Pd4S/SiO2: A Sulfur-Tolerant Palladium Catalyst for Catalytic Complete Oxidation of Methane
Abstract
1. Introduction
2. Results and Discussion
2.1. Fresh Catalysts
2.1.1. Catalyst Characterization
2.1.2. Activity Studies
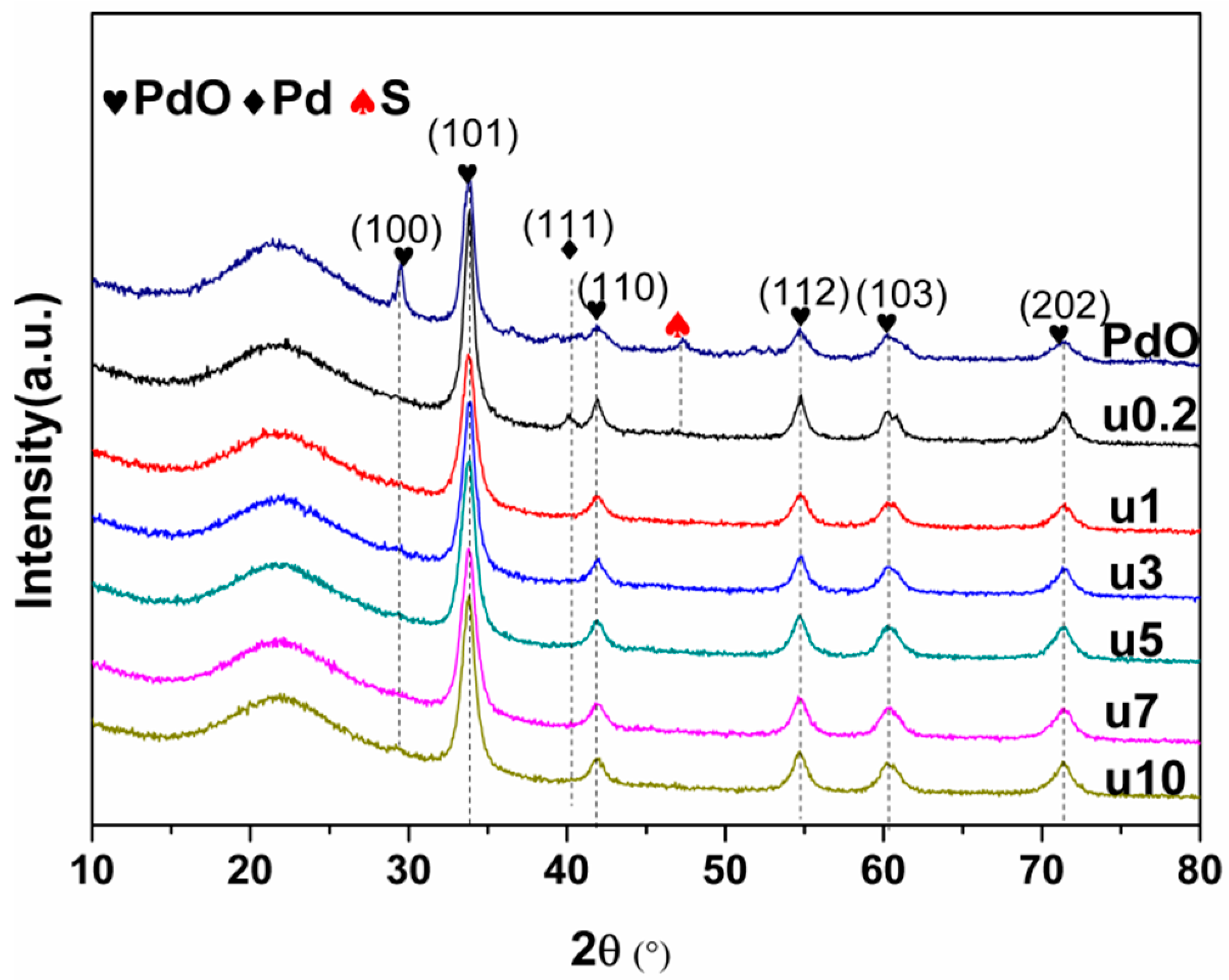
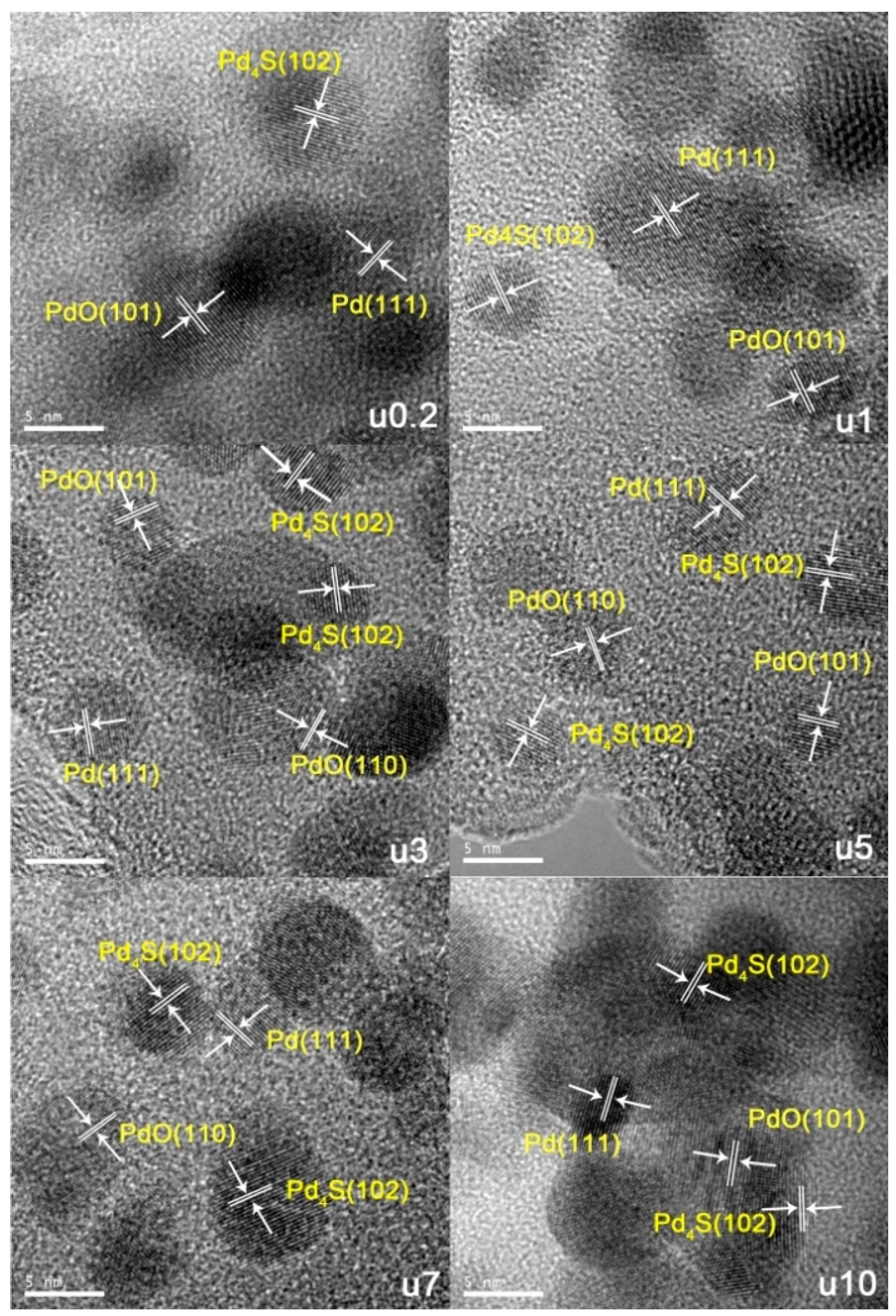
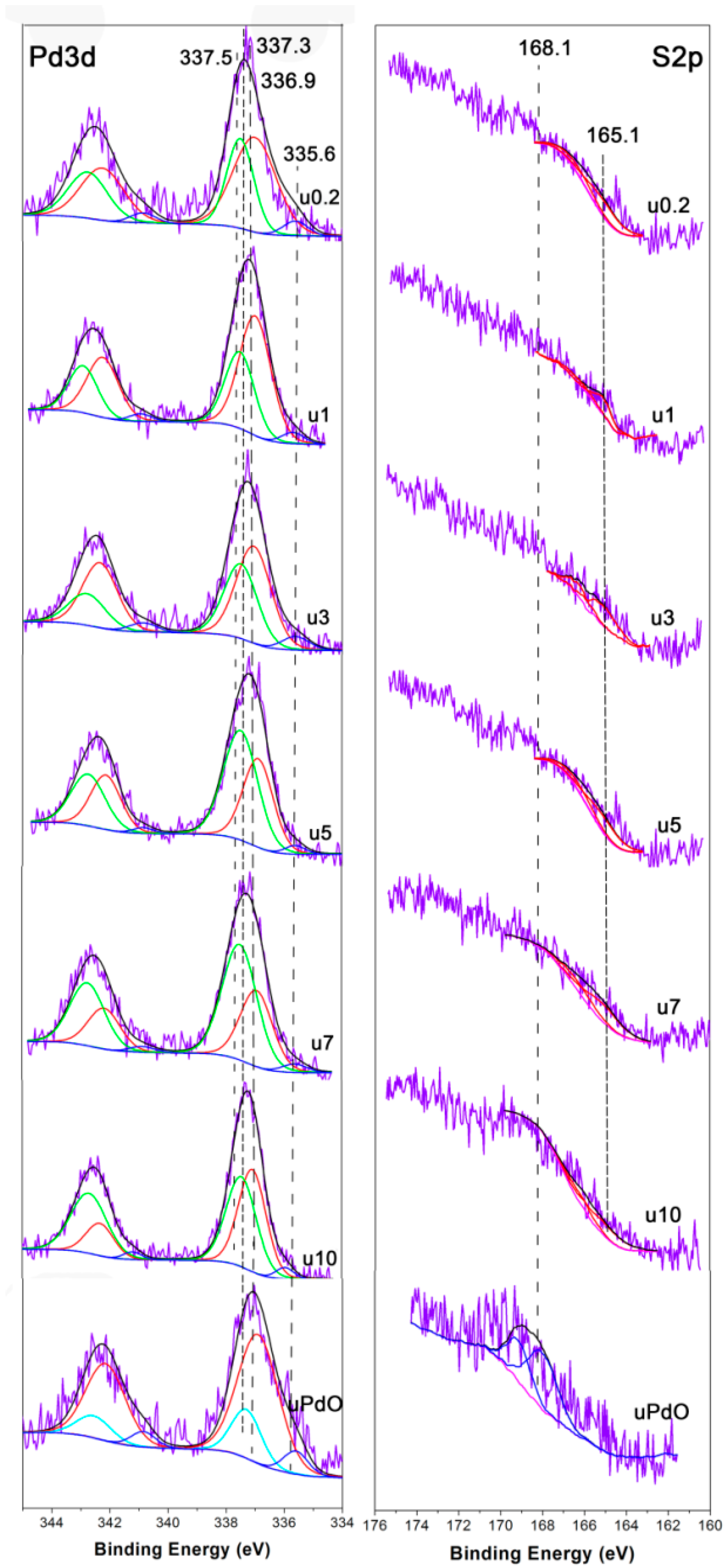
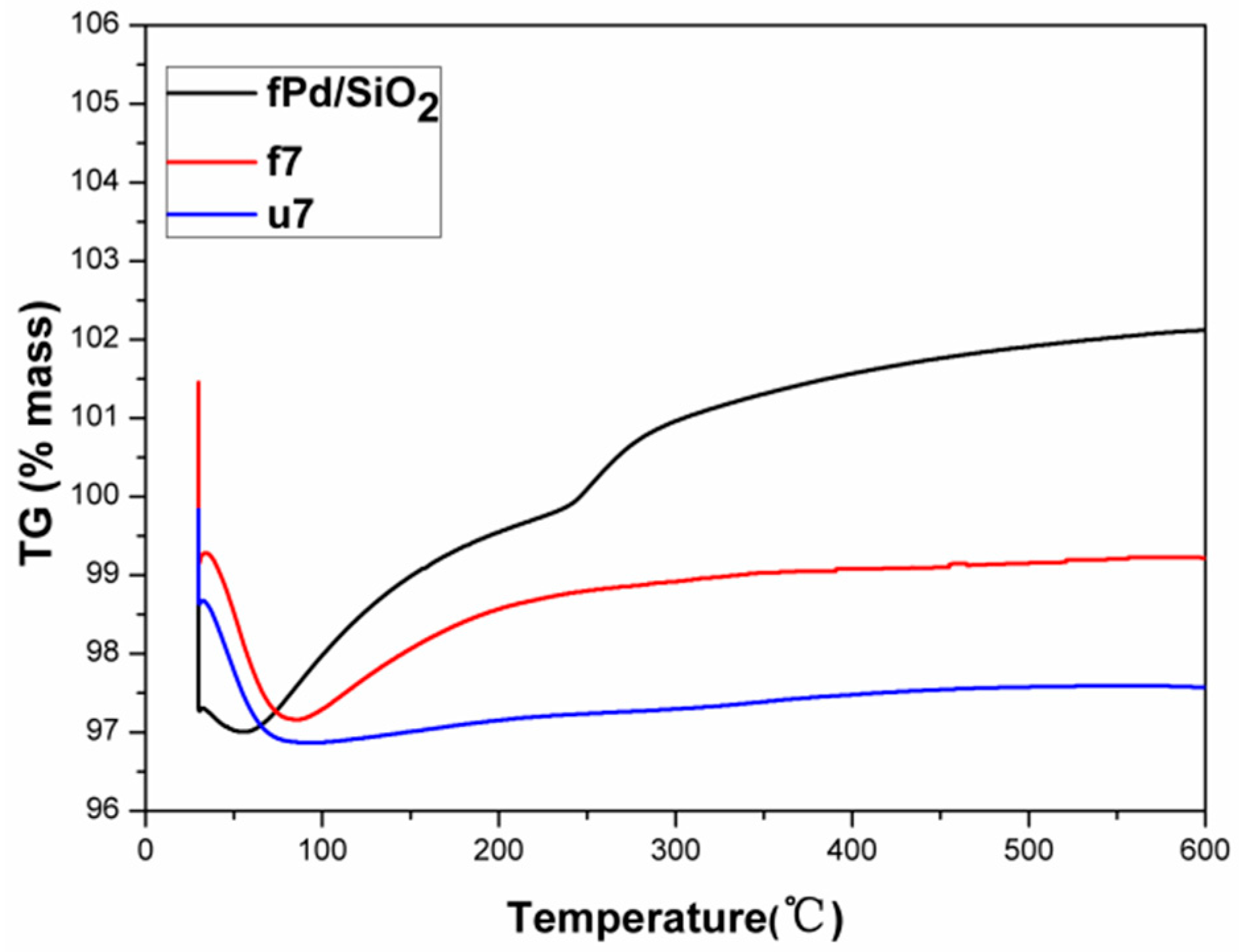
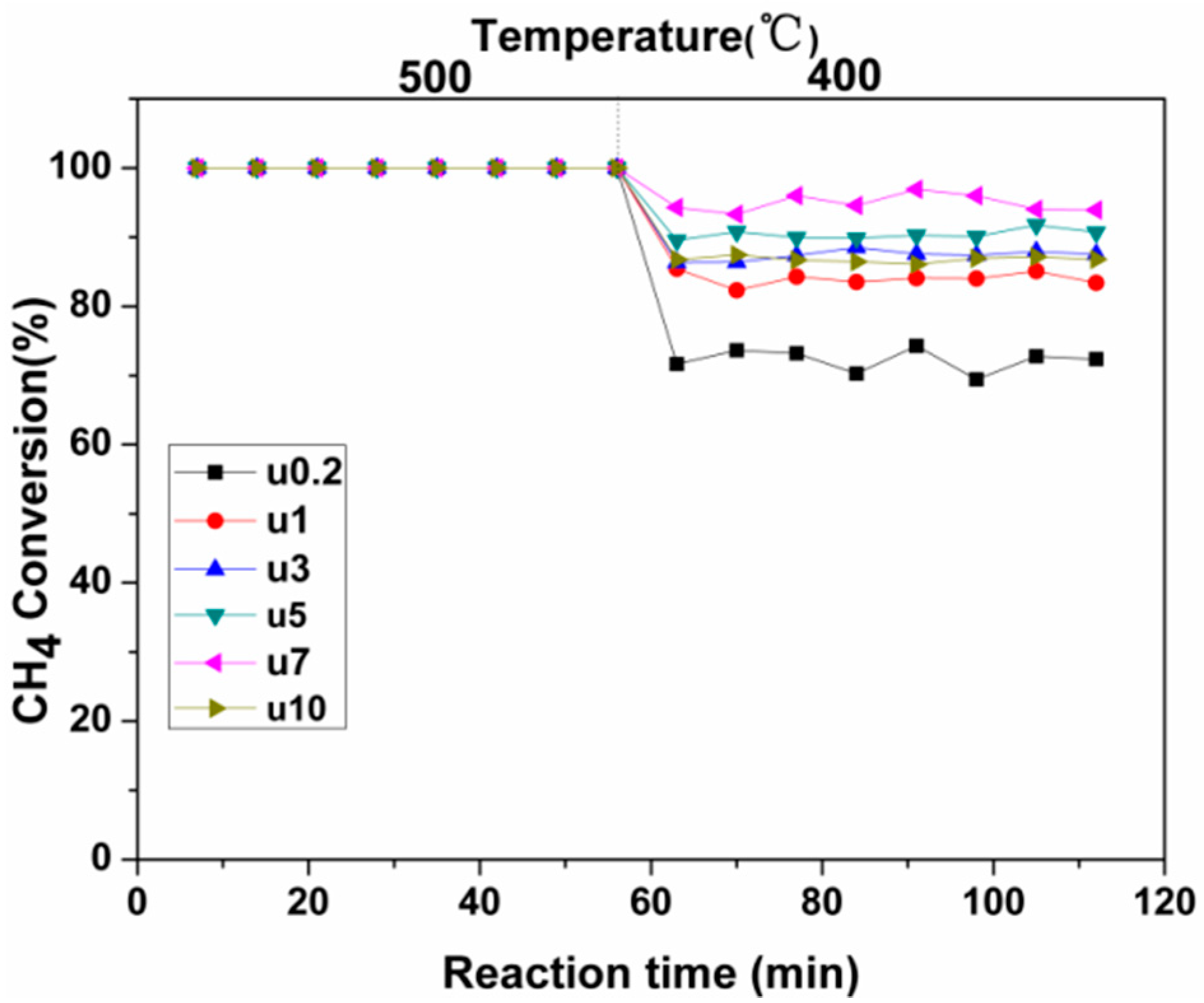
2.2. Used Catalyst
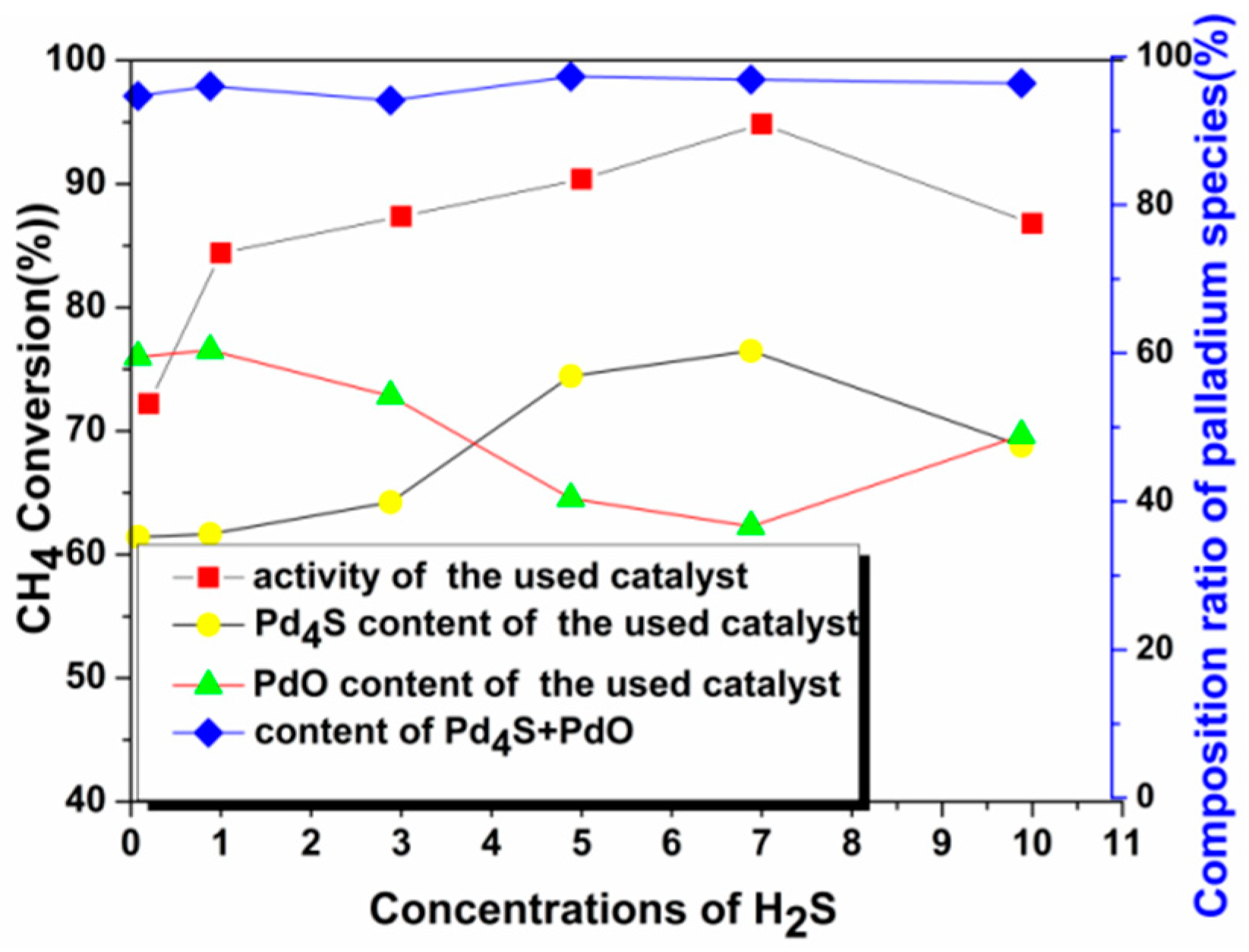
2.2.1. Catalyst Characterization
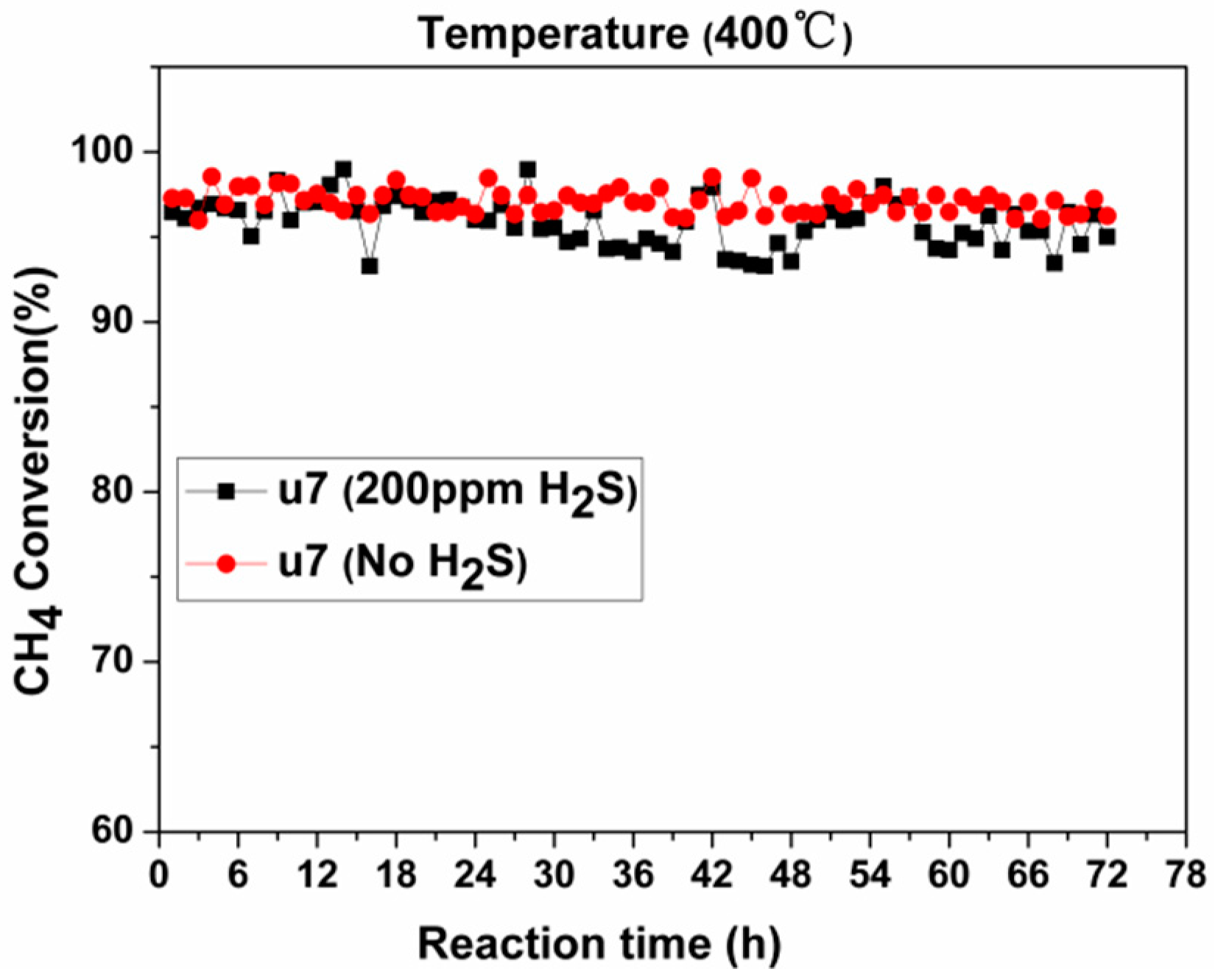
2.2.2. Activity Studies
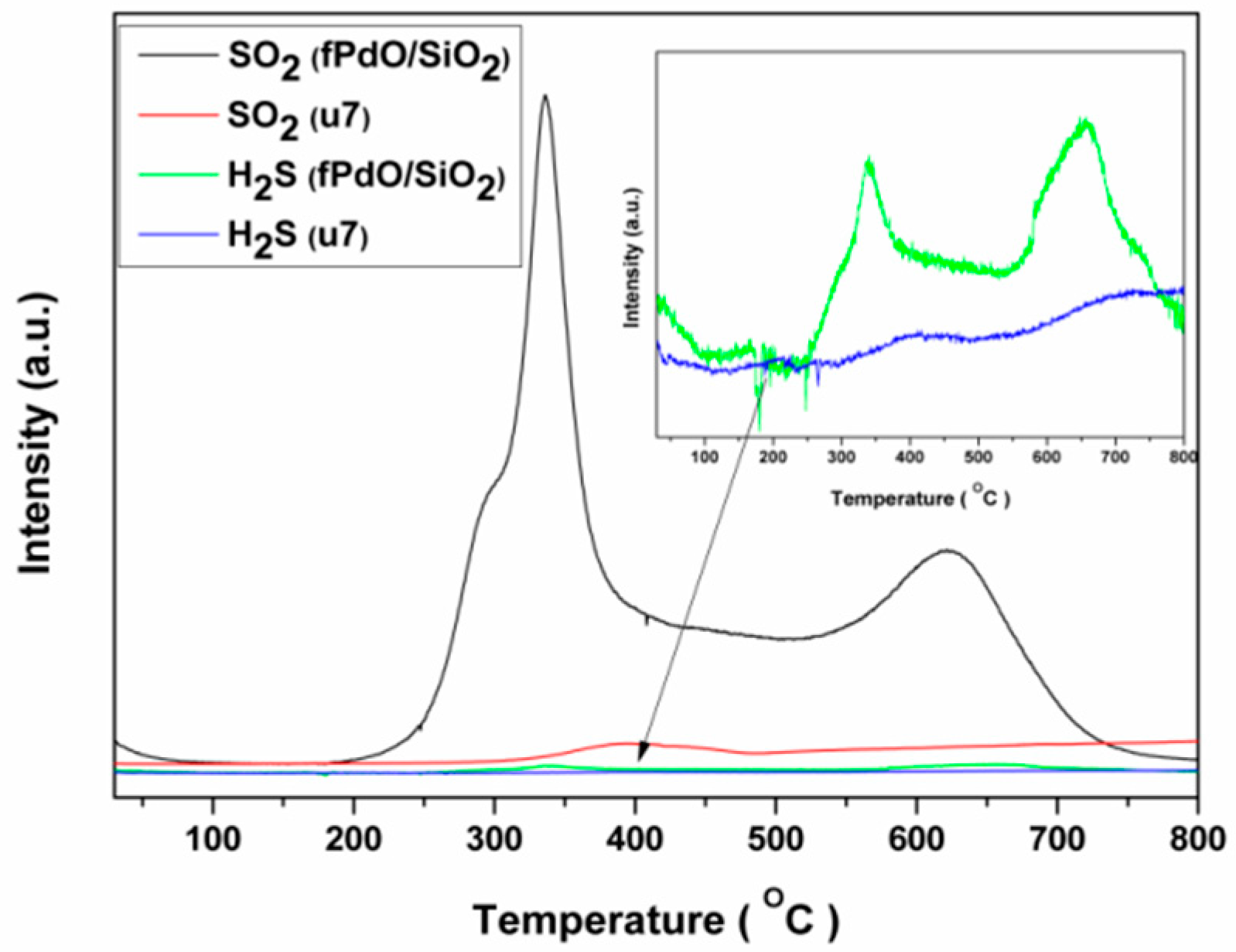
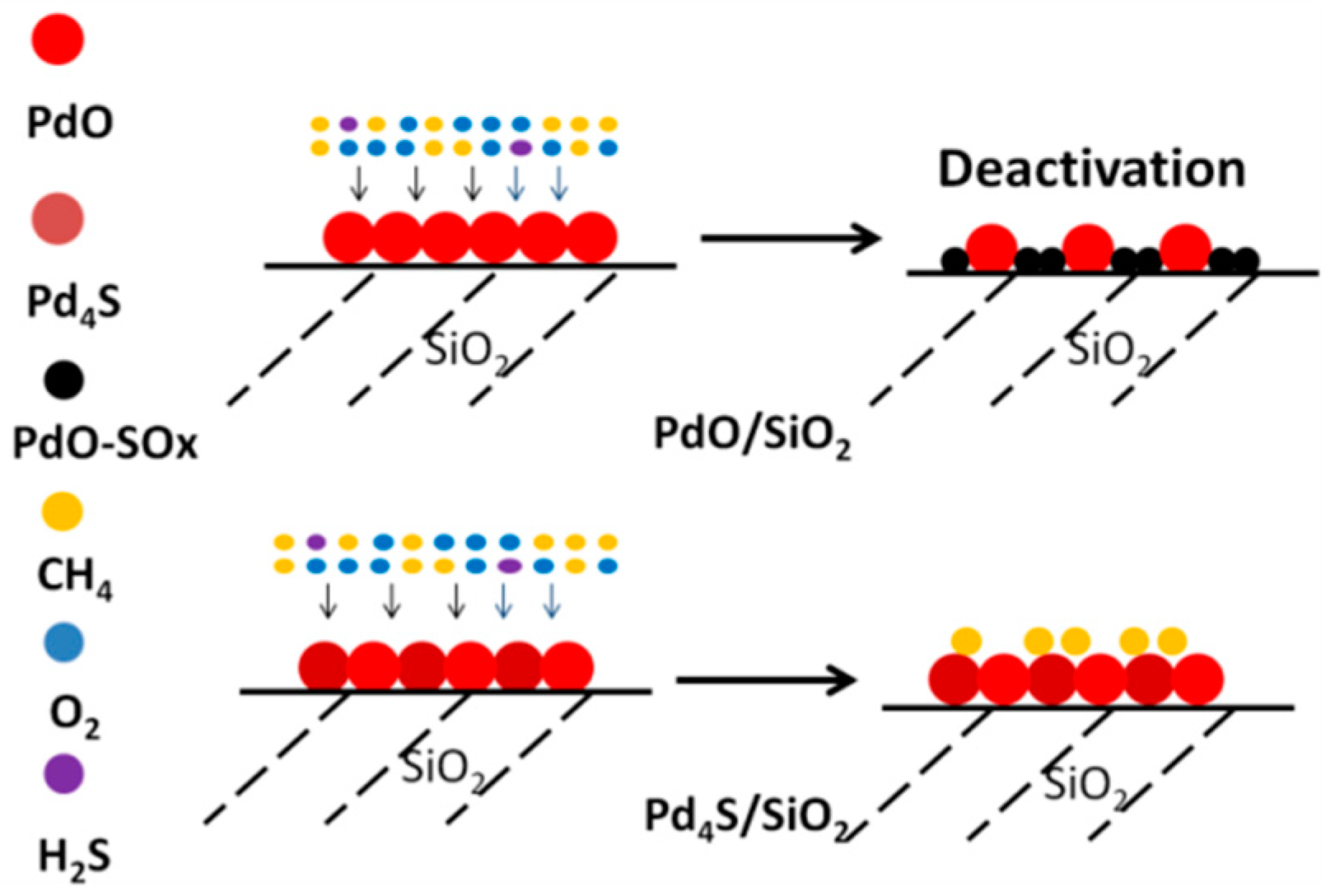
2.3. Mechanism of Sulfur-Tolerance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Evaluation of Catalysts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Trinchero, A.; Hellman, A.; Grönbeck, H. Methane oxidation over Pd and Pt studied by DFT and kinetic modeling. Surf. Sci. 2013, 616, 206–213. [Google Scholar] [CrossRef]
- Mahara, Y.; Ohyama, J.; Tojo, T.; Murata, K.; Ishikawa, H.; Satsuma, K. Enhanced activity for methane combustion over a Pd/Co/Al2O3 catalyst prepared by a galvanic deposition method. Catal. Sci. Technol. 2016, 6, 4773–4776. [Google Scholar] [CrossRef]
- Chen, J.; Arandiyan, H.; Gao, X.; Li, J. Recent Advances in Catalysts for Methane Combustion. Catal. Surv. Asia 2015, 19, 140–171. [Google Scholar] [CrossRef]
- Colussi, S.; Gayen, A.; Camellone, M.F.; Boaro, M.; Llorca, J.; Fabris, S.; Trovarelli, A. Nanofaceted Pd-O Sites in Pd-Ce Surface Superstructures: Enhanced Activity in Catalytic Combustion of Methane. Angew. Chem. 2009, 121, 8633–8636. [Google Scholar] [CrossRef]
- Ji, Y.; Guo, Y.B. Nanostructured perovskite oxides as promising substitutes of noble metals catalysts for catalytic combustion of methane. Chin. Chem. Lett. 2018, 29, 252–260. [Google Scholar]
- Bossche, M.V.D.; Gronbeck, H. Methane Oxidation over PdO(101) Revealed by First-Principles Kinetic Modeling. J. Am. Chem. Soc. 2015, 137, 12035–12044. [Google Scholar] [CrossRef] [PubMed]
- Monai, M.; Montini, T.; Gorte, R.J.; Fornasiero, P. Catalytic Oxidation of Methane: Pd and Beyond. Eur. J. Inorg. Chem. 2018, 25, 2884–2893. [Google Scholar] [CrossRef]
- Jiang, L.; Zheng, Y.; Chen, X.; Xiao, Y.; Cai, G.; Zheng, Y.; Zhang, Y.; Huang, F. Catalytic Activity and Stability over Nanorod-Like Ordered Mesoporous Phosphorus-Doped Alumina Supported Palladium Catalysts for Methane Combustion. ACS Catal. 2018, 8, 11016–11028. [Google Scholar]
- Fouladvand, S.; Skoglundh, M.; Carlsson, P.A. A transient in situ infrared spectroscopy study on methane oxidation over supported Pt catalysts. Catal. Sci. Technol. 2014, 4, 3463–3473. [Google Scholar] [CrossRef]
- Florén, C.R.; Bossche, M.V.D.; Creaser, D.; Grobeck, H.; Carlsson, P.A.; Korpi, H.; Skoglundh, M. Modelling complete methane oxidation over palladium oxide in a porous catalyst using first-principles surface kinetics. Catal. Sci. Technol. 2018, 8, 508–520. [Google Scholar] [CrossRef]
- Banerjee, A.C.; Golub, K.W.; Abdul, Md. H.; Billor, M.Z. Comparative study of the characteristics and activities of Pd/γ-Al2O3 catalysts prepared by Vortex and Incipient Wetness Methods. Catalysts 2019, 9, 336. [Google Scholar] [CrossRef]
- Banerjee, A.C.; McGuire, M.M.; Lawnick, O.; Bozack, M.J. Low-temperature activity and PdO/PdOx transition in methane combustion by a PdO-PdOx/γ-Al2O3 catalyst. Catalysts 2018, 8, 266. [Google Scholar] [CrossRef]
- Ciuparu, D.; Lyubovsky, M.R.; Altman, E.; Pfefferle, L.D.; Datye, A. Catalytic combustion of methane over palladium based catalysts. Catal. Rev. Sci. Eng. 2002, 44, 593–649. [Google Scholar] [CrossRef]
- Persson, K.; Pfefferle, L.D.; Schwartz, W.; Ersson, A.; Jaras, S.G. Stability of palladium-based catalysts during catalytic combustion of methane: The influence of water. Appl. Catal. B 2007, 74, 242–250. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Gao, D.; Sun, T.; Zhang, C.; Wang, S.D. Influence of metal oxides on the performance of Pd/Al2O3 catalysts for methane combustion under lean-fuel conditions. Fuel Process. Technol. 2013, 111, 55–61. [Google Scholar] [CrossRef]
- Baylet, A.; Royer, S.; Marecot, P.; Tatibouet, J.M.; Duprez, D. High catalytic activity and stability of Pd doped hexaaluminate catalysts for the CH4 catalytic combustion. Appl. Catal. B 2008, 77, 237–247. [Google Scholar] [CrossRef]
- Ma, J.; Lou, Y.; Cai, Y.; Zhao, Z.; Wang, L.; Zhan, W.; Guo, Y.; Guo, Y. The relationship between the chemical state of Pd species and the catalytic activity for methane combustion on Pd/CeO2. Catal. Sci. Technol. 2018, 8, 2567–2577. [Google Scholar] [CrossRef]
- Mihai, O.; Smedler, G.; Nylén, U.; Olofsson, M.; Olsson, L. The effect of water on methane oxidation over Pd/Al2O3 under lean, stoichiometric and rich conditions. Catal. Sci. Technol. 2017, 7, 3084–3096. [Google Scholar] [CrossRef]
- Zi, X.; Liu, L.; Xue, B.; Dai, H.; He, H. The durability of alumina supported Pd catalysts for the combustion of methane in the presence of SO2. Catal. Today 2011, 175, 223–230. [Google Scholar] [CrossRef]
- Wilburn, M.S.; Epling, W.S. Sulfur deactivation and regeneration of mono- and bimetallic Pd-Pt methane oxidation catalysts. Appl. Catal. B 2017, 206, 589–598. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Melchionna, M.; Ducchon, T.; Kus, P.; Chen, C.; Tsud, N.; Nasi, L.; Prince, K.C.; Veltruska, K.; et al. The effect of sulfur dioxide on the activity of hierarchical Pd-based catalysts in methane combustion. Appl. Catal. B 2017, 202, 72–83. [Google Scholar] [CrossRef]
- Yin, F.; Ji, S.; Wu, P.; Zhao, F.; Liu, H.; Li, C. Preparation of Pd-Based Metal Monolithic Catalysts and a Study of Their Performance in the Catalytic Combustion of Methane. ChemSusChem 2008, 1, 311–319. [Google Scholar] [CrossRef]
- Hoyos, L.J.; Praliaud, H.; Primet, M. Catalytic combustion of methane over palldium supported on alumina and silica in presence of hydrogen-sulfie. Appl. Catal. A 1993, 98, 125–138. [Google Scholar] [CrossRef]
- Venezia, A.M.; Carlo, G.D.; Liotta, L.F.; Pantaleo, G.; Kantcheva, M. Effect of Ti(IV) loading on CH4 oxidation activity and SO2 tolerance of Pd catalysts supported on silica SBA-15 and HMS. Appl. Catal. B 2011, 106, 529–539. [Google Scholar] [CrossRef]
- Ortloff, F.; Bohnau, J.; Kramar, U.; Graf, F.; Kolb, T. Studies on the influence of H2S and SO2 on the activity of a PdO/Al2O3 catalyst for removal of oxygen by total oxidation of (bio-)methane at very low O2:CH4 ratios. Appl. Catal. B 2016, 182, 550–561. [Google Scholar] [CrossRef][Green Version]
- Meeyoo, V.; Trimm, D.L.; Cant, N.W. The effect of sulfur containing pollutants on the oxidation activity of precious metals used in vehicle exhaust catalysts. Appl. Catal. B 1998, 16, L101–L104. [Google Scholar] [CrossRef]
- Ordóñez, S.; Hurtado, P.; Díez, F.V. Methane catalytic combustion over Pd/Al2O3 in presence of sulfur dioxide: development of a regeneration procedure. Catal. Lett. 2005, 100, 27–34. [Google Scholar] [CrossRef]
- Corro, G.; Cano, C.; Fierro, J.L.G. A study of Pt–Pd/γ-Al2O3 catalysts for methane oxidation resistant to deactivation by sulfur poisoning. J. Mol. Catal. A: Chem 2010, 315, 35–42. [Google Scholar] [CrossRef]
- Simon, L.J.; Ommen, J.G.V.; Jentys, A.; Lercher, J.A. Sulfur-Tolerant Pt-Supported Zeolite Catalysts for Benzene Hydrogenation: I. Influence of the Support. J. Catal. 2001, 201, 60–69. [Google Scholar] [CrossRef]
- Ferrer, I.J.; Diazchao, P.; Pascual, A.; Sánchez, C. An investigation on palladium sulphide (PdS) thin films as a photovoltaic material. Thin Solid Films 2007, 515, 5783–5786. [Google Scholar] [CrossRef]
- Xu, W.; Ni, J.; Zhang, Q.F.; Feng, F.; Xiang, Y.Z.; Li, X.N. Tailoring supported palladium sulfide catalysts through H2-assisted sulfidation with H2S. J. Mater. Chem. A 2013, 1, 12811–12817. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Xu, W.; Li, X.N.; Jiang, D.H.; Xiang, Y.Z.; Wang, J.G.; Cen, J.; Romano, S.; Ni, J. Catalytic hydrogenation of sulfur-containing nitrobenzene over Pd/C catalysts: In situ sulfidation of Pd/C for the preparation of PdxSy catalysts. Appl. Catal. A 2015, 497, 17–21. [Google Scholar] [CrossRef]
- Mccue, A.J.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I.; Anderson, J.A. Palladium sulphide – A highly selective catalyst for the gas phase hydrogenation of alkynes to alkenes. J. Catal. 2016, 340, 10–16. [Google Scholar] [CrossRef]
- Menegazzo, F.; Canton, P.; Pinna, F.; Pernicone, N. Bimetallic Pd–Au catalysts for benzaldehyde hydrogenation: Effects of preparation and of sulfur poisoning. Catal. Commun. 2008, 9, 2353–2356. [Google Scholar] [CrossRef]
- Mccue, A.J.; Anderson, J.A. Sulfur as a catalyst promoter or selectivity modifier in heterogeneous catalysis. Cheminform 2014, 4, 272–294. [Google Scholar] [CrossRef]
- O’Brien, C.P.; Gellman, A.J.; Morreale, B.D.; Miller, J.B. The hydrogen permeability of Pd4S. J. Membr. Sci. 2011, 371, 263–267. [Google Scholar] [CrossRef]
- O’Brien, C.P.; Howard, B.H.; Miller, J.B.; Morreale, B.D.; Gellman, A.J. Inhibition of hydrogen transport through Pd and Pd47Cu53 membranes by H2S at 350 degrees. J. Membr. Sci. 2010, 349, 380–384. [Google Scholar] [CrossRef]
- Morreale, B.D.; Howard, B.H.; Iyoha, O.; Enick, R.M.; Ling, C.; Sholl, D.S. Experimental and Computational Prediction of the Hydrogen Transport Properties of Pd4S. Ind. eng. chem. res 2007, 46, 6313–6319. [Google Scholar] [CrossRef]
- Zubkov, A.; Fujino, T.; Sato, N.; Yamada, K. Enthalpies of formation of the palladium sulphides. J. Chem. Thermodyn. 1998, 30, 571–581. [Google Scholar] [CrossRef]
- Iyoha, O.; Enick, R.; Killmeyer, R.; Morreale, B. The influence of hydrogen sulfide-to-hydrogen partial pressure ratio on the sulfidization of Pd and 70 mol% Pd–Cu membranes. J. Membr. Sci. 2007, 305, 77–92. [Google Scholar] [CrossRef]
- Hensen, E.J.M.; Brans, H.J.A.; Lardinois, G.M.H.J.; deBeer, V.H.J.; vanVeen, J.A.R.; van Santen, R.A. Periodic Trends in Hydrotreating Catalysis: Thiophene Hydrodesulfurization over Carbon-Supported 4d Transition Metal Sulfides. J. Catal. 2000, 192, 98–107. [Google Scholar] [CrossRef]
- Gerson, A.R.; Bredow, T. Interpretation of sulfur 2p XPS spectra in sulfide minerals by means of ab initio calculations. Surf. Interface Anal. 2000, 29, 145–150. [Google Scholar] [CrossRef]
- Bhatt, R.; Bhattacharya, S.; Basu, R.; Singh, A.; Deshpande, U.; Surger, C.; Basu, S.; Aswal, D.K.; Gupta, S.K. Growth of Pd4S, PdS and PdS2 films by controlled sulfurization of sputtered Pd on native oxide of Si. Thin Solid Films 2013, 539, 41–46. [Google Scholar] [CrossRef]
- Senftle, T.P.; Van Duin, A.C.T.; Janik, M.J. The Role of Site Stability in Methane Activation on PdxCe1-xOδ Surfaces. ACS Catal. 2015, 5, 6187–6199. [Google Scholar] [CrossRef]
- Beketov, G.; Heinrichs, B.; Pirard, J.P.; Chenakin, S.; Kruse, N. XPS structural characterization of Pd/SiO2 catalysts prepared by cogelation. Appl. Surf. Sci. 2013, 287, 293–298. [Google Scholar] [CrossRef]
- Romanchenko, A.S.; Mikhlin, Y.L. An XPS study of products formed on pyrite and pyrrhotine by reacting with palladium (II) chloride solutions. J. Struct. Chem. 2015, 56, 531–537. [Google Scholar] [CrossRef]
- Corro, G.; Vázquez-Cuchillo, O.; Banuelos, F.; Fierro, J.L.G.; Azomoza, M. An XPS evidence of the effect of the electronic state of Pd on CH4 oxidation on Pd/gamma-Al2O3 catalysts. Catal. Commun. 2007, 8, 1977–1980. [Google Scholar] [CrossRef]
- Chenakin, S.P.; Melaet, G.; Szukiewicz, R.; Kruse, N. XPS study of the surface chemical state of a Pd/(SiO2+TiO2) catalyst after methane oxidation and SO2 treatment. J. Catal. 2014, 312, 1–11. [Google Scholar] [CrossRef]
- Weng, X.; Ren, H.; Chen, M.; Wan, H. Effect of Surface Oxygen on the Activation of Methane on Palladium and Platinum Surfaces. ACS Catal. 2014, 4, 2598–2604. [Google Scholar] [CrossRef]
- Cargnello, M.; Jaén, J.J.D.; Garrido, J.C.H.; Bakhmutsky, K.; Montini, T.; Gámez, J.J.C.; Gorte, R.J.; Fornasiero, P. Exceptional Activity for Methane Combustion over Modular Pd@CeO2 Subunits on Functionalized Al2O3. Science 2012, 337, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, H.; Sekizawa, K.; Eguchi, K.; Arai, H. Oxidation of methane over Pd/mixed oxides for catalytic combustion. Catal. Today 1999, 47, 95–101. [Google Scholar] [CrossRef]
- Sekizawa, K.; Widjaja, H.; Maeda, S.; Ozawa, Y.; Eguchi, K. Low temperature oxidation of methane over Pd catalyst supported on metal oxides. Catal. Today 2000, 59, 69–74. [Google Scholar] [CrossRef]
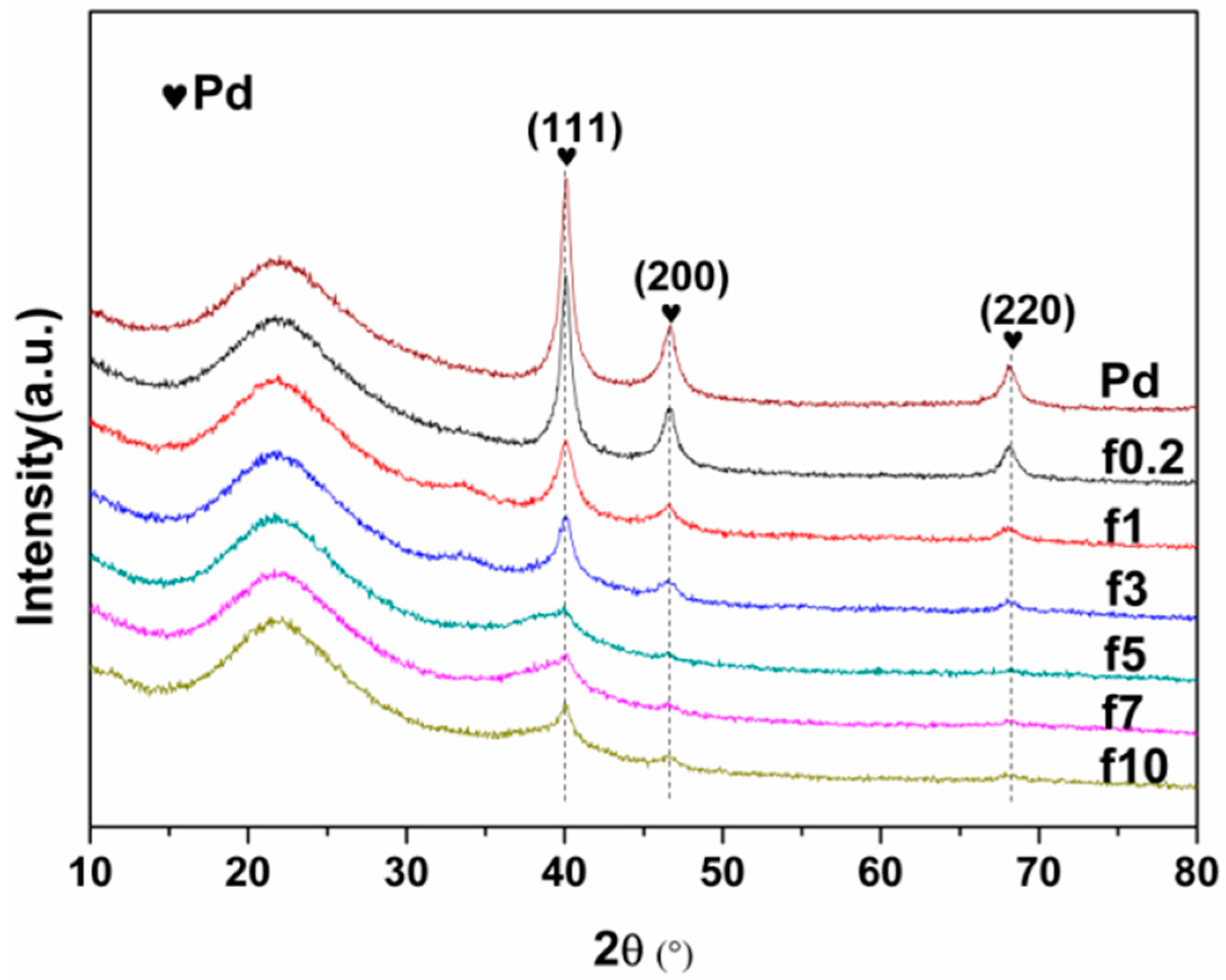
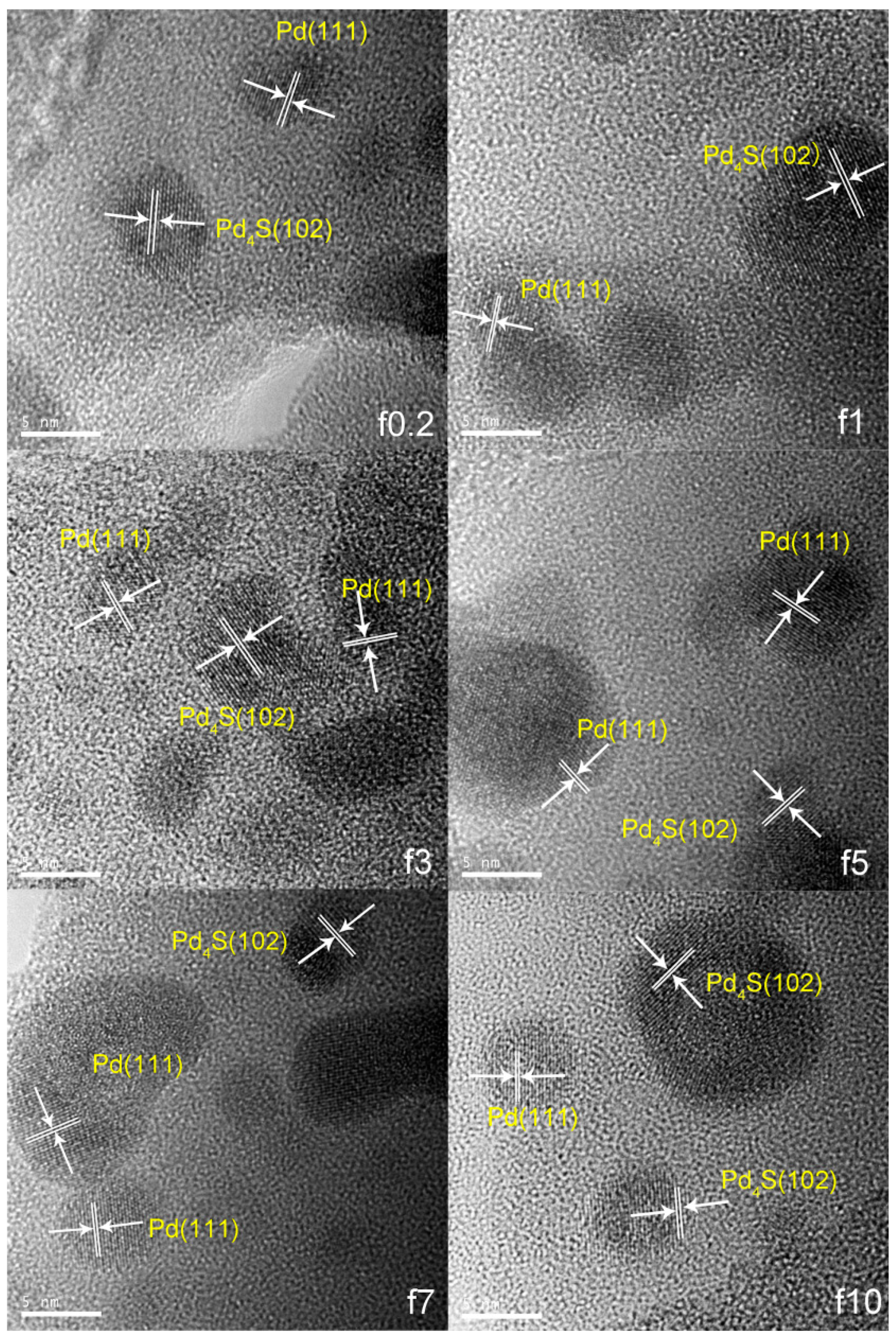
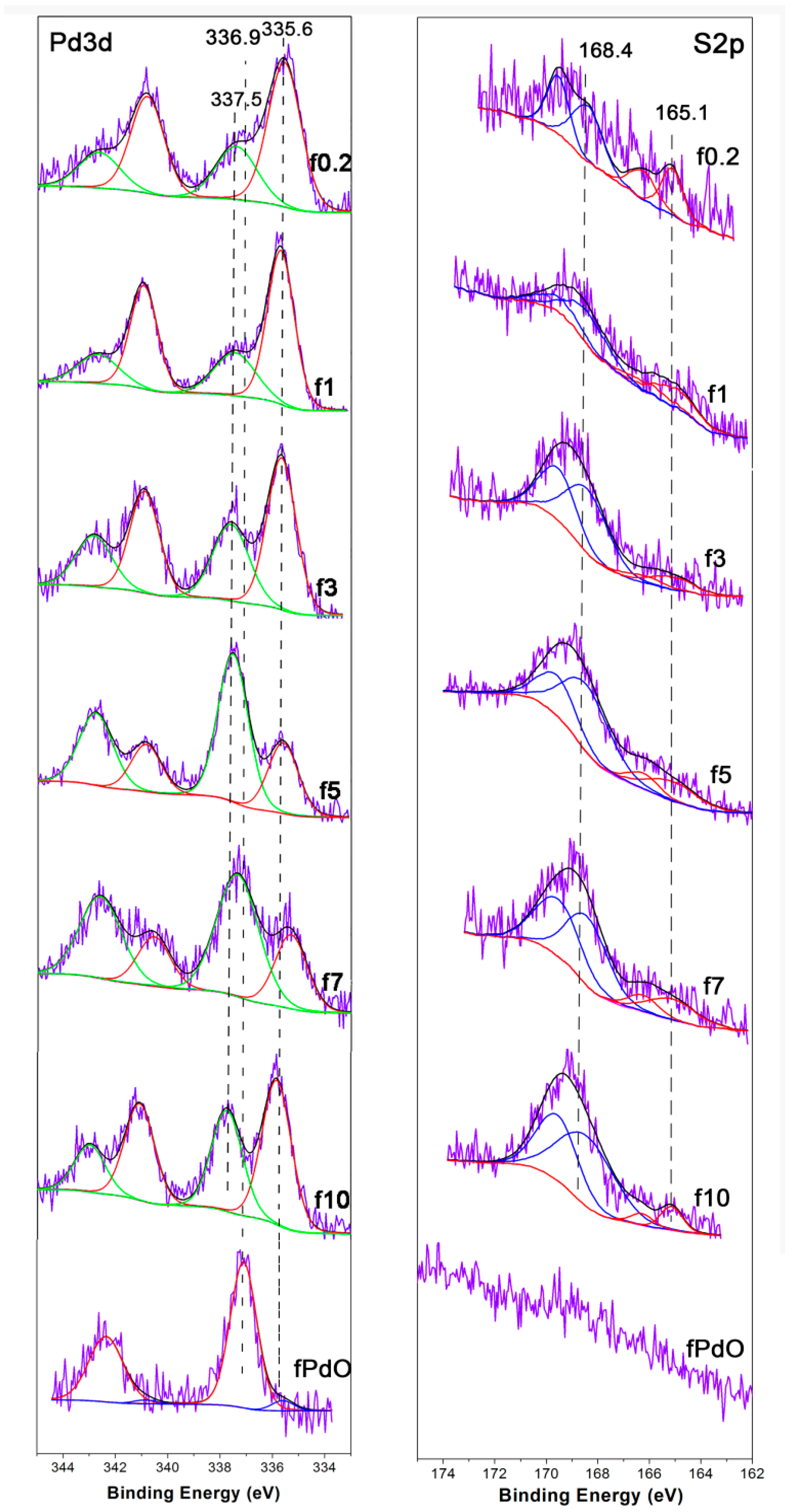
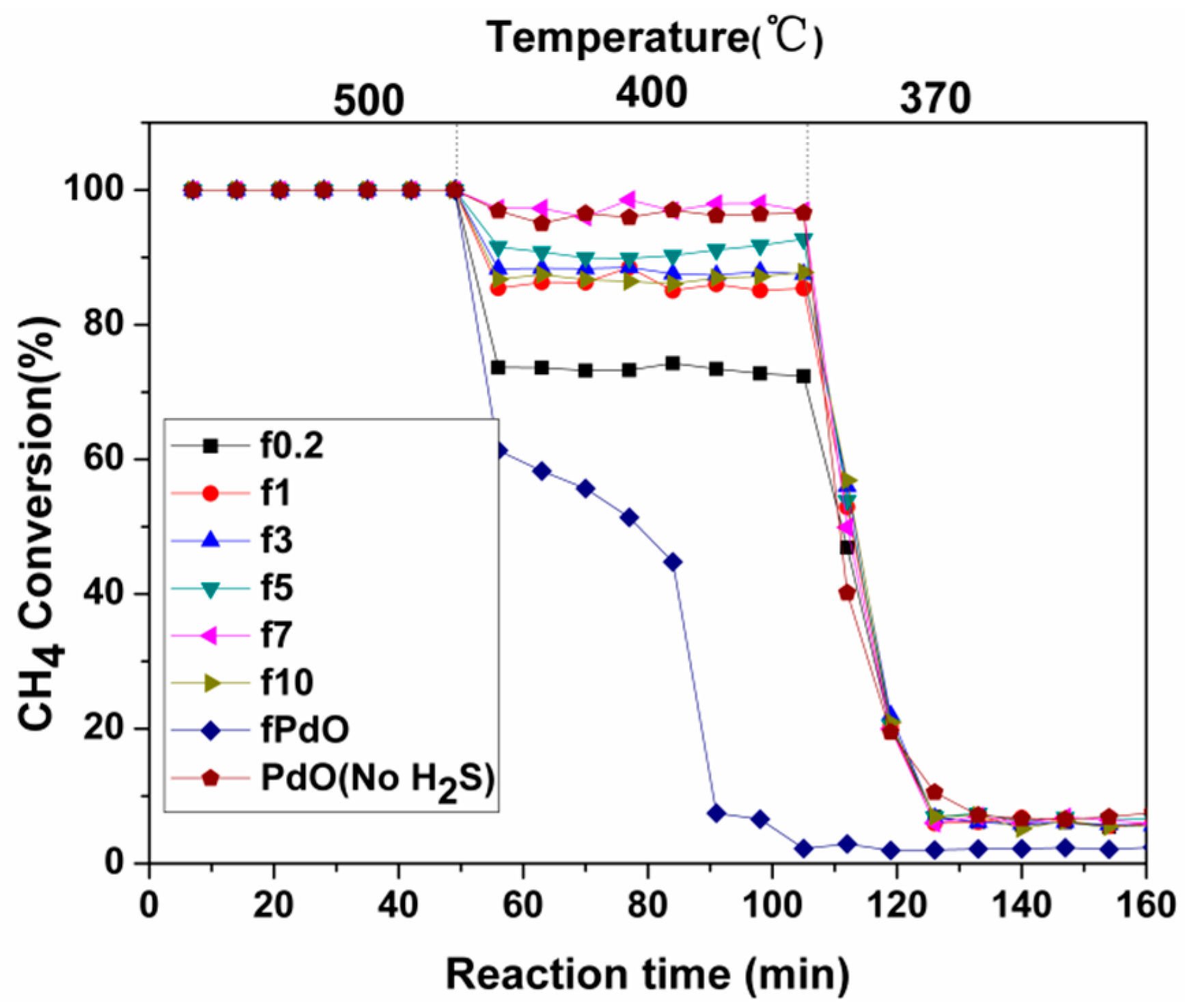
| Catalyst | FWHM | Size (nm) |
|---|---|---|
| Pd | 0.761 | 12.2 |
| f0.2 | 0.803 | 11.5 |
| f1 | 1.328 | 6.9 |
| f3 | 1.297 | 6.7 |
| f5 | 3.329 | 2.6 |
| f7 | 3.262 | 2.6 |
| f10 | 2.163 | 4.0 |
| Catalyst | Composition Ratio of Palladium Species (%) | |
|---|---|---|
| Pd0 | Pd4S | |
| f0.2 | 68.9 | 31.1 |
| f1 | 68.6 | 31.4 |
| f3 | 61.6 | 38.4 |
| f5 | 33.4 | 66.6 |
| f7 | 32.1 | 67.9 |
| f10 | 57.9 | 42.1 |
| Catalyst | Composition Ratio of Palladium Species (%) | ||
|---|---|---|---|
| Pd | PdO | Pd4S | |
| u0.2 | 5.3 | 59.5 | 35.2 |
| u1 | 4.0 | 60.4 | 35.6 |
| u3 | 5.9 | 54.2 | 39.9 |
| u5 | 2.7 | 40.4 | 56.9 |
| u7 | 3.1 | 36.6 | 60.3 |
| u10 | 3.6 | 48.9 | 47.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, L.; Yuan, S.; Jiang, T.; Zhu, X.; Lu, C.; Li, X. Pd4S/SiO2: A Sulfur-Tolerant Palladium Catalyst for Catalytic Complete Oxidation of Methane. Catalysts 2019, 9, 410. https://doi.org/10.3390/catal9050410
Ma L, Yuan S, Jiang T, Zhu X, Lu C, Li X. Pd4S/SiO2: A Sulfur-Tolerant Palladium Catalyst for Catalytic Complete Oxidation of Methane. Catalysts. 2019; 9(5):410. https://doi.org/10.3390/catal9050410
Chicago/Turabian StyleMa, Lei, Shiyan Yuan, Taotao Jiang, Xiangming Zhu, Chunshan Lu, and Xiaonian Li. 2019. "Pd4S/SiO2: A Sulfur-Tolerant Palladium Catalyst for Catalytic Complete Oxidation of Methane" Catalysts 9, no. 5: 410. https://doi.org/10.3390/catal9050410
APA StyleMa, L., Yuan, S., Jiang, T., Zhu, X., Lu, C., & Li, X. (2019). Pd4S/SiO2: A Sulfur-Tolerant Palladium Catalyst for Catalytic Complete Oxidation of Methane. Catalysts, 9(5), 410. https://doi.org/10.3390/catal9050410




