. Catal. B Environ. 1998, 18, 105–114. [Google Scholar] [CrossRef]
- Arosio, F.; Colussi, S.; Trovarelli, A.; Groppi, G. Effect of alternate CH4-reducing/lean combustion treatments on the reactivity of fresh and S-poisoned Pd/CeO2/Al2O3 catalysts. Appl. Catal. B Environ. 2008, 80, 335–342. [Google Scholar] [CrossRef]
- Nissinen, V.H.; Nissinen, N.; Suvanto, M. Regeneration of a sulfur-poisoned methane combustion catalyst: Structural evidence of Pd4S formation. Appl. Catal. B Environ. 2018, 237, 110–115. [Google Scholar] [CrossRef]
- Heck, R.M.; Farrauto, R.J.; Gulati, S.T. Catalytic Air Pollution Control: Commercial Technology, 3rd ed.; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Kinnunen, N.M.; Nissinen, V.H.; Hirvi, J.T.; Kallinen, K.; Maunula, T.; Keenan, M.; Suvanto, M. Decomposition of Al2O3 supported PdSO4 and Al2(SO4)3 in regeneration of model methane combustion catalyst: A fundamental study. Catalysts 2019. submitted. [Google Scholar]
- Bruker AXS TOPAS V2.0: General Profile Analysis Software for Powder Diffraction Data. 2000.
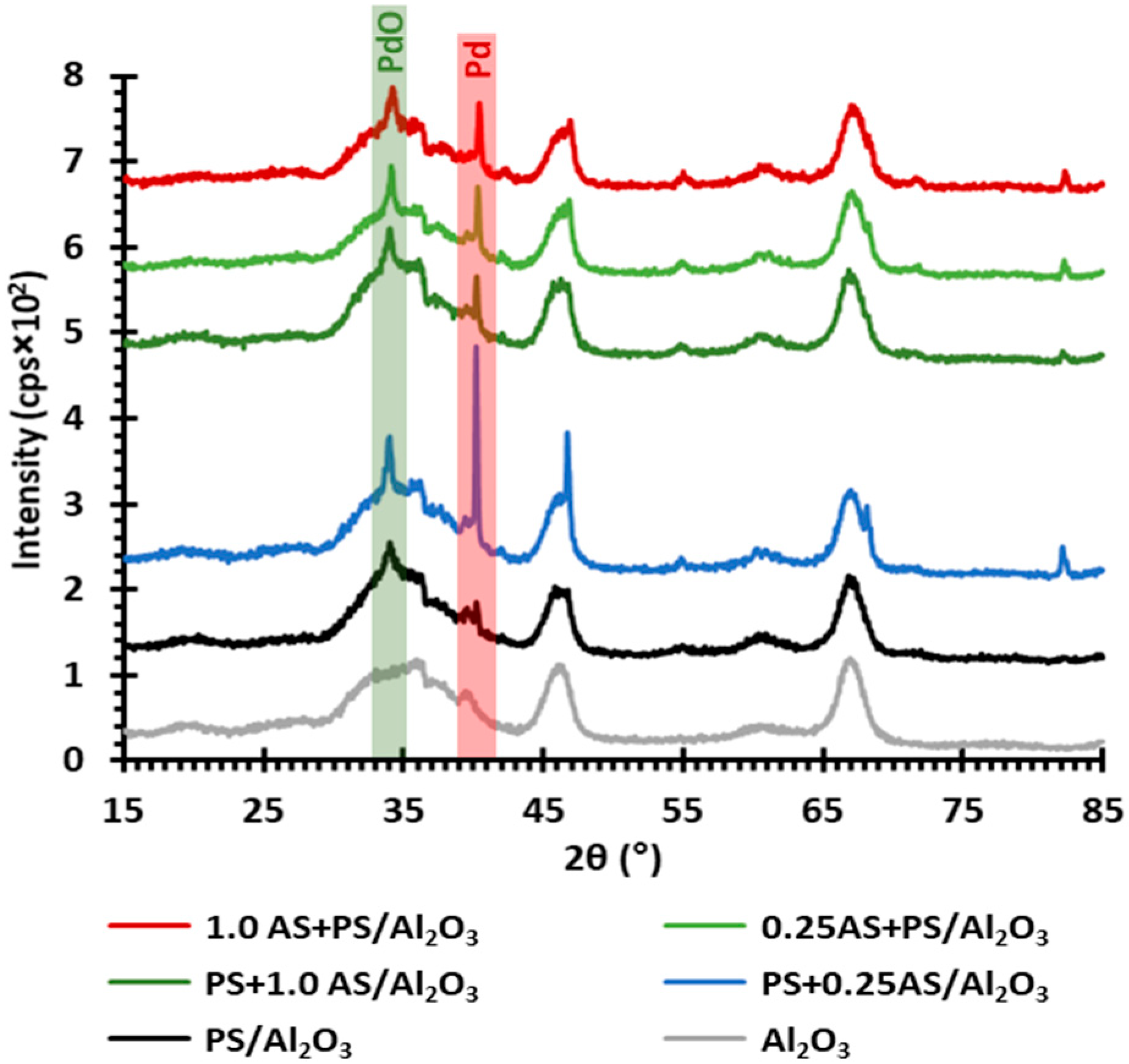

| Catalyst 1 | PdO(101) Peak Area 2 | PdO(101) Crystallite Size (nm) 2 | Pd(111) Peak Area 2 | Pd(111) Crystallite Size (nm) 2 | PdO(101):Pd(111) 3 |
|---|---|---|---|---|---|
| PS/Al2O3 | 55.3 | 4.7 | 17.3 | 13.1 | 3.20 |
| PS + 0.25 AS/Al2O3 | 41.0 | 15.6 | 52.1 | 111.0 | 0.79 |
| PS + 1.0 AS/Al2O3 | 51.5 | 11.2 | 20.0 | 50.9 | 2.58 |
| 0.25 AS + PS/Al2O3 | 27.0 | 23.6 | 24.7 | 60.8 | 1.09 |
| 1.0 AS + PS/Al2O3 | 31.1 | 13.9 | 20.6 | 56.8 | 1.51 |
| Catalyst 1 | Oxygen Uptake (μmol gcat−1) | O:Pd Mole Ratio | Regenerated CH4 Conversion (%) 2 |
|---|---|---|---|
| PS/Al2O3 | 19.2 | 0.43 | 63.7 |
| PS + 0.25 AS/Al2O3 | 11.1 | 0.25 | 37.6 |
| PS + 1.0 AS/Al2O3 | 15.7 | 0.36 | 53.4 |
| 0.25 AS + PS/Al2O3 | 9.0 | 0.22 | 42.7 |
| 1.0 AS + PS/Al2O3 | 11.4 | 0.22 | 47.0 |
| Catalyst | Total Sulfur Content of the Catalyst (wt.%) |
|---|---|
| PS/Al2O3 1 | 0.88 |
| PS + 0.25 AS/Al2O3 1,2 | 1.39 |
| PS + 1.0 AS/Al2O3 1,2 | 2.10 |
| 0.25 AS + PS/Al2O3 1,2 | 1.03 |
| 1.0 AS + PS/Al2O3 1,2 | 1.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinnunen, N.M.; Kallinen, K.; Maunula, T.; Keenan, M.; Suvanto, M. Fundamentals of Sulfate Species in Methane Combustion Catalyst Operation and Regeneration—A Simulated Exhaust Gas Study. Catalysts 2019, 9, 417. https://doi.org/10.3390/catal9050417
Kinnunen NM, Kallinen K, Maunula T, Keenan M, Suvanto M. Fundamentals of Sulfate Species in Methane Combustion Catalyst Operation and Regeneration—A Simulated Exhaust Gas Study. Catalysts. 2019; 9(5):417. https://doi.org/10.3390/catal9050417
Chicago/Turabian StyleKinnunen, Niko M., Kauko Kallinen, Teuvo Maunula, Matthew Keenan, and Mika Suvanto. 2019. "Fundamentals of Sulfate Species in Methane Combustion Catalyst Operation and Regeneration—A Simulated Exhaust Gas Study" Catalysts 9, no. 5: 417. https://doi.org/10.3390/catal9050417
APA StyleKinnunen, N. M., Kallinen, K., Maunula, T., Keenan, M., & Suvanto, M. (2019). Fundamentals of Sulfate Species in Methane Combustion Catalyst Operation and Regeneration—A Simulated Exhaust Gas Study. Catalysts, 9(5), 417. https://doi.org/10.3390/catal9050417





