Abstract
In this study, a critical comparison between two low metal (Ni) loading catalysts is presented, namely Ni/Al2O3 and Ni/AlCeO3 for the glycerol steam reforming (GSR) reaction. The surface and bulk properties of the catalysts were evaluated using a plethora of techniques, such as N2 adsorption/desorption, Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP–AES), X-ray Diffraction (XRD), X-ray Photoelectron Spectroscopy (XPS), Scanning Electron Microscopy / Energy Dispersive X-Ray Spectroscopy (SEM/EDX, Transmission Electron Microscopy (TEM), CO2 and NH3– Temperature Programmed Desorption (TPD), and Temperature Programmed Reduction (H2–TPR). Carbon deposited on the catalyst’s surfaces was probed using Temperature Programmed Oxidation (TPO), SEM, and TEM. It is demonstrated that Ce-modification of Al2O3 induces an increase of the surface basicity and Ni dispersion. These features lead to a higher conversion of glycerol to gaseous products (60% to 80%), particularly H2 and CO2, enhancement of WGS reaction, and a higher resistance to coke deposition. Allyl alcohol was found to be the main liquid product for the Ni/AlCeO3 catalyst, the production of which ceases over 700 °C. It is also highly significant that the Ni/AlCeO3 catalyst demonstrated stable values for H2 yield (2.9–2.3) and selectivity (89–81%), in addition to CO2 (75–67%) and CO (23–29%) selectivity during a (20 h) long time-on-stream study. Following the reaction, SEM/EDX and TEM analysis showed heavy coke deposition over the Ni/Al2O3 catalyst, whereas for the Ni/AlCeO3 catalyst TPO studies showed the formation of more defective coke, the latter being more easily oxidized.
1. Introduction
Biofuels are expected to play an important role in meeting the combined challenge of providing adequate energy supplies, while simultaneously combating the threat posed by climate change, with projections estimating that their use will grow from 1.3 million barrels of oil equivalent (BOE) in 2012 to 4.6 BOE in 2040 [1]. Although biodiesel had captured approximately 25% of total biofuel output in 2015 [2], the high costs associated with its production means that the sector remains uncompetitive, with favourable government policies underpinning much of its growth [3]. The industry is also facing the challenge of dealing, in a sustainable way, with the co-production of crude glycerol (C3H8O3), which constitutes its major by-product and amounts to approximately 10 wt % of the oil undergoing transesterification [4].
However, glycerol can be converted into hydrogen, a fuel that finds a variety of present-day applications in transport, building and other industry sectors, while at the same time is expected to have a leading role in a future carbon-free energy mix [5]. Although different thermochemical processes may be used for the conversion of C3H8O3 into H2—e.g., aqueous phase reforming (APR), autothermal reforming (ATR) and super critical water reforming (SCWR)—the process that appears most promising is that of the steam reforming of glycerol (GSR) [6,7,8]. This is because GSR has a high H2 production capacity per mol of C3H8O3 reformed (Equation (1)) and is a mature industrial technology unlikely to require major technical adjustments in switching feedstocks [9,10]. The GSR (Equation (1)), a strongly endothermic reaction (ΔHΘ = 123 kJ/mole), combines the decomposition of glycerol (Equation (2)) and the water-gas shift reaction (WGS, Equation (3)), but a number of parallel reactions that include methanation (Equation (4)), methane dry reforming (Equation (5)) and carbon formation reactions (Equations (6)–(9)) also take place [11,12]. According to the thermodynamic studies undertaken, the GSR should be undertaken at high temperature (>630 °C), high water to glycerol feed ratio (WGFR < 9:1, molar) and at atmospheric pressure [13,14].
C3H8O3 + 3H2O → 3CO2 + 7H2,
C3H8O3 → 3CO + 4H2,
CO + H2O ↔ CO2 + H2,
CO + 3H2 ↔ CH4 + H2O,
CH4 + CO2 ↔ 2H2 + 2CO,
2CO ↔ CO2 + C,
CH4 ↔ 2H2 + C,
H2O + C ↔ CO + H2,
C3H8O3 → H2 + 3H2O + 3C,
To be effective, the catalysts for use in the GSR should not promote C–O cleavage and CO or CO2 hydrogenation, but they should favour the cleavage of the C–H, C–C and O–H bonds [15,16]. As Ni-based systems are known to be highly active in reforming reactions, most efforts currently found in the GSR related literature have focused on the development of such catalysts, using a variety of metal oxides (e.g., Al2O3, ZrO2, SiO2) as supports [17,18,19,20]. The most commonly used support is Al2O3 as it possesses a number of desirable properties, such as chemical and mechanical resistance under reaction conditions and a high specific surface area, which favours the dispersion of the active phase on the support [20,21]. However, there are two main disadvantages associated with the use of alumina; firstly, the Lewis type acid sites that it possesses promote acid-base-catalyzed reactions, which in turn favour the formation of a filamentous type of carbon, and secondly, it fosters the sintering of metallic particles [22,23]. For example, Chiodo et al. [24], using a low steam to glycerol ratio (3 mol/mol) and testing the stability at 800 °C for 20 h, reported rapid deactivation for a Ni/Al catalyst (30 wt % Ni). Cheng et al. [25], reported significant carbon deposition for a Ni/Al (15 wt % Ni) at 550 °C tested for approximately 4 h, even under excess steam conditions. Previous work by our group [15] showed severe deactivation for a Ni/Al (8 wt % Ni) during 20 h time-on-stream at 600 °C (WGFR of 9/1, molar), which was attributed to the high degree of crystallinity of the carbon deposits. Thus, much effort has been spent on overcoming the disadvantages associated with the use of alumina by inducing support-mediated promotional effects using different alkaline earth metals (e.g., Mg, Ca), lanthanide metals (e.g., Ce, La), and/or transition metals (e.g., Zr, Cu) as promoters for Ni/Al catalysts [26,27,28].
As is well understood, ceria can affect the surface acidity of alumina due to its higher point of zero charge (PZC) [29]. It also has the ability to act as an O2 buffer, storing/releasing O2 via the Ce3+/Ce4+ redox couple in CeO2 [30], and thus promote water dissociation and the water gas shift reaction [31]. Recent research also suggests that a [Oδ−, δ+] dipolar layer is created on the surface of Ni particles that can protect them against thermal sintering [32,33,34]. For the GSR, a handful of works exist where ceria was used as promoter to alumina for Ni-based catalysts [35,36,37,38]. The general conclusion from these papers is that the addition of CeO2 as alumina modifier enhances the activity of the non-promoted catalyst [35,36,37]. This is mainly related to the ability of ceria to stabilize the nickel particles and to promote steam reforming of the oxygenated hydrocarbons intermediates, leading to a reduction in coke deposition. It has also been suggested that ceria hinders secondary dehydration reactions (favoured by the presence of acid sites in the support), and lead to the formation of hydrocarbons that are coke precursors (and thus, responsible for catalyst deactivation) [38]. It has also been suggested that the formation of a CeAlO3 perovskite structure can suppress the interaction between Ni and Al2O3 and increase the number of active Brønsted acid sites. This, in turn, improves the bifunctional metal-acid properties of Ni/CeO2–Al2O3 and favours the hydrogenolysis and dehydrogenation-dehydration of condensable intermediate products that produce more H2 [39].
In the present investigation, low metal loading (8 wt %) Ni catalysts supported on γ-Al2O3 and γ-Al2O3 modified with CeO2 were investigated in terms of catalytic activity and time-on-stream stability during the GSR. Different characterization techniques, i.e., N2-physisorption, ICP, XRD, temperature programmed reduction (H2–TPR), CO2–TPD, NH3–TPD, XPS, TPO, SEM and TEM were used in an effort to identify the catalyst surface and bulk properties which affect the reaction and its products. The performance of the catalysts was investigated with the aim of identifying the effect of temperature on the total conversion of glycerol and the conversion of glycerol to gaseous products, the selectivity towards H2, CO2, CO, CH4 and the liquid effluents produced during the reaction and for the determination of the H2/CO and CO/CO2 molar ratio in the gaseous products mixture. Time-on-stream experiments were conducted for 20 h under harsh reaction conditions in order to induce carbon deposition and the main liquid effluents were quantified.
2. Results and Discussion
2.1. Characterization Results
2.1.1. Chemical Analysis and Textural Properties
The physicochemical, structural and textural properties of the calcined and reduced catalysts are summarized in Table 1. As a first observation, both catalysts have a similar Ni metal loading, which was measured for the calcined samples at 7.14 and 7.69 wt % for Ni/Al2O3 and Ni/AlCeO3, respectively. Regarding the properties of the calcined supports used herein, N2-physisorption measurements showed that Al2O3 has a much higher specific surface area (SSA) than AlCeO3 (195 m2 g−1 compared to 48 m2 g−1) and pore volume (0.65 cm g−1 compared to 0.24 cm g−1). Following Ni impregnation and then catalyst calcination, the SSA of the Ni/Al2O3 catalyst dropped to 158 m2 g−1 and then further to 136 m2 g−1 after the reduction procedure. For Ni/AlCeO3, this drop was less pronounced after calcination, but somewhat sharper after reduction (43 m2 g−1 after calcination and 26 m2 g−1 after reduction).

Table 1.
Physicochemical, structural and textural properties of calcined and reduced catalysts.
Also, for both catalysts, the pore volume remained almost unchanged following calcination, but dropped substantially after reduction. However, the doping of Al2O3 with CeO2 resulted in enhanced basicity, as shown from the potentiometric titration curves obtained for the Al2O3 and AlCeO3 suspensions, with the PZC values recorded at 6.8 and 8.2, respectively (Figure 1). It is noted that the PZC is defined as the pH value where the basic and acidic sites on the surface are in equilibrium [40].
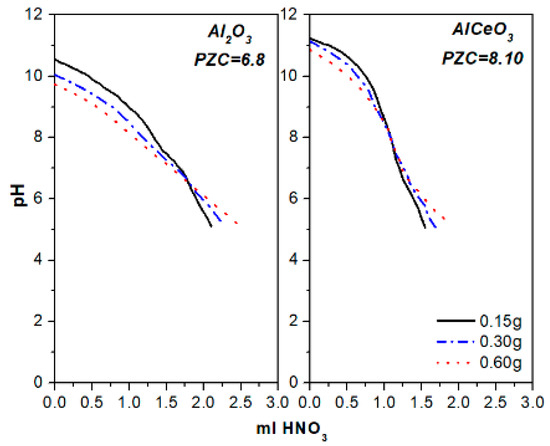
Figure 1.
Potentiometric titration curves and point of zero charge (PZC) of the Al2O3 and AlCeO3 supports.
The N2 adsorption—desorption isotherms of both catalysts, displayed as an inset in Figure 2, are of Type IV with an H4-type hysteresis loop [41], and show major adsorption at high pressures (0.7 < P/P0 < 1.0) and only minor adsorption at low pressure (P/P0 < 0.05). This is indicative of mainly mesoporous material with some macroporosity [42] and is corroborated by the corresponding pore size distributions.
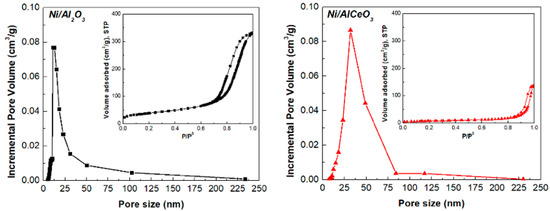
Figure 2.
Pore size distribution and N2 adsorption-desorption isotherms (inset) of the reduced Ni/Al2O3 and Ni/AlCeO3 catalysts.
Figure 3 presents the XRD patterns for the supports (after calcination) and catalytic samples (after calcination and after reduction). Regarding the Al2O3 support and Ni/Al2O3 catalyst, the characteristic peaks of γ-Al2O3 are clearly identified at 2θ = 37.2°, 47.2° and 67.2°. The nickel aluminate phase (NiAl2O4) was observed at 2θ = 19.0°, 32.0°, 37.0°, 45.0°, 60.2° and 65.9° for both calcined and reduced catalysts [43]. However, it is also clear from the diffractograms that the Al2O3 and NiAl2O4 peaks are not as intense on the reduced sample in comparison to the calcined one. Moreover, on the reduced catalyst, two small peaks corresponding to metallic nickel (Ni0), at 2θ = 44.0° and 51.2° [43], can be observed. For the calcined AlCeO3 support, γ-Al2O3 was detected only at 2θ = 67.2° and the crystal phase of CeO2 dominated with peaks at 2θ = 28.5°, 33.0°, 47.5°, 56.5° and 59.0° (Figure 3b) [44]. The XRD patterns of the Ni/AlCeO3 catalyst after calcination and after reduction (Figure 3b) are nearly identical to the pattern of the calcined AlCeO3 support with the only differences being two small peaks corresponding to NiAl2O4 detected on the calcined catalyst (2θ = 32.0° and 37.0°), and two peaks, corresponding to Ni0, detected at 2θ = 43.5ο and 50.8° on the reduced sample. It is noted that the reaction 4CeAlO3 + O2 ↔ 4CeO2 + 2Al2O3 is reversible, i.e., CeAlO3 can be oxidised to CeO2 by heating in air and CeO2 can be reduced by heating in H2 flow [45]. The Ni species mean particle size (Table 1), for the reduced samples, was determined from the XRD spectra using Scherrer analysis and was found at 16.8 nm for the Ni/Al2O3 and at 14.2 nm for the Ni/AlCeO3 catalyst.
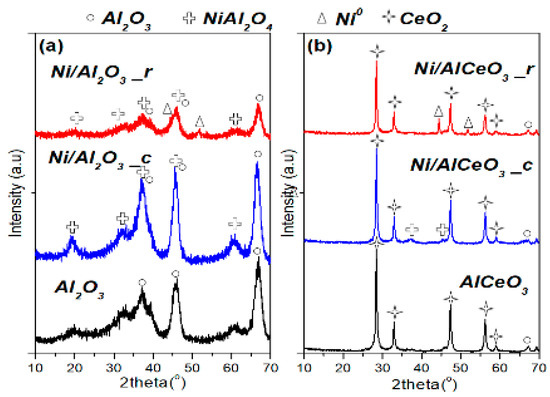
Figure 3.
XRD patterns of supports, calcined and reduced catalysts: (a) Al2O3 and Ni/Al2O3 and (b) AlCeO3 and Ni/AlCeO3.
2.1.2. Surface Acidity-Basicity Estimation
Figure 4a presents the CO2–TPD profiles obtained over the Ni/Al2O3 and Ni/AlCeO3 catalysts. The Ni/Al2O3 catalyst presents mainly two types of basic sites of weak and medium strength. This is reflected by the peaks that appeared in the temperature regimes of 30 °C < Tmax < 220 °C and 220 °C < Tmax < 450 °C. The strong basic site population is very limited. On the other hand, it seems that the modification of Al2O3 support with Ce leads to a catalyst with a limited population of weak basic sites and a wider distribution of medium strength basic sites, whereas the population of strong basic sites is enhanced significantly. This result is in good agreement with the PZC studies, discussed earlier.
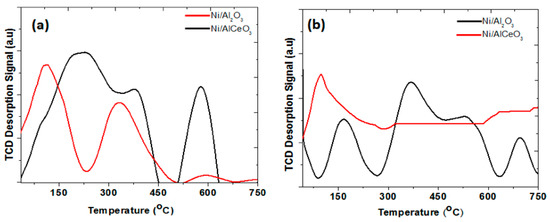
Figure 4.
(a) CO2–TPD and (b) NH3–TPD profiles of the Ni/Al2O3 and Ni/AlCeO3 catalysts.
Figure 4b presents the NH3–TPD profiles obtained over the Ni/Al2O3 and Ni/AlCeO3 catalysts. The Ni/Al2O3 presents a rather rich surface in terms of acid sites of weak, medium and high strength. Incorporation of Ce into the catalyst support leads to a drastic re-distribution of the acid sites of the catalyst, with only the weak sites dominating the surface. It is commonly accepted that on the surface of pure Al2O3 only the Lewis-type acid centres are observed. Miranda et al. [46] attributed desorption peaks at 77–277 °C and at 277–627 °C to the presence of two kinds of acid sites having different strengths. According to the authors, the lower desorption peak is linked to desorption of NH3 bridge species which are bonded to penta-coordinated aluminum (Lewis weak sites), while the higher desorption peak to terminally bonded NH3 on tri-coordinated aluminum (Lewis strong sites) [47]. Thus, it can be concluded that the high temperature acid sites correspond to the Brønsted acid sites of the solid surface [48] and the strong Lewis acid sites are associated with Al2O3 [47,49], whereas the low temperature acid sites are weak acid sites and mainly associated with CeO2 [50] due to its generally weak basicity [39,51].
2.1.3. Ni species Reducibility
Metal-support interactions (electronic or geometrical) are crucial in defining the catalytic properties. In general, NiO interaction with the support, as well as the size of NiO, regulates the extent of its reduction. Weak interaction with the support is considered to give rise to reduction peaks at low temperatures, whereas peaks at high temperatures are due to a strong NiO-support interaction. In the H2-TPR profile of the Ni/Al2O3 catalyst (Figure 5), two predominant peaks at 420 °C and 690 °C are apparent (Figure 5). The peak at 690 °C is due to the reduction of nickel aluminate (NiAl2O4), while the peak at 420 °C is due to small NiO crystallites interacting with the support (supported NiO). The small hump at 390 °C for the Ni/Al2O3 catalyst can be assigned to the reduction of bulk NiO [52]. The addition of Ce causes a redistribution of species and most likely changes the support size thus affecting the NiO-support contact area, with the latter being increased. This causes a reduction profile with peaks at lower temperatures compared with the Ni/Al2O3 catalyst.
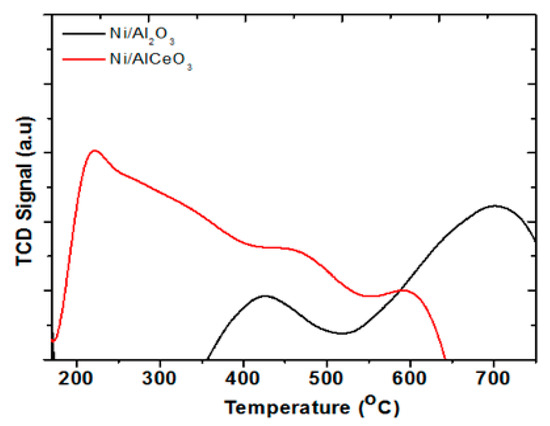
Figure 5.
Temperature programmed reduction (TPR) profiles of Ni/Al2O3 and Ni/AlCeO3 catalysts.
These TPR reduction profiles are in agreement with the ones presented by Yang et al. [53] where Ni/Al2O3 reduction takes place between 400–900 °C, whereas the addition of Ce gave rise to another reduction peak in the 200–400 °C region. This is due to the excellent redox properties of the Ce-related phases formed [54]. Thus, it can be concluded that the nickel species on the AlCeO3 support retained a higher reducibility than Al2O3; the higher reducibility being a result of the incorporation of ceria into the alumina lattice [39]. Also, the complex profile of NiO/AlCeO3 catalyst can be due to the fact that the nucleation and growth of the NiO over the Ce-modified Al2O3 is different compared to that of Al2O3. This could be due to the presence of new sites (e.g., Ce3+, Al–O–Ce site, defects), which can act as anchoring sites for the growth of NiO, which affect the kinetics of the growth and ultimately the NiO size (distribution of NiO sizes). Furthermore, a support that exhibits strong metal-support interactions is anticipated to show less sintering during oxidation/reduction.
2.1.4. Surface Analysis
The XPS Ni 2p and Ce 3d peaks for both the calcined and reduced Ni/Al2O3 and Ni/AlCeO3 catalysts are shown in Figure 6a–f. The Ni 2p3/2 peak for the calcined Ni/Al2O3 sample shows a peak at a binding energy of 856.1 eV, associated with the presence of NiAl2O4 (Figure 6a). The reduced catalyst also shows the presence of a main Ni 2p3/2 peak at a binding energy of 856.1 eV due to NiAl2O4/Ni2O3 but also a shoulder at lower binding energies at around 853 eV, due to the presence of metallic Ni (Figure 6b). For the Ni/AlCeO3 catalysts, only the Ni 2p3/2 peak and satellite is shown in the Figure 6c,d as the 2p1/2 peak and satellite overlap with the Ce 3d peaks.
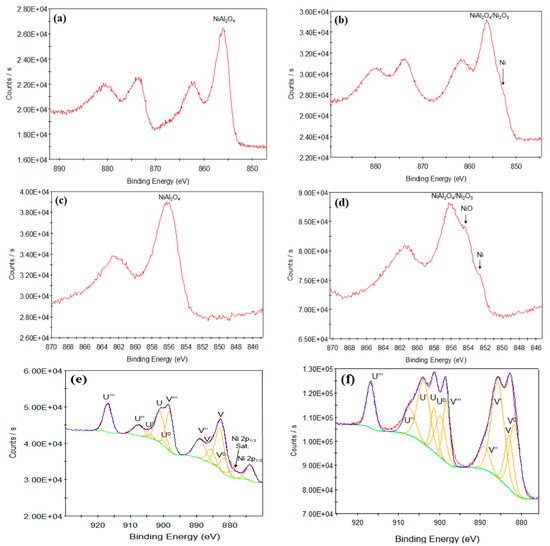
Figure 6.
XPS Ni 2p for: (a) calcined Ni/Al2O3, (b) reduced Ni/Al2O3, (c) calcined Ni/AlCeO3, (d) reduced Ni/AlCeO3, and Ce 3d peaks for the calcined (e) and reduced (f) Ni/AlCeO3 catalyst.
The calcined Ni/AlCeO3 catalyst exhibits a Ni 2p3/2 peak at a binding energy of 856.2 eV due to NiAl2O4. The reduced Ni/AlCeO3 catalyst shows a more complex peak shape with 3 components at binding energies of 856.2, 854.1 and around 852.6 eV. The complexity of the Ni 2p spectra in terms of their photoelectron and satellite features (particularly Ni oxide) precludes a sensible peak fit of the Ni 2p envelope [55]. The different components can be tentatively assigned to NiAl2O4/Ni2O3 (856.2 eV), NiO (854.1 eV) and Ni metal (852.6 eV). XPS Ce 3d photoelectron spectra for CeO2 (Ce4+) and Ce2O3 (Ce3+) (Figure 6e,f) are also well known for their complex photoelectron and satellite peak structure [56,57,58]. Furthermore, for the catalyst surfaces examined here, overlap with the main Ni 2p1/2 satellite further complicates the spectral shape. Nevertheless, using standard spectra for CeO2 and Ce2O3 recorded on this XPS instrument as a reference, and with the assistance of other XPS work on cerium oxides [56,57,58] the Ce 3d spectra have been peak fitted with a reasonable degree of certainty. The photoelectron and satellite peaks for the calcined Ni/AlCeO3 catalyst are considered first (Figure 6e). The calcined catalyst surface shows peaks generally representative of Ce4+ (CeO2). Using the nomenclature employed in previous XPS work on cerium oxide [56,57], the peaks are labelled v and u, corresponding to transitions associated with the Ce 3d5/2 and Ce 3d3/2 respectively. The peaks observed are those corresponding to the v, v’’, v’’’, u, u’’ and u’’’, corresponding to the Ce4+ species, hence CeO2 is clearly the predominant oxide present. However, the peak fit shows the presence of weak peaks associated with the Ce3+ species (v0, v’, u0 and u’) and approximate fractions of the two oxide forms is 85% CeO2 and 15% Ce2O3, corresponding to a stoichiometry of CeO1.94. The peak shape agrees well with that given by Henderson et al., for a reported stoichiometry of CeO1.95 [58]. For the reduced catalyst (Figure 6f), the Ce 3d spectrum is very different, with clear peaks present for both the Ce4+ (v, v’’’, u, u’’ and u’’’) and Ce3+ (v’ and u’) species. The peak fit gives a ratio of 41% CeO2 and 59% Ce2O3, corresponding to a stoichiometry of CeO1.80. The peak shape recorded for the reduced Ni/AlCeO3 catalyst agrees very well with the Ce 3d peak shapes shown for samples with reported stoichiometries of CeO1.78 [56] and CeO1.82 [58], having relative peak intensities in-between those observed for CeO1.78 and CeO1.82, as would be expected for a stoichiometry of CeO1.80.
As is widely accepted, CeO2 increases the dispersion of the metallic phase [59] and this is also observed for the catalysts tested herein with the results shown in Table 2. It has also been suggested that a high dispersion causes a specific interaction between the nickel and ceria species, were the Ni particles close to CeO2 activate the dissociation of H2 and by spillover favour the reduction of the ceria surface [60].

Table 2.
Ni2p XPS data of calcined and reduced Ni/Al2O3 and Ni/AlCeO3 catalysts.
2.2. Catalytic Performance
2.2.1. Total Conversion and Conversion to Gaseous Products
Figure 7a presents the results obtained following the catalytic tests, performed under experimental protocol #1, in terms of glycerol total conversion (XC3H8O3, %) and glycerol conversion to gaseous products (XC3H8O3 gaseous, %) in relation to reaction temperature. It is clear that a very high conversion of glycerol was achieved by both catalysts, but also for the AlCeO3 support, over the whole temperature range (from ≈85 at 400 °C to > 95% at 750 °C). In contrast, the total conversion of glycerol was relatively low for the bare alumina support, ranging from approximately 70% at 400 °C to 80% at 750 °C. The high conversion values recorded are due not only to the reforming of glycerol but also to its thermal decomposition, which can take place simultaneously during the GSR process [61,62,63]. The endothermic nature of the GSR reaction can be clearly deduced from the conversion of glycerol to gaseous products, which increases sharply with increasing temperature (Figure 7a).
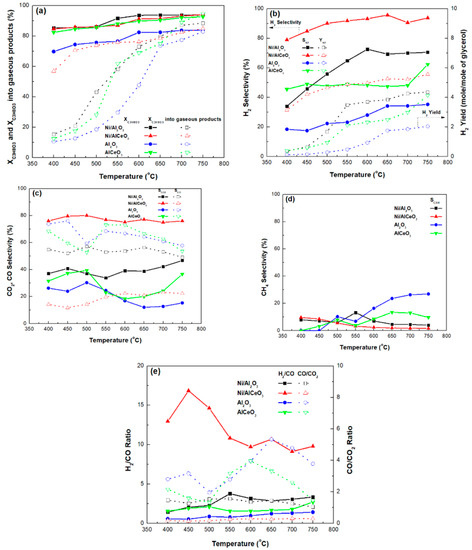
Figure 7.
(a) Total glycerol conversion and glycerol conversion into gaseous products, (b) H2 selectivity and H2 yield, (c) CO2 and CO selectivity, (d) CH4 selectivity, and (e) H2/CO and CO/CO2 molar ratios (samples tested under experimental protocol #1).
However, it is clear that the Ni/AlCeO3 catalyst produces a far greater number of gaseous products in comparison to Ni/Al2O3, especially for temperatures lower than 600 °C. Interestingly, the values of Xgaseous for the Ni/AlCeO3 sample ranged from 60% to 80% for the whole temperature range. Furthermore, the AlCeO3 supporting material seems to produce more or less the same number of gaseous products as the Ni/Al2O3 catalytic sample. Previously published works [39] argued that the formation of CeAlO3 perovskite structures suppresses any strong interaction between the nickel species and the Al2O3 support, thereby increasing the number of active sites and the reducibility of the active nickel species. From the results presented herein, the superior behaviour of the Ni/AlCeO3 can be related to its higher dispersion (XPS), i.e., smaller Ni crystallites, and also to higher electron density and accessibility of the active sites caused by the close contact between nickel and the cerium species.
2.2.2. Gaseous Products’ Selectivity
The influence of reaction temperature on H2 selectivity (SH2) and yield (YH2), following experimental protocol #1, for both catalysts and supports is presented in Figure 7b. As a first observation, the H2 production of the Ni/AlCeO3 catalyst is markedly higher in comparison to the Ni/Al2O3 sample for the whole temperature range, as its SH2 and YH2 take the values of 80–90% and 4–6 mol/mol (6 mol/mol is very close to the value predicted by thermodynamics). This improved performance can also be observed for the AlCeO3 support in comparison to the pure alumina. Thus, it can be suggested that the Ni active sites combined with the Brønsted acid sites promote the hydrogenolysis and dehydrogenation-dehydration of the bifunctional metal-acid Ni/AlCeO3 catalyst, thereby converting more condensable intermediates into gaseous products, as well as leading to higher glycerol conversion and H2 yield.
The influence of reaction temperature on the selectivity to CO2 and CO (SCO2 and SCO) for all samples is presented in Figure 7c. It is obvious that the Ni/AlCeO3 sample is more selective towards CO2 and less selective towards CO for the whole temperature range. This is in contrast to the trend observed for the Ni/Al2O3 catalyst and supporting materials, as these materials appear more selective towards CO and less selective towards CO2. Thus, it seems that the ability to transform OHCs into H2 and CO2 is significantly higher for the Ni/AlCeO3 catalyst probably due to its enhanced basic character (higher PZC value), which was introduced by the addition of CeO2 to the alumina. Moreover, from Figure 7d it can be seen that both catalytic samples exhibit very low SCH4 for the whole temperature range; in contrast, SCH4 increases for the supports with increasing temperature. This was rather expected as according to the literature [36] at high water to glycerol feed ratios and at temperatures higher than 650 °C, the formation of CH4 is strongly inhibited due to the methane steam reforming reaction being catalysed by the metallic active phase. Keeping in mind that glycerol decomposition to CH4 during the reforming process is highly favoured [64], it appears that both catalysts have the capacity to reform the produced CH4 into H2 and CO.
Finally, the influence of reaction temperature on the H2/CO and the CO/CO2 molar ratios in the gaseous products’ mixture is presented in Figure 7e. For the Ni/AlCeO3 catalyst, the CO/CO2 molar ratio is close to zero for the whole temperature range, while the H2/CO molar ratio value decreases with increasing temperature from a value of about 17 (450 °C) to a value of about 10 (550–750 °C). In contrast, for the Ni/Al2O3 catalyst, both ratios are equal to 2–3 and appear relatively stable over the whole temperature range under investigation. The H2/CO molar ratio value is more or less negligible for the supporting materials, whereas the CO/CO2 molar ratio values range from about 2 between 400 and 500 °C, increases from 500 to 600 °C and decreases again for 650 °C < T < 750 °C (its maximum value was ~5.5 for the Al and ~4 for the AlCeO3 sample). It is known that in the reaction process, dehydrogenation of the adsorbed glycerol molecule first takes place on the metal surface of catalyst to give adsorbed intermediates for the cleavage of C–C or C–O bonds.
From the results presented above it is obvious that the presence of ceria in the alumina supporting material has an important effect on the conversion and the gaseous product distribution, mainly by increasing H2 and CO2 production to the detriment of CH4 and CO formation. It is likely that for the Ni/AlCeO3 catalyst there is a first reaction step that involves rapid C–C breaking and CO formation, which is then followed by the WGS reaction, enhancing H2 and CO2 production. Sad et al. [65] has argued that acidic supports (such as alumina) favour the production of oxygenates (such as acrolein, acetol, acetaldehyde and acetic acid) via dehydration and dehydrogenation reactions, and that H2 production is favoured by the use of non-acidic supports. However, the GSR process is also influenced by pyrolysis phenomena, given that the glycerol molecule is not thermally stable, which means that the intermediates formed by glycerol cracking are reformed on the catalyst surface [66].
2.2.3. Liquid Products’ Selectivity
In accordance with previous research (e.g., [10,12,31]), a variety of liquid products were identified for the GSR however, only the main ones, i.e., acetone, acrolein, acetaldehyde, acetic acid, allyl alcohol, and acetol were quantified. The influence of temperature on liquid product selectivity for the Ni/Al2O3 and Ni/AlCeO3 catalysts and the corresponding supports is shown in Figure 8. For the Ni/AlCeO3 catalyst and the AlCeO3 support, production of liquid effluents ceases over 700 °C, 50 °C higher compared to the Ni/Al2O3 sample. In contrast, pure alumina produces liquid effluents even at temperatures as high as 750 °C. Another important observation is the differences in the liquid product distribution between modified and unmodified samples. Specifically, for the Ni/AlCeO3 and AlCeO3, allyl alcohol seems to be the main product (at least for high temperatures), with acetaldehyde and acetone the secondary products. On the other hand, acetone is the main product at the high temperature range for the Ni/Al2O3 sample and acetic acid and acetol for pure alumina.
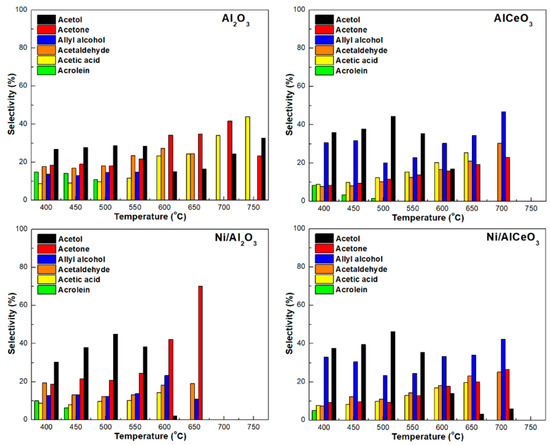
Figure 8.
Liquid product selectivity for all samples tested herein (samples tested under experimental protocol #1).
According to the literature, there are four main reaction routes of glycerol consisting of three types of reactions, i.e., dehydrogenation, hydrogenolysis, and dehydration. These reactions represent the effects of the bifunctional metal–acid properties of the catalysts [47]. The first and the second routes of glycerol dehydrogenation and hydrogenolysis generate a common product, such as ethylene glycol, which is converted to acetaldehyde, glycolaldehyde, and ethanol. The hydrogenolysis of glycerol also produces methanol. The third route is glycerol dehydration to hydroxyacetone (acetol), which can be converted simultaneously in three ways to form a variety of products such as acetaldehyde, acetone, ethanol, acetic acid, propionic acid, formic acid, phenol, 1,2-propanediol, propanal, 1-propanol, and 2-cyclo-pentanone. This pathway is caused by the activation of the terminal OH of glycerol on the Lewis acid sites, which is represented by the strong acid sites of the catalysts. The fourth pathway is another glycerol dehydration reaction producing 3-hydroxypropanal, which is a starting reactant for the production of several chemicals. The species observed and confirming this pathway include allyl alcohol, acetaldehyde, methanol, formic acid, C2H4, and C2H6. This pathway involves the protonation of the secondary OH of glycerol to form acrolein, which favours the Brønsted acid sites mainly represented by the moderate acid sites [67].
2.3. Catalytic Stability
The results for catalytic stability are presented in Figure 9 and Table 3. It is noted that these experiments were carried out under more severe conditions (experimental protocol #2) in order to provoke carbon deposition and catalyst deactivation. From the results presented herein it is clear that although the Ni/AlCeO3 catalyst suffers a slight decrease concerning glycerol total conversion (94–77%) and glycerol conversion to gaseous products (48–37%), it maintains remarkably stable values for H2 yield (2.9–2.3) and selectivity (89–81%), as well as CO2 (75–67%) and CO (23–29%) selectivity. As a result, the H2/CO and CO/CO2 molar ratios remain reasonably constant for the duration of the experiment, with values ranging between 9 to 6.5 and 0.3 to 0.4, respectively. The catalytic performance observed indicated that the effect of the WGS reaction do not weaken with time. It is accepted that the addition of basic modifiers, such as CeO2 to Ni/Al2O3 catalysts can prevent carbon formation by favouring both the adsorption of H2O, O2, CO2 or–OH fragments and the spillover of such fragments from the support to the metal particles [67], and thus facilitate carbon gasification.
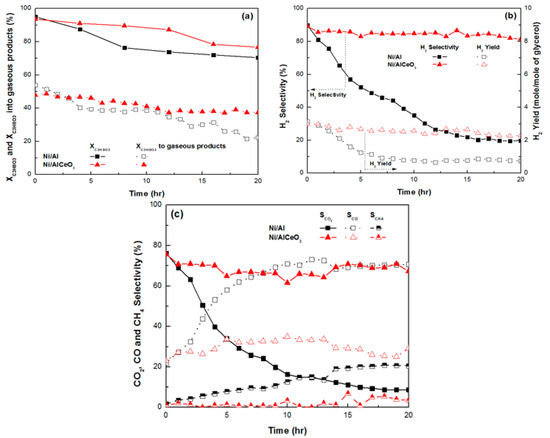
Figure 9.
Time on stream experiments for Ni/Al2O3 and Ni/AlCeO3 catalysts: (a) Total glycerol conversion and glycerol conversion into gaseous products, (b) H2 selectivity and yield, (c) CO2, CO and CH4 selectivity (samples tested under experimental protocol #2).

Table 3.
Catalytic performance of the Ni/Al2O3 and Ni/AlCeO3 catalysts during time-on-stream at 600 °C (first and last measurement).
In contrast, although the decrease in total XC3H8O3 and XC3H8O3 gaseous products for the Ni/Al2O3 catalyst was similar to the Ni/AlCeO3, SH2, YH2 and SCO2 decreased substantially; this decrease was accompanied by an increase in the SCO values. As a result, for the Ni/Al2O3 catalyst, the H2/CO molar ratio decreased from 9.5 to 0.6 and the CO/CO2 ratio increased from 0.3 to 8.0 with time on stream; a significant increase in methane production was also observed. The deactivation of Ni catalysts supported on pure alumina during the GSR reaction has also been reported by other research groups and has been attributed to carbon formation and sintering [68,69,70].
The difference in the catalytic behavior of the samples can be strongly affected by the nature of the carrier, suggesting that the activity and the rate of deactivation is likely to be related to the different extent of electronic interaction between supported metal and support, influencing the bonding and reactivity of the chemisorbed species. The presence of Ni2+/Ni0 and Ce4+/Ce3+ couples means that different species can participate in the activation of the glycerol molecules. According to Bazin et al. [71] an increase of the local electron density is expected with increased metal dispersion, as has been proven for the Ni/AlCeO3 catalyst; it exhibits a great number of active sites accessible to the reactant molecules compared to that observed for the Ni/Al2O3 catalyst.
2.4. Characterization of Used Catalysts
The nature of the carbonaceous deposits formed on to the spent catalytic samples following the time-on-stream experiments (experimental protocol #2) was examined using TPO, SEM, and TEM.
The TPO results for both catalysts are presented in Figure 10. As a first observation, significantly more carbon was deposited on to the Ni/Al2O3 catalyst (0.41 gcoke/gcatalyst) in comparison to the Ni/AlCeO3 sample (0.14 gcoke/gcatalyst). The Ni/Al2O3 catalyst shows a weak peak at 470 °C and two broad peaks at 550 °C and 650 °C, which indicate the existence of different co-existing carbon allotropes. For the Ni/AlCeO3 catalyst, there is a clear peak at ~375 °C, whereas a larger peak at 490 °C is observed; this points to the existence of coke that contains a higher fraction of defective carbon, which is known to be more easily oxidized. In addition, the high temperature decomposition peak appears as one single thermal event centred at 625 °C, i.e., slightly lower than the corresponding peak of the Ni/Al2O3 catalyst. It has been reported that the thermal decomposition of physisorbed carbonaceous products, termed as soft (or amorphous) coke, occurs at temperatures between 200 and 500 °C [39], while the gasification of hard coke (comprising of bulky carbonaceous species and often referred to as filamentous carbon) [72,73,74] takes place between 500 and 800 °C.
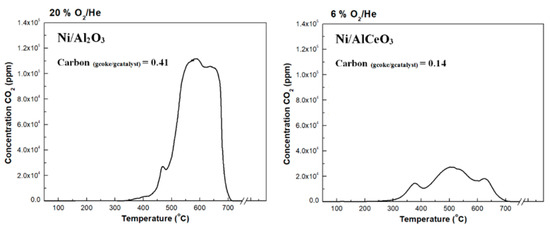
Figure 10.
Temperature Programmed Oxidation (TPO) profiles and total amount of deposited carbon obtained for the spent Ni/Al2O3 and Ni/AlCeO3 catalytic samples (samples tested under experimental protocol #2).
The hard coke species are 500–600 °C and can be associated with filamentous coke (carbon nanotubes, CNTs, and nanofibers, CNFs, that contain defects), whilst the coke gasified at over 600 °C can be associated with graphitic carbon species. This graphitic carbon is an inert coke that does not easily react with oxygen or steam [75]. Thus, not only was a higher quantity of coke deposited onto the spent Ni/Al2O3 catalyst, but also the fractions of graphitic carbon were greater and more difficult to oxidize than on the Ni/AlCeO3 catalyst. These results explain the excellent stability observed for the Ni/AlCeO3 catalyst. The different coke species on the catalyst surface can be formed by the Boudouard, methane decomposition and polymerization reactions [72,73]. Thus, the redox properties of CeO2 and a higher dispersion of the active phase for the Ni/AlCeO3 catalyst has resulted in markedly lower coke yields.
The morphology of the carbon deposits was initially examined using SEM/EDX; representative SEM micrographs and the carbon EDX images of the corresponding areas are shown in Figure 11. Although it is not straightforward to determine the precise nature of the coke deposits from this initial examination (i.e., whether these are nano-fibers, micro-fibers or carbon nanotubes), entangled tubular arrangements (filaments) can be discerned from the images. Also, the orientation of these filaments is quite random, which makes it difficult to estimate their length with any degree of accuracy. However, the EDX carbon images (red) show heavy coke deposition, particularly on the Ni/Al2O3 spent catalyst, in good agreement with the TPO results discussed above.
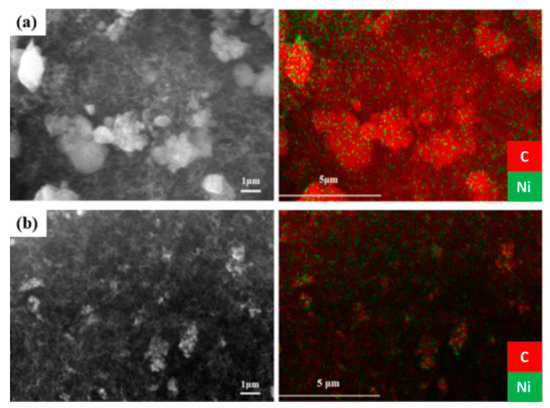
Figure 11.
SEM images and carbon EDX images of the spent catalysts: (a) Ni/Al2O3, and (b) Ni/AlCeO3 (samples tested under experimental protocol #2).
Figure 12 presents representative TEM images of the two spent catalytic samples and the corresponding particle size distribution histograms. The images confirm the formation of different carbon allotropes on the catalytic surface (indicated by black dashed circles), but also show heavy filament formation on the Ni/Al2O3 system, in excellent agreement with the discussion above. Encapsulating carbon can also be observed in the lower image for this system (examples of Ni particles are shown by red dashed circles), which is known to lead to a loss of activity as the Ni particle is no longer available during the reaction. Moreover, the particle size distribution histograms show that the mean Ni size of the Ni/AlCeO3 catalyst is substantially smaller than that of the Ni/Al2O3, helping to explain the improved activity and stability characteristics of the former sample.
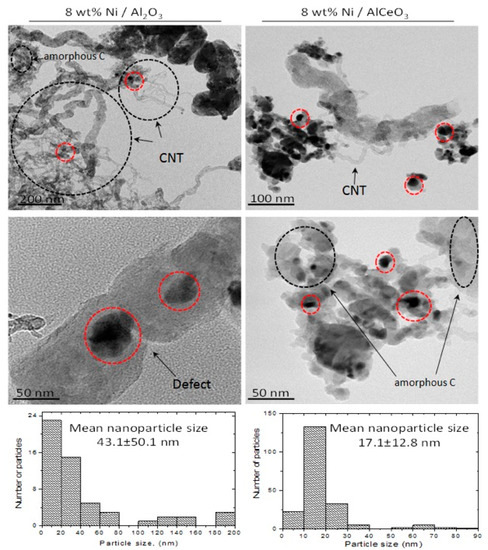
Figure 12.
TEM images and particle size distribution of the Ni/Al2O3 and Ni/AlCeO3 spent catalysts (samples tested under experimental protocol #2).
3. Materials and Methods
3.1. Catalyst Preparation
The alumina support (pellets) was acquired from Akzo, while the AlCeO3 (powder) was sourced from Sigma Aldrich. The pelletized support was crushed and sieved to particle sizes in the range 350–500 μm, while the powder was first pelletized and then crushed to the same size. The catalysts were produced via the wet impregnation technique using an aqueous solution of Ni(NO3)2 6H2O, obtained from Sigma Aldrich, with a concentration of 0.17 M to obtain catalysts with metal content of 8 wt %. The water contained in the slurries was evaporated under continuous stirring at 75 °C over 5 h. After, the suspensions were dried for 12 h at 120 °C and the calcination of the catalysts was carried out at 800 °C for 4 h.
3.2. Catalyst Characterization
For the determination of the catalysts’ surface and bulk properties, different characterization techniques were employed. These included the calculation/determination of: (a) the Point of Zero Charge (PZC) for the calcined supports via Potentiometric Mass Titrations (PMTs), (b) the total specific surface area (SSA) of the catalytic materials and corresponding supports via the Brunauer–Emmet–Teller (BET) method, (c) the pore size distribution (PSD) of the catalytic materials and corresponding supports using the Barrett, Joyner, and Halenda (BJH) method, (d) the total metal loading (wt %) of the calcined catalysts using Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP-AES), (e) the crystalline structure of the calcined supports, as well as the calcined and reduced catalysts by X-ray Diffraction (XRD) analysis, (f) the degree of Ni species reducibility using Temperature Programmed Reduction (H2-TPR), (g) the acid and basic properties of the catalysts via CO2- and NH3-TPD experiments, (h) the nature of the various surface species and their oxidation states, on the reduced catalysts, using X-ray Photoelectron Spectroscopy (XPS) analysis, (i) the morphological characteristics of the spent catalysts using Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM), and (j) the amount and nature of the carbonaceous deposits on the spent catalysts using Temperature Programmed Oxidation (TPO). The methodology and equipment used has been described in detail in previous publications by our group, and in particular: PZC in [26], XRD in [11], ICP at [76], and N2 adsorption-desorption, H2–TPR, CO2–TPD, NH3–TPD, XPS, SEM, TEM and TPO in [17,23].
3.3. Catalytic Tests
For the catalytic testing a continuous flow fixed-bed reactor was employed; the exact system and procedure has been described in detail in previous publications (e.g., [17,23]). Succinctly, using glycerol with 99.5% purity (Sigma-Aldrich, St. Louis, MO, USA) and a Weight Hourly Space Velocity (WHSV) of 50,000 mL g−1 h−1, two different experimental protocols were employed. For the first protocol, the gas feed at the inlet of the reactor was a gas mixture of 73% H2O, 4% C3H8O3 and 23% He (i.e., 20 v.v. % of C3H8O3 diluted in H2O) and the catalytic performance was investigated between 400–750 °C. The second protocol was used for the investigation of catalytic stability during time-on-stream and was carried out for 20 h at 600 °C. The conditions chosen were more severe and the gas mixture at the reactor’s inlet consisted of 63% H2O, 7% C3H8O3, 30% He (31° of C3H8O3 diluted in H2O). Before commencing the experiments, catalytic activation was undertaken in situ using a flow (100 mL min−1) of high purity H2 (5.0) at 800 °C for 1h. The system was then purged with He and the temperature lowered according to the protocol that was to be followed. The liquid products were analysed via a combination of Gas Chromatography (Agilent, Santa Clara, CA, USA, 7890A) and Mass Spectroscopy (Agilent 5975C). The gaseous products were determined via an Agilent gas chromatographer (7890A). Detailed information regarding the analysis of liquid and gaseous products can be found in Reference [11].
3.4. Reaction Metrics
The investigation of catalytic performance necessitated the calculation of total glycerol conversion, conversion of glycerol into gaseous products, and determination of the H2 yield, H2, CH4, CO2 and CO selectivity. For the calculations Equations (10) and (14) shown below were used. The selectivity of acetone [(CH3)2CO], acetaldehyde (C2H4O), acetol (C3H6O2), allyl alcohol (CH2=CHCH2OH), acrolein (C3H4O) and acetic acid (C2H4O) was calculated based on Equation (15):
where, RR is the reforming ratio (7/3), defined as the ratio of moles of H2 to CO2 formed.
where, species refers to CO, CO2 and CH4.
where, species refers to acetol, acetone, allyl alcohol, acetaldehyde, acrolein and acetic acid.
4. Conclusions
A critical assessment of the effect of the Ce-modification of Al2O3 on the catalytic performance of Ni/Al2O3 and Ni/AlCeO3 catalysts towards glycerol steam reforming reaction and H2 production has been performed. A thorough comparison of the AlCeO3, Al2O3 (supports) as well as Ni/Al2O3 and Ni/AlCeO3 (catalysts) has been presented.
The study has shown that Ce-modification of Al2O3 leads to a Ni catalyst with increased basicity (PZC and CO2–TPD studies) and a higher Ni dispersion (H2–TPR, XPS studies). The Ni/AlCeO3 sample was more selective towards CO2 and less selective towards CO. The opposite trend was observed for the Ni/Al2O3 catalyst. Importantly, both Ni/Al2O3 and Ni/AlCeO3 had low CH4 selectivity. For the Ni/AlCeO3 catalyst the CO/CO2 molar ratio was almost zero, while the H2/CO molar ratio value decreases (from 17 to 10) with increasing temperature (450 °C to 750 °C). Regarding the liquid products for the Ni/AlCeO3 and AlCeO3, allyl alcohol was found to be the main product (at least for high temperatures), with acetaldehyde and acetone the secondary ones. On the other hand, acetone was the main product at the high T range for the Ni/Al2O3 sample and acetic acid and acetol for pure alumina. Stability studies performed for over than 20 h on stream showed that the Ni/AlCeO3 catalyst experienced a slight decrease on glycerol total conversion (94–77%) and glycerol conversion to gaseous products (48–37%), but it maintained remarkably stable values for H2 yield (2.9–2.3) and selectivity (89–81%), as well as for the CO2 (75–67%) and CO (23–29%) selectivity. Characterization of the exhausted catalysts was performed in order to understand the nature of the deposited coke. It was found that Ni/AlCeO3 was far more resistant to coke, while at the same time, it favored the formation of more defective carbon compared to the coke deposited onto Ni/Al2O3.
Author Contributions
Data curation, N.D.C., K.P. and M.A.G.; Formal analysis, N.D.C., B.D., V.S., S.J.H., M.A.B., K.P. and M.A.G.; Funding acquisition, K.P. and M.A.G.; Investigation, N.D.C. and G.I.S.; Methodology, N.D.C. and M.A.G.; Supervision, N.D.C., K.P. and M.A.G.; Writing—original draft, N.D.C., M.A.B., K.P. and M.A.G.; Writing—review & editing, N.D.C., M.A.B., K.P. and M.A.G.
Funding
This research received no external funding.
Acknowledgments
MAG: NDC and GIS are grateful for financial support by the program THALIS implemented within the framework of Education and Lifelong Learning Operational Programme, co-financed by the Hellenic Ministry of Education, Lifelong Learning and Religious Affairs and the European Social Fund, Project Title: “Production of Energy Carriers from Biomass by Products: Glycerol Reforming for the Production of Hydrogen, Hydrocarbons and Superior Alcohols”. KP acknowledges the Abu Dhabi Department of Education and Knowledge (ADEK) through the Award for Research Excellence (AARE) 2017 and the Khalifa University through the RCII-2018-024 grant.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hosseinzadeh-Bandbafha, H.; Tabatabaei, M.; Aghbashlo, M.; Khanali, M.; Demirbas, A. A comprehensive review on the environmental impacts of diesel/biodisel additives. Energy Convers. Manag. 2018, 174, 579–614. [Google Scholar] [CrossRef]
- Boutesteijn, C.; Drabik, D.; Venus, T.J. The interaction between EU biofuel policy and first-and second-generation biodiesel production. Ind. Crop. Prod. 2017, 106, 124–129. [Google Scholar] [CrossRef]
- Gebremariam, S.N.; Marchetti, J.M. Economics of biodiesel production: Review. Energy Convers. Manag. 2018, 168, 74–84. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Papageridis, K.N.; Siakavelas, G.; Tzounis, L.; Goula, M.A. Effect of active metal supported on SiO2 for selective hydrogen production from the glycerol steam reforming reaction. Bioresources 2016, 11, 10173–10189. [Google Scholar] [CrossRef]
- Weger, L.; Abanades, A.; Butler, T. Methane cracking as a bridge technology to the hydrogen economy. Int. J. Hydrogen Energy 2017, 42, 720–731. [Google Scholar] [CrossRef]
- Callison, J.; Subramanian, N.D.; Rogers, S.M.; Chutia, A.; Gianolio, D.; Catlow, C.R.A.; Wells, P.P.; Dimitratos, N. Directed aqueous-phase reforming of glycerol through tailored platinum nanoparticles. Appl. Catal. B Environ. 2018, 238, 618–628. [Google Scholar] [CrossRef]
- Jiang, B.; Li, L.; Bian, Z.; Li, Z.; Sun, Y.; Sun, Z.; Tang, D.; Kawi, S.; Dou, B.; Goula, M.A. Chemical looping glycerol reforming for hydrogen production by Ni@ZrO2 nano-composite oxygen carriers. Int. J. Hydrogen Energy 2018, 43, 13200–13211. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Charisiou, N.D.; Papageridis, K.N.; Sebastian, V.; Hinder, S.J.; Dabbawala, A.; AlKhoori, A.A.; Baker, M.A.; Goula, M.A. The effect of Ni addition onto a Cu-based ternary support on the H2 production over glycerol steam reforming reaction. Nanomaterials 2018, 8, 931. [Google Scholar] [CrossRef]
- Goula, Μ.A.; Charisiou, N.D.; Pandis, P.K.; Stathopoulos, V.N. Ni/apatite-type lanthanum silicate supported catalyst for the glycerol steam reforming reaction. RSC Adv. 2016, 6, 78954–78958. [Google Scholar] [CrossRef]
- Bepari, S.; Pradhan, N.C.; Dalai, A.K. Selective production of hydrogen by steam reforming of glycerol over Ni/Fly ash catalyst. Catal. Today 2017, 291, 36–46. [Google Scholar] [CrossRef]
- Goula, Μ.A.; Charisiou, N.D.; Papageridis, K.N.; Siakavelas, G. Influence of the synthesis method parameters used to prepare nickel-based catalysts on the catalytic performance for the glycerol steam reforming reaction. Chin. J. Catal. 2016, 37, 1949–1965. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Charisiou, N.D.; Siakavelas, G.; Avraam, D.G.; Tzounis, L.; Kousi, K.; Goula, M.A. Comparative study of Ni, Co, Cu supported on γ-alumina catalysts for hydrogen production via the glycerol steam reforming reaction. Fuel Process. Technol. 2016, 152, 156–175. [Google Scholar] [CrossRef]
- Chen, H.; Ding, Y.; Cong, N.T.; Dou, B.; Dupont, V.; Ghadiri, M.; Williams, P.T. A comparative study on hydrogen production from steam-glycerol reforming: Thermodynamics and experimental. Renew. Energy 2011, 36, 779–788. [Google Scholar] [CrossRef]
- Silva, J.M.; Soria, M.A.; Madeira, L.M. Challenges and strategies for optimization of glycerol steam reforming process. Renew. Sustain. Energy Rev. 2015, 42, 1187–1213. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Papageridis, K.N.; Siakavelas, G.; Tzounis, L.; Kousi, K.; Baker, M.A.; Hinder, S.J.; Sebastian, V.; Polychronopoulou, K.; Goula, M.A. Glycerol steam reforming for hydrogen production over nickel supported on alumina, zirconia and silica catalysts. Top. Catal. 2017, 60, 1226–1250. [Google Scholar] [CrossRef]
- Dou, B.; Song, Y.; Wang, C.; Chen, H.; Xu, Y. Hydrogen production from catalytic steam reforming of biodiesel byproduct glycerol: Issues and challenges. Renew. Sustain. Energy Rev. 2014, 30, 950–960. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Polychronopoulou, K.; Goula, M.A. The influence of SiO2 doping on the Ni/ZrO2 supported catalyst for hydrogen production through the glycerol steam reforming reaction. Catal. Today 2019, 319, 206–219. [Google Scholar] [CrossRef]
- Aman, D.; Radwan, D.; Ebaid, M.; Mikhail, S.; van Steen, E. Comparing nickel and cobalt perovskites for steam reforming of glycerol. Mol. Catal. 2018, 452, 60–67. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Polychronopoulou, K.; Asif, A.; Goula, M.A. The potential of glycerol and phenol towards H2 production using steam reforming reaction: A review. Surf. Coat. Technol. 2018, 352, 92–111. [Google Scholar] [CrossRef]
- Senseni, A.Z.; Meshkani, F.; Rezaei, M. Steam reforming of glycerol on mesoporous nanocrystalline Ni/Al2O3 catalysts for H2 production. Int. J. Hydrogen Energy 2016, 41, 20137–20146. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Papageridis, K.N.; Delimitis, A.; Pachatouridou, E.; Iliopoulou, E.F. Nickel on alumina catalysts for the production of hydrogen rich mixtures via the biogas dry reforming reaction: Influence of the synthesis method. Int. J. Hydrogen Energy 2015, 40, 9183–9200. [Google Scholar] [CrossRef]
- da Menezes, S.Q.J.P.; Manfro, R.L.; Souza, M.M.V.M. Hydrogen production from glycerol steam reforming over nickel catalysts supported on alumina and niobia: Deactivation process, effect of reaction conditions and kinetic modeling. Int. J. Hydrogen Energy 2018, 43, 15064–15082. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Papageridis, K.N.; Tzounis, L.; Sebastian, V.; Baker, M.A.; Hinder, S.J.; AlKetbi, M.; Polychronopoulou, K.; Goula, M.A. Ni supported on CaO-MgO-Al2O3 as a highly selective and stable catalyst for H2 production via the glycerol steam reforming reaction. Int. J. Hydrogen Energy 2019, 44, 256–273. [Google Scholar] [CrossRef]
- Chiodo, V.; Freni, S.; Galvagno, A.; Mondello, N.; Frusteri, F. Catalytic features of Rh and Ni supported catalysts in the steam reforming of glycerol to produce hydrogen. Appl. Catal. A Gen. 2010, 381, 1–7. [Google Scholar] [CrossRef]
- Cheng, C.K.; Foo, S.Y.; Adesina, A.A. Steam reforming of glycerol over Ni/Al2O3 catalyst. Catal. Today 2011, 178, 25–33. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Polychronopoulou, K.; Goula, M.A. Hydrogen production via the glycerol steam reforming reaction over nickel supported on alumina and lanthana-alumina catalysts. Int. J. Hydrogen Energy 2017, 42, 13039–13060. [Google Scholar] [CrossRef]
- Yurdakul, M.; Ayas, N.; Bizkarra, K.; El Doukkali, M.; Cambra, J.F. Preparation of Ni-based catalysts to produce hydrogen from glycerol by steam reforming process. Int. J. Hydrogen Energy 2016, 41, 8084–8091. [Google Scholar] [CrossRef]
- Zamzuri, N.H.; Mat, R.; Amin, N.A.S.; Talebian-Kiakalaieh, A. Hydrogen production from catalytic steam reforming of glycerol over various supported nickel catalysts. Int. J. Hydrogen Energy 2017, 42, 9087–9098. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Avraam, D.G.; Goula, M.A. Syngas production via the biogas dry reforming reaction over nickel supported on modified with CeO2 and/or La2O3 alumina catalysts. J. Nat. Gas Sci. Eng. 2016, 31, 164–183. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrogen Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Demsash, H.D.; Mohan, R. Steam reforming of glycerol to hydrogen over ceria promoted nickel–alumina catalysts. Int. J. Hydrogen Energy 2016, 41, 22732–22742. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Katsoni, A.; Diamadopoulos, E.; Matzavinos, D.; Delimitis, A. Dry reforming of methane: Catalytic performance and stability of Ir catalysts supported on γ-Al2O3, Zr0.92Y0.08O2−δ (YSZ) or Ce0.9Gd0.1O2−δ (GDC) supports. Top. Catal. 2015, 58, 1228–1241. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Panagiotopoulou, P.; Kampouri, S.; Taylor, M.J.; Kyriakou, G.; Lambert, R.M. Stabilization of catalyst particles against sintering on oxide supports with high oxygen ion lability exemplified by Ir-catalyzed decomposition of N2O. Appl. Catal. B Environ. 2016, 192, 357–364. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in depth investigation of deactivation through carbon formation during the biogas dry reforming reaction for Ni supported on modified with CeO2 and La2O3 zirconia catalysts. Int. J. Hydrogen Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Iriondo, A.; Barrio, V.L.; Cambra, J.F.; Arias, P.L.; Guemez, M.B.; Sanchez-Sanchez, M.C.; Navarro, R.M.; Fierro, J.L.G. Glycerol steam reforming over Ni catalysts supported on ceria and ceria-promoted alumina. Int. J. Hydrogen Energy 2010, 35, 11622–11633. [Google Scholar] [CrossRef]
- Iriondo, A.; Barrio, V.L.; Cambra, J.F.; Arias, P.L.; Güemez, M.B.; Navarro, R.M.; Sanchez-Sanchez, M.C.; Fierro, J.L.G. Hydrogen production from glycerol over nickel catalysts supported on Al2O3 modified by Mg, Zr, Ce or La. Top. Catal. 2008, 49, 46–58. [Google Scholar] [CrossRef]
- Buffoni, I.N.; Pompeo, F.; Santori, G.F.; Nichio, N.N. Nickel catalysts applied in steam reforming of glycerol for hydrogen production. Catal. Commun. 2009, 10, 1656–1660. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Mastro, S.L.; Sodo, A. Ni/CeO2-Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Kamonsuangkasem, K.; Therdthianwong, S.; Therdthianwong, A.; Thammajak, N. Remarkable activity and stability of Ni catalyst supported on CeO2-Al2O3 via CeAlO3 perovskite towards glycerol steam reforming for hydrogen production. Appl. Catal. B Environ. 2017, 218, 650–663. [Google Scholar] [CrossRef]
- Bourikas, K.; Kordulis, C.; Lycourghiotis, A. Differential potentiometric titration: Development of a methodology for determining the point of zero charge of metal (hydro)oxides by one titration curve. Environ. Sci. Technol. 2005, 11, 4100–4108. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haull, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids; Academic Press: Marseille, France, 1999. [Google Scholar]
- Dou, B.; Wang, C.; Song, Y.; Chen, H.; Xu, Y. Activity of Ni–Cu–Al based catalyst for renewable hydrogen production from steam reforming of glycerol. Energy Convers. Manag. 2014, 78, 253–259. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Tzounis, L.; Sebastian, V.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Goula, M.A. Investigating the correlation between deactivation and the carbon deposited on the surface of Ni/Al2O3 and Ni/La2O3-Al2O3 catalysts during the biogas reforming reaction. Appl. Surf. Sci. 2019, 474, 42–56. [Google Scholar] [CrossRef]
- Aruna, S.T.; Kini, N.S.; Rajam, K.S. Solution combustion synthesis of CeO2–CeAlO3 nano-composites by mixture-of-fuels approach. Matter. Res. Bull. 2009, 44, 728–733. [Google Scholar] [CrossRef]
- Miranda, B.C.; Chimentao, R.J.; Santos, J.B.O.; Gispert-Guirado, F.; Llorca, J.; Medina, F.; Bonillo, F.L.; Sueiras, J.E. Conversion of glycerol over 10%Ni/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2014, 147, 464–480. [Google Scholar] [CrossRef]
- Lif, J.; Odenbrand, I.; Skoglundh, M. Sintering of alumina-supported nickel particles under amination conditions: Support effects. Appl. Catal. A Gen. 2007, 317, 62–69. [Google Scholar] [CrossRef]
- Guo, R.; Zhou, Y.; Pan, W.; Hong, J.; Zhen, W.; Jin, Q.; Ding, C.; Guo, S. Effect of preparation methods on the performance of CeO2/Al2O3 catalysts for selective catalytic reduction of NO with NH3. J. Ind. Eng. Chem. 2013, 19, 2022–2025. [Google Scholar] [CrossRef]
- Shen, Y.; Zhu, S.; Qiu, T.; Shen, S. A novel catalyst of CeO2/Al2O3 for selective catalytic reduction of NO by NH3. Catal. Commun. 2009, 11, 20–23. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Rane, V.H. Acidity/basicity of rare-earth oxides and their catalytic activity in oxidative coupling of methane to C2-hydrocarbons. J. Catal. 1991, 130, 411–422. [Google Scholar] [CrossRef]
- Sato, S.; Takahashi, R.; Kobune, M.; Gotoh, H. Basic properties of rare earth oxides. Appl. Catal. A Gen. 2009, 356, 57–63. [Google Scholar] [CrossRef]
- Li, G.; Hu, L.; Hill, J.M. Comparison of reducibility and stability of alumina-supported Ni catalysts prepared by impregnation and co-precipitation. Appl. Catal. A Gen. 2006, 301, 16–24. [Google Scholar] [CrossRef]
- Yang, L.; Pastor-Perz, L.; Gu, S.; Sepulveda-Escribano, A.; Reina, T.R. Highly efficient Ni/CeO2-Al2O3 catalysts for CO2 upgrading via reverse water-gas shift: Effect of selected transition metal promoters. Appl. Catal. B Environ. 2018, 232, 464–471. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Zedan, A.F.; Katsiotis, M.S.; Baker, M.A.; AlKhoori, A.A.; AlQaradawi, S.Y.; Hinder, S.J.; AlHassan, S. Rapid microwave assisted sol-gel synthesis of CeO2 and CexSm1−xO2 nanoparticle catalysts for CO oxidation. Mol. Catal. 2017, 428, 41–55. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf. Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Pfau, A.; Schierbaum, K.D. The electronic structure of stoichiometric and reduced CeO2 surfaces: An XPS, UPS and HREELS study. Surf. Sci. 1994, 321, 71–80. [Google Scholar] [CrossRef]
- Ardelean, H.; Frateur, I.; Marcus, P. Corrosion protection of magnesium alloys by cerium, zirconium and niobium-based coatings. Corros. Sci. 2008, 50, 1907–1918. [Google Scholar] [CrossRef]
- Henderson, M.A.; Perkins, C.L.; Engelhard, M.H.; Thevuthasan, A.; Peden, C.H.F. Redox properties of water on the oxidized and reduced surfaces of CeO2. Surf. Sci. 2003, 526, 1–18. [Google Scholar] [CrossRef]
- Polychronopoulou, K.; Charisiou, N.D.; Siakavelas, G.I.; Alkhoori, A.A.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Goula, M.A. Ce-Sm-x Cu cost-efficient catalysts for H2 production through the glycerol steam reforming reaction. Sustain. Energy Fuels 2019, 3, 673–691. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Palcheva, R.; Karakirova, Y.; Capel Sanchez, M.C.; Tyuliev, G.; Gaigneaux, E.; Fierro, J.L.G. Structure and surface properties of ceria-modified Ni-based catalysts for hydrogen production. Appl. Catal. B Environ. 2018, 225, 340–353. [Google Scholar] [CrossRef]
- Valliyappan, T.; Bakhshi, N.N.; Dalai, A.K. Pyrolysis of glycerol for the production of hydrogen or syngas. Bioresour. Technol. 2008, 99, 4476–4483. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. Pyrolysis-gasification of post-consumer municipal solid plastic waste for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 949–957. [Google Scholar] [CrossRef]
- Bobadilla, L.F.; Penkova, A.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A. Influence of the acid-base properties over NiSn/MgO-Al2O3 catalysts in the hydrogen production from glycerol steam reforming. Int. J. Hydrogen Energy 2014, 39, 5704–5712. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Chen, B.; Cao, Y. Thermodynamic analysis of hydrogen production via glycerol steam reforming with CO2 adsorption. Int. J. Hydrogen Energy 2010, 35, 7768–7777. [Google Scholar] [CrossRef]
- Sad, M.E.; Duarte, H.A.; Vignatti, C.; Padro, C.L.; Apesteguıa, C.L. Steam reforming of glycerol: Hydrogen production optimization. Int. J. Hydrogen Energy 2015, 40, 6097–6105. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, Z.; Wang, Y.; Liang, T.; Li, X.; Yang, Z.; Chen, M.; Wang, J. Effects of attapulgite-supported transition metals catalysts on glycerol steam reforming for hydrogen production. Int. J. Hydrogen Energ. 2018, 43, 20451–20464. [Google Scholar] [CrossRef]
- Stosic, D.; Bennici, S.; Sirotin, S.; Calais, C.; Couturier, J.-L.; Doubois, J.-L.; Travert, A.; Auroux, A. Glycerol dehydration over calcium phosphate catalysts: Effect of acidic–basic features on catalytic performance. Appl. Catal. A Gen. 2012, 447–448, 124–134. [Google Scholar] [CrossRef]
- Sanchez, E.A.; Comelli, R.A. Hydrogen production by glycerol steam-reforming over nickel and nickel-cobalt impregnated on alumina. Int. J. Hydrogen Energy 2014, 39, 8650–8655. [Google Scholar] [CrossRef]
- Dieuzeide, M.L.; Jobbagy, M.; Amadeo, N. Glycerol steam reforming over Ni/γ-Al2O3 catalysts, modified with Mg (II). Effect of Mg (II) content. Catal. Today 2013, 213, 50–57. [Google Scholar] [CrossRef]
- Iriondo, A.; Barrio, V.L.; El Doukkali, M.; Cambra, J.F.; Guemez, M.B.; Requies, J.; Arias, P.L.; Sachez-Sanchez, M.C.; Navarro, R.M.; Fiero, J.L.G. Biohydrogen production by gas phase reforming of glycerine and ethanol mixtures. Int. J. Hydrogen Energy 2012, 37, 2028–2036. [Google Scholar] [CrossRef]
- Bazin, D.; Sayers, D.; Rehr, J.J.; Matteri, C. Numerical Simulation of the Platinum LIII Edge White Line Relative to Nanometer Scale Clusters. J. Phys. Chem. B 1997, 101, 5332–5336. [Google Scholar] [CrossRef]
- Srisiriwat, N.; Therdthianwong, S.; Therdthianwong, A. Oxidative steam reforming of ethanol over Ni/Al2O3 catalysts promoted by CeO2, ZrO2 and CeO2–ZrO2. Int. J. Hydrogen Energy 2009, 34, 2224–2234. [Google Scholar] [CrossRef]
- Zhang, H.; Shao, S.; Xiao, R.; Shen, D.; Zeng, J. Characterization of coke deposition in the catalytic fast pyrolysis of biomass derivates. Energy Fuels 2014, 28, 52–57. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Ray, S.S.; Singh, I.D. Structural characterization of coke on spent hydroprocessing catalysts used for processing of vacuum gas oils. Appl. Catal. A Gen. 2004, 278, 83–91. [Google Scholar] [CrossRef]
- Koo, K.Y.; Lee, S.; Jung, U.H.; Roh, H.; Yoon, W.L. Syngas production via combined steam and carbon dioxide reforming of methane over Ni–Ce/MgAl2O4 catalysts with enhanced coke resistance. Fuel Process. Technol. 2014, 119, 151–157. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Baklavaridis, A.; Papadakis, V.G.; Goula, M.A. Synthesis gas production via the biogas reforming reaction over Ni/MgO-Al2O3 and Ni/CaO-Al2O3 catalysts. Waste Biomass Valorization 2016, 7, 725–736. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).