Ultra-Small Pd Nanoparticles on Ceria as an Advanced Catalyst for CO Oxidation
Abstract
1. Introduction
2. Results and Discussion
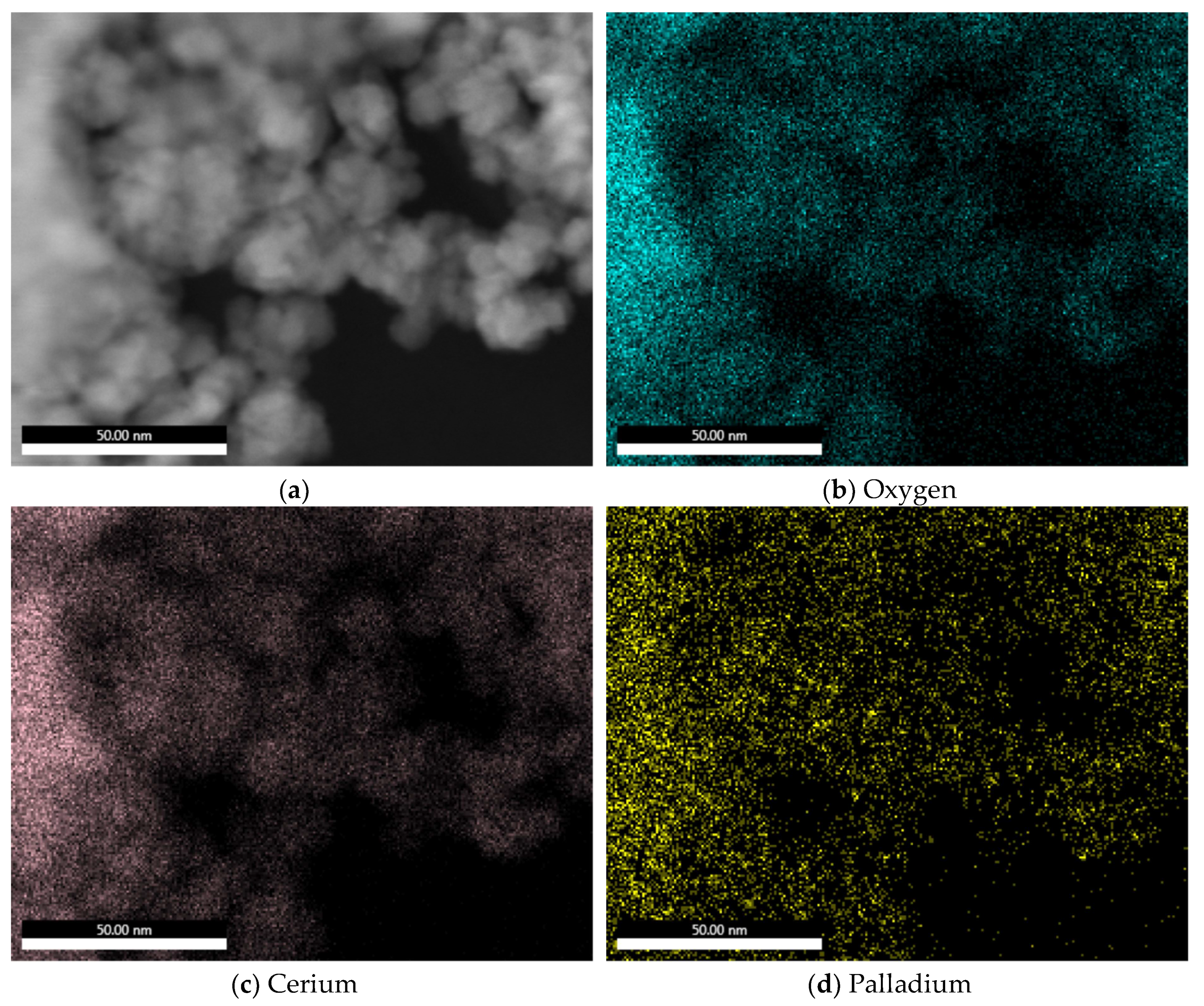
2.1. Elemental Composition
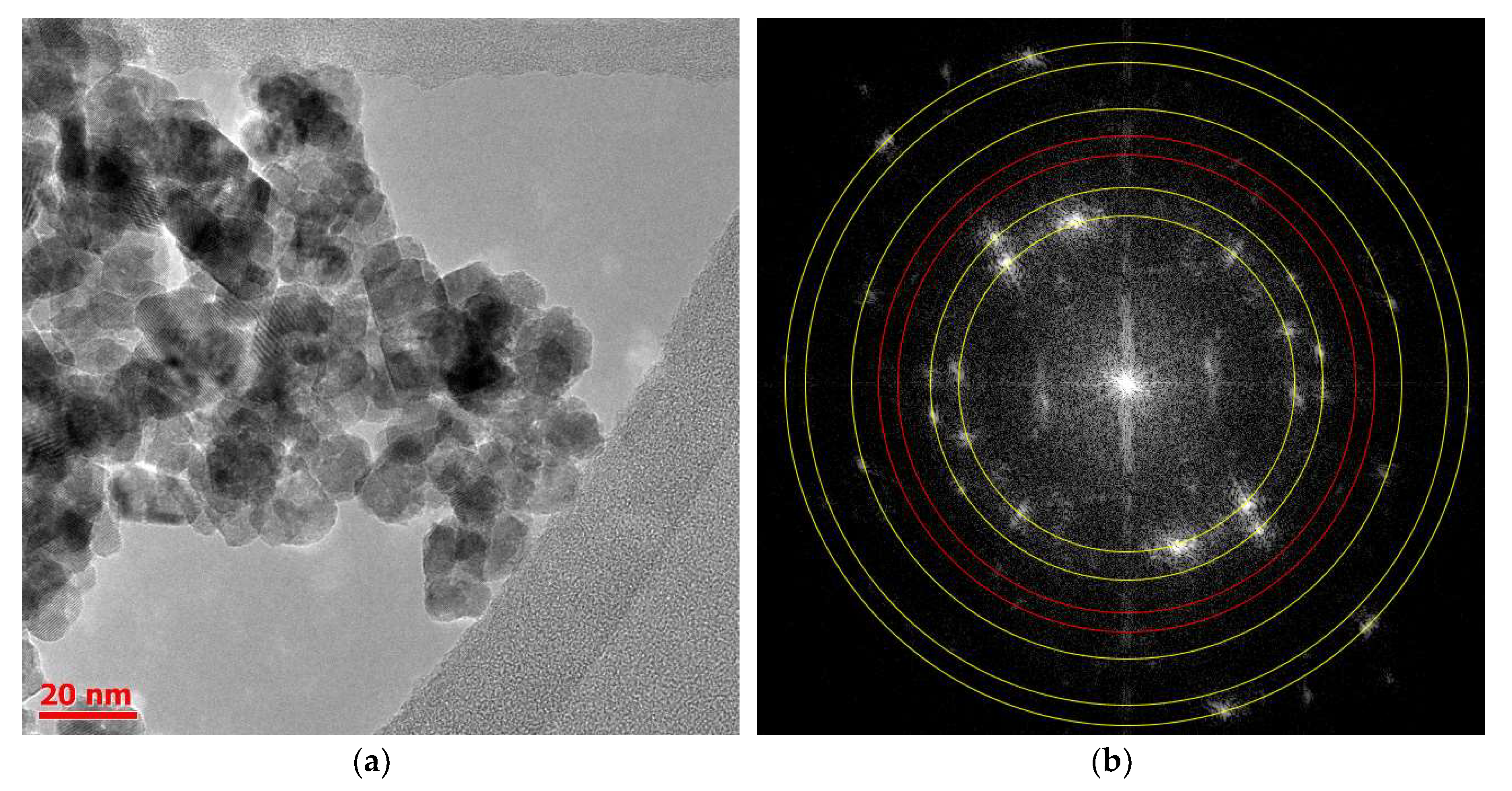
2.2. Structure and Size
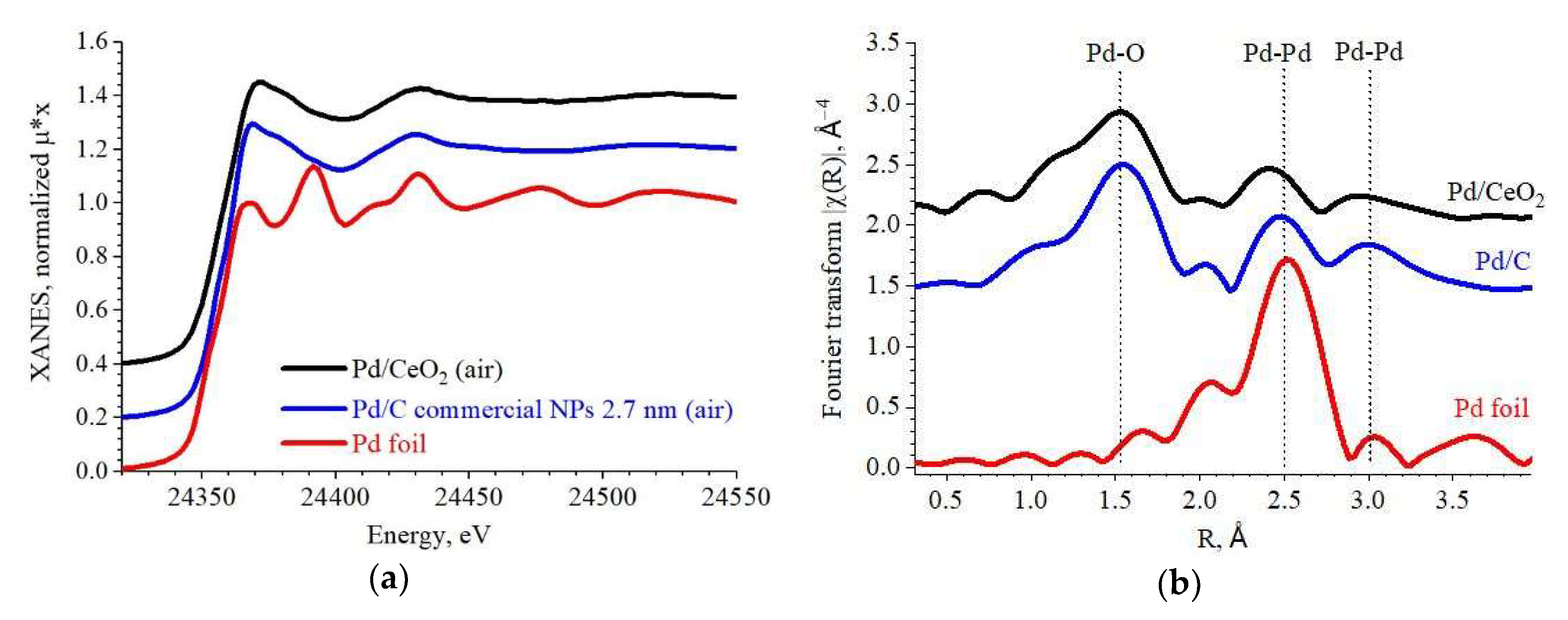
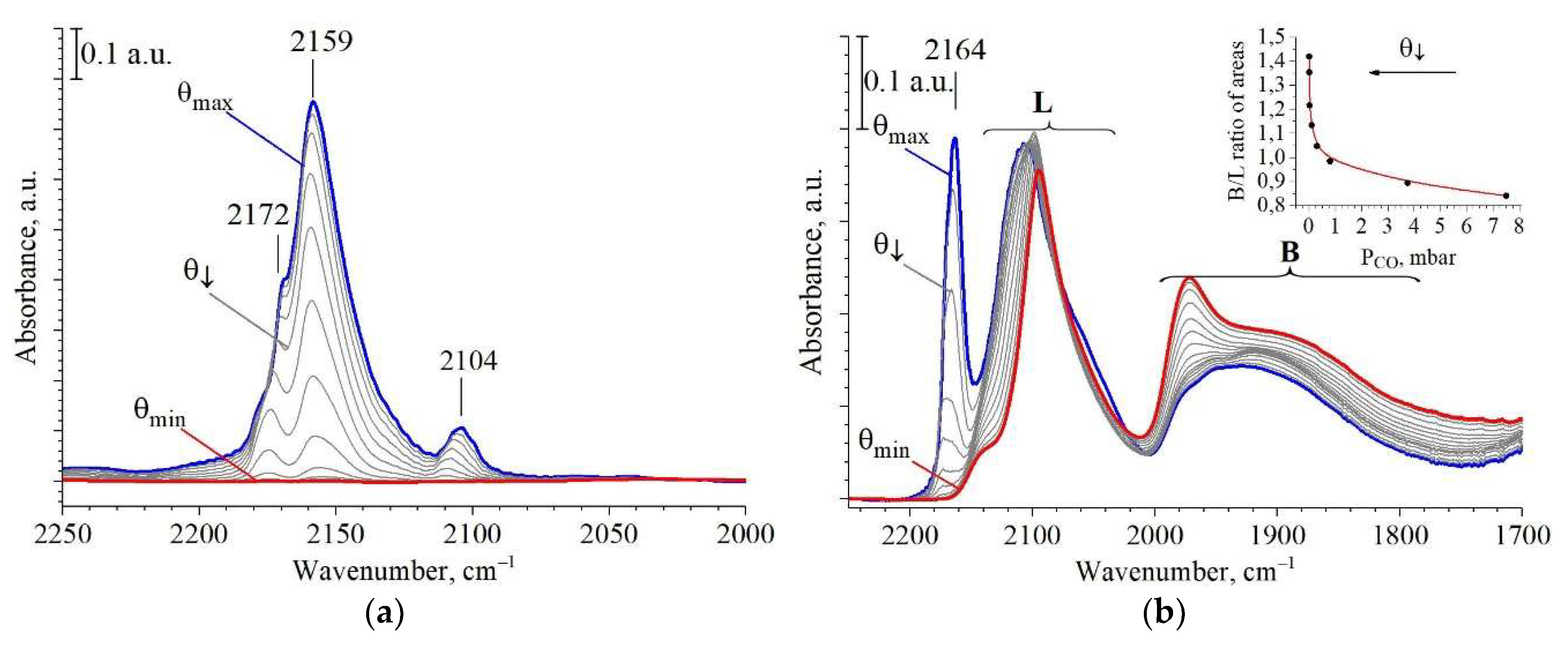
2.3. Active Sites Characterization
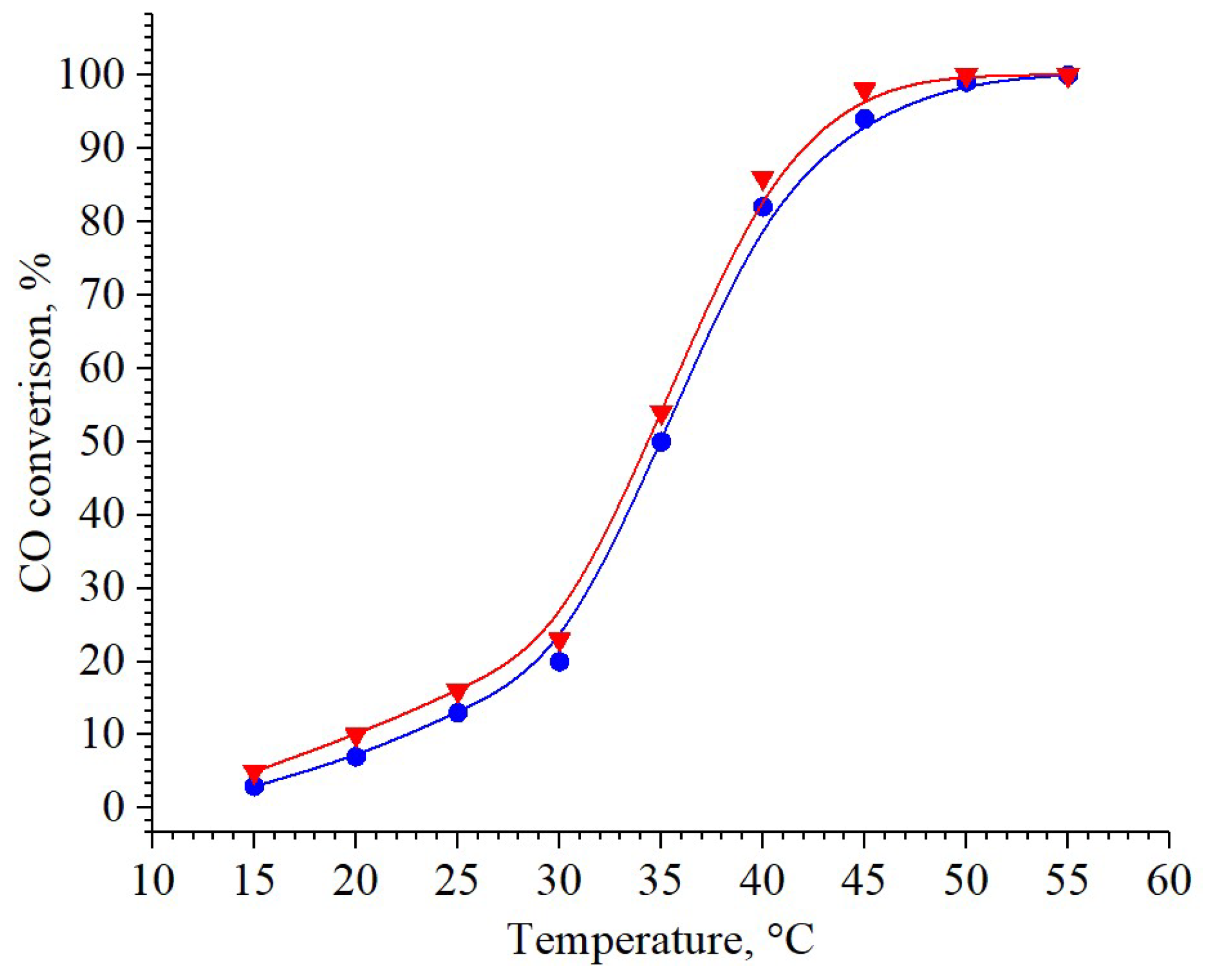
2.4. Catalytic Activity
3. Materials and Methods
3.1. Materials
3.1.1. Synthesis of a Modified Support
3.1.2. Synthesis of Supported Pd NPs
3.2. Methods
Catalytic Activity Tests
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
References
- Tang, Z.; Edwards, J.; Bartley, J.; Taylor, S.; Carley, A.; Herzing, A.; Kiely, C.; Hutchings, G. Nanocrystalline cerium oxide produced by supercritical antisolvent precipitation as a support for high-activity gold catalysts. J. Catal. 2007, 249, 208–219. [Google Scholar] [CrossRef]
- Yang, Y.; Saoud, K.M.; Abdelsayed, V.; Glaspell, G.; Deevi, S.; El-Shall, M.S. Vapor phase synthesis of supported Pd, Au, and unsupported bimetallic nanoparticle catalysts for CO oxidation. Catal. Commun. 2006, 7, 281–284. [Google Scholar] [CrossRef]
- Glaspell, G.; Fuoco, L.; El-Shall, M.S. Microwave synthesis of supported Au and Pd nanoparticle catalysts for CO oxidation. J. Phys. Chem. B 2005, 109, 17350–17355. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Si, Z.; Cao, Y.; Ran, R.; Wu, X.; Weng, D. Localized surface plasmon resonance assisted photothermal catalysis of CO and toluene oxidation over Pd–CeO2 catalyst under visible light irradiation. J. Phys. Chem. C 2016, 120, 29116–29125. [Google Scholar] [CrossRef]
- Jeong, H.; Bae, J.; Han, J.W.; Lee, H. Promoting effects of hydrothermal treatment on the activity and durability of Pd/CeO2 catalysts for CO oxidation. ACS Catal. 2017, 7, 7097–7105. [Google Scholar] [CrossRef]
- Slavinskaya, E.M.; Gulyaev, R.V.; Zadesenets, A.V.; Stonkus, O.A.; Zaikovskii, V.I.; Shubin, Y.V.; Korenev, S.V.; Boronin, A.I. Low-temperature CO oxidation by Pd/CeO2 catalysts synthesized using the coprecipitation method. Appl. Catal. B Environ. 2015, 166, 91–103. [Google Scholar] [CrossRef]
- Spezzati, G.; Benavidez, A.D.; DeLaRiva, A.T.; Su, Y.; Hofmann, J.P.; Asahina, S.; Olivier, E.J.; Neethling, J.H.; Miller, J.T.; Datye, A.K.; et al. CO oxidation by Pd supported on CeO2(100) and CeO2(111) facets. Appl. Catal. B Environ. 2019, 243, 36–46. [Google Scholar] [CrossRef]
- Zhu, H.; Qin, Z.; Shan, W.; Shen, W.; Wang, J. Low-temperature oxidation of CO over Pd/CeO2–TiO2 catalysts with different pretreatments. J. Catal. 2005, 233, 41–50. [Google Scholar] [CrossRef]
- Kinoshita, K.J. Particle size effects for oxygen reduction on highly dispersed platinum in acid electrolytes. J. Electrochem. Soc. 1990, 137, 845–848. [Google Scholar] [CrossRef]
- Rojluechai, S.; Chavadej, S.; Schwank, J.W.; Meeyoo, V. Catalytic activity of ethylene oxidation over Au, Ag and Au–Ag catalysts: Support effect. Catal. Commun. 2007, 8, 57–64. [Google Scholar] [CrossRef]
- Buil, M.L.; Esteruelas, M.A.; Niembro, S.; Oliván, M.; Orzechowski, L.; Pelayo, C.; Vallribera, A. Dehalogenation and hydrogenation of aromatic compounds catalyzed by nanoparticles generated from rhodium bis(imino)pyridine complexes. Organometallics 2010, 29, 4375–4383. [Google Scholar] [CrossRef]
- Shim, J.; Yoo, D.-Y.; Lee, J.-S. Characteristics for electrocatalytic properties and hydrogen–oxygen adsorption of platinum ternary alloy catalysts in polymer electrolyte fuel cell. Electrochim. Acta 2000, 45, 1943–1951. [Google Scholar] [CrossRef]
- Nie, L.; Mei, D.; Xiong, H.; Peng, B.; Ren, Z.; Hernandez, X.I.P.; DeLaRiva, A.; Wang, M.; Engelhard, M.H.; Kovarik, L.; et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 2017, 358, 1419–1423. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, C.; Yang, Q. Accelerated catalytic activity of Pd NPs supported on amine-rich silica hollow nanospheres for quinoline hydrogenation. Catal. Sci. Technol. 2017, 7, 2221–2227. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Xia, C.; Li, F. Nitrogen-functionalized ordered mesoporous carbons as multifunctional supports of ultrasmall Pd nanoparticles for hydrogenation of phenol. ACS Catal. 2013, 3, 2440–2448. [Google Scholar] [CrossRef]
- Wong, A.; Liu, Q.; Griffin, S.; Nicholls, A.; Regalbuto, J.R. Synthesis of ultrasmall, homogeneously alloyed, bimetallic nanoparticles on silica supports. Science 2017, 358, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zou, L.; Li, Y. Facile synthesis of hollow structured mesoporous silica nanoreactors with confined ultra-small Pd NPs for efficient hydrogenation reactions. J. Porous Mater. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Chaudhary, S.; Sharma, P.; Singh, D.; Umar, A.; Kumar, R. Chemical and pathogenic cleanup of wastewater using surface-functionalized CeO2 nanoparticles. ACS Sustain. Chem. Eng. 2017, 5, 6803–6816. [Google Scholar] [CrossRef]
- Xu, J.; Li, L.; Li, G. A facile approach to well-dispersible CeO2 nanoparticles. J. Dispers. Sci. Technol. 2008, 29, 1072–1076. [Google Scholar] [CrossRef]
- Jorge, A.B.; Sakatani, Y.; Boissière, C.; Laberty-Roberts, C.; Sauthier, G.; Fraxedas, J.; Sanchez, C.; Fuertes, A. Nanocrystalline N-doped ceria porous thin films as efficient visible-active photocatalysts. J. Mater. Chem. 2012, 22, 3220. [Google Scholar] [CrossRef]
- Safonova, O.V.; Guda, A.A.; Paun, C.; Smolentsev, N.; Abdala, P.M.; Smolentsev, G.; Nachtegaal, M.; Szlachetko, J.; Soldatov, M.A.; Soldatov, A.V.; et al. Electronic and geometric structure of Ce3+ forming under reducing conditions in shaped ceria nanoparticles promoted by platinum. J. Phys. Chem. C 2014, 118, 1974–1982. [Google Scholar] [CrossRef]
- Groppo, E.; Bertarione, S.; Rotunno, F.; Agostini, G.; Scarano, D.; Pellegrini, R.; Leofanti, G.; Zecchina, A.; Lamberti, C. Role of the support in determining the vibrational properties of carbonyls formed on Pd supported on SiO2−Al2O3, Al2O3, and MgO. J. Phys. Chem. C 2007, 111, 7021–7028. [Google Scholar] [CrossRef]
- Lamberti, C.; Zecchina, A.; Groppo, E.; Bordiga, S. Probing the surfaces of heterogeneous catalysts by in situ ir spectroscopy. Chem. Soc. Rev. 2010, 39, 4951–5001. [Google Scholar] [CrossRef] [PubMed]
- Abbott, H.L.; Aumer, A.; Lei, Y.; Asokan, C.; Meyer, R.J.; Sterrer, M.; Shaikhutdinov, S.; Freund, H.J. CO adsorption on monometallic and bimetallic Au−Pd nanoparticles supported on oxide thin films. J. Phys. Chem. C 2010, 114, 17099–17104. [Google Scholar] [CrossRef]
- Baylet, A.; Marécot, P.; Duprez, D.; Castellazzi, P.; Groppi, G.; Forzatti, P. In situ raman and in situ XRD analysis of Pdo reduction and Pd° oxidation supported on γ-Al2O3 catalyst under different atmospheres. Phys. Chem. Chem. Phys. 2011, 13, 4607–4613. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lee, J.Y. Particle size effects in Pd-catalyzed electrooxidation of formic acid. J. Phys. Chem. C 2008, 112, 3789–3793. [Google Scholar] [CrossRef]
- Xia, Y.; Ye, J.; Cheng, D.-G.; Chen, F.; Zhan, X. Identification of a flattened pd–ce oxide cluster as a highly efficient catalyst for low-temperature Co oxidation. Catal. Sci. Technol. 2018, 8, 5137–5147. [Google Scholar] [CrossRef]
- Cargnello, M.; Montini, T.; Polizzi, S.; Wieder, N.L.; Gorte, R.J.; Graziani, M.; Fornasiero, P. Novel embedded Pd@CeO2 catalysts: A way to active and stable catalysts. Dalton Trans. 2010, 39, 2122–2127. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to imagej: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Agostini, G.; Lamberti, C.; Pellegrini, R.; Leofanti, G.; Giannici, F.; Longo, A.; Groppo, E. Effect of pre-reduction on the properties and the catalytic activity of Pd/carbon catalysts: A comparison with Pd/Al2O3. ACS Catal. 2014, 4, 187–194. [Google Scholar] [CrossRef]
- Qi, W.; Huang, B.; Wang, M. Structure of unsupported small palladium nanoparticles. Nanoscale Res. Lett. 2009, 4, 269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Binet, C.; Daturi, M.; Lavalley, J.-C. Ir study of polycrystalline ceria properties in oxidised and reduced states. Catal. Today 1999, 50, 207–225. [Google Scholar] [CrossRef]
- Zaki, M.I.; Knözlngem, H. Characterization of oxide surfaces by adsorption of carbon monoxide—a low temperature infrared spectroscopy study. Spectrochim. Acta Part A Mol. Spectrosc. 1987, 43, 1455–1459. [Google Scholar] [CrossRef]
- Badri, A.; Binet, C.; Lavalley, J.-C. Metal–support interaction in Pd/CeO2 catalysts. Part 2.—ceria textural effects. J. Chem. Soc. Faraday Trans. 1996, 92, 1603–1608. [Google Scholar] [CrossRef]
- Bozon-Verduraz, F.; Bensalem, A. Ir studies of cerium dioxide: Influence of impurities and defects. J. Chem. Soc. Faraday Trans. 1994, 90, 653–657. [Google Scholar] [CrossRef]
- Li, C.; Sakata, Y.; Arai, T.; Domen, K.; Maruya, K.-I.; Onishi, T. Carbon monoxide and carbon dioxide adsorption on cerium oxide studied by fourier-transform infrared spectroscopy. Part 1.—formation of carbonate species on dehydroxylated CeO2, at room temperature. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 929–943. [Google Scholar] [CrossRef]
- Mudiyanselage, K.; Kim, H.Y.; Senanayake, S.D.; Baber, A.E.; Liu, P.; Stacchiola, D. Probing adsorption sites for CO on ceria. Phys. Chem. Chem. Phys. 2013, 15, 15856–15862. [Google Scholar] [CrossRef]
- Chen, S.; Cao, T.; Gao, Y.; Li, D.; Xiong, F.; Huang, W. Probing surface structures of CeO2, TiO2, and Cu2O nanocrystals with CO and CO2 chemisorption. J. Phys. Chem. C 2016, 120, 21472–21485. [Google Scholar] [CrossRef]
- Bensalem, A.; Bozon-Verduraz, F.; Perrichon, V. Palladium–ceria catalysts: Reversibility of hydrogen chemisorption and redox phenomena. J. Chem. Soc. Faraday Trans. 1995, 91, 2185–2189. [Google Scholar] [CrossRef]
- Padilla, J.M.; Del Angel, G.; Navarrete, J. Improved Pd/γ-Al2O3–Ce catalysts for benzene combustion. Catal. Today 2008, 133, 541–547. [Google Scholar] [CrossRef]
- Di Gregorio, F.; Bisson, L.; Armaroli, T.; Verdon, C.; Lemaitre, L.; Thomazeau, C. Characterization of well faceted palladium nanoparticles supported on alumina by transmission electron microscopy and ft-ir spectroscopy of CO adsorption. Appl. Catal. A Gen. 2009, 352, 50–60. [Google Scholar] [CrossRef]
- Yudanov, I.V.; Sahnoun, R.; Neyman, K.M.; Rösch, N.; Hoffmann, J.; Schauermann, S.; Johánek, V.; Unterhalt, H.; Rupprechter, G.; Libuda, J.; et al. CO adsorption on Pd nanoparticles: Density functional and vibrational spectroscopy studies. J. Phys. Chem. B 2003, 107, 255–264. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Guan, N.; Li, L. Supported Pd catalysts for solvent-free benzyl alcohol selective oxidation: Effects of calcination pretreatments and reconstruction of Pd sites. Appl. Catal. B Environ. 2012, 115, 7–15. [Google Scholar] [CrossRef]
- Baidya, T.; Bera, P.; Mukri, B.; Kumar Parida, S.; Kröcher, O.; Elsener, M.; Hegde, M.S. Drifts studies on CO and NO adsorption and NO plus CO reaction over Pd2+-substituted CeOSS and Ce0.75Sn0.25O2 catalysts. J. Catal. 2013, 303, 117–129. [Google Scholar] [CrossRef]
- Spezzati, G.; Su, Y.; Hofmann, J.P.; Benavidez, A.D.; DeLaRiva, A.T.; McCabe, J.; Datye, A.K.; Hensen, E.J.M. Atomically dispersed Pd–O species on CeO2(111) as highly active sites for low-temperature CO oxidation. ACS Catal. 2017, 7, 6887–6891. [Google Scholar] [CrossRef] [PubMed]
- Sheu, L.; Karpinski, Z.; Sachtler, W.M.H. Effects of palladium particle size and palladium silicide formation on fourier transform infrared spectra of CO adsorbed on Pd/SiO2 catalysts. J. Phys. Chem. (USA) 1989, 93, 4890–4894. [Google Scholar] [CrossRef]
- Fan, Q.; He, S.; Hao, L.; Liu, X.; Zhu, Y.; Xu, S.; Zhang, F. Photodeposited Pd nanoparticles with disordered structure for phenylacetylene semihydrogenation. Sci. Rep. 2017, 7, 42172. [Google Scholar] [CrossRef]
- Fu, Q.; Weber, A.; Flytzani-Stephanopoulos, M. Nanostructured Au–CeO2 catalysts for low-temperature water–gas shift. Catal. Lett. 2001, 77, 87–95. [Google Scholar] [CrossRef]
- Iglesias-Juez, A.; Hungría, A.B.; Martínez-Arias, A.; Anderson, J.A.; Fernández-García, M. Pd-based (Ce,Zr)ox-supported catalysts: Promoting effect of base metals (Cr, Cu, Ni) in CO and NO elimination. Catal. Today 2009, 143, 195–202. [Google Scholar] [CrossRef]
- Qi, J.; Chen, J.; Li, G.; Li, S.; Gao, Y.; Tang, Z. Facile synthesis of core–shell Au@CeO2 nanocomposites with remarkably enhanced catalytic activity for CO Oxidation. Energy Environ. Sci. 2012, 5, 8937. [Google Scholar] [CrossRef]
- Cargnello, M.; Doan-Nguyen, V.V.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 2013, 341, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Benmouhoub, C.; Kadri, A.; Benbrahim, N.; Hadji, S. Synthesis and characterization of cerium Oxide (CeO2) nanoparticles. Mater. Sci. Forum 2009, 609, 189–194. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic computing system jana2006: General features. Zeitschrift für Kristallographie-Crystalline Materials 2014, 229, 345. [Google Scholar] [CrossRef]
- Chernyshov, A.A.; Veligzhanin, A.; Zubavichus, Y. Structural materials science end-station at the kurchatov synchrotron radiation source: Recent instrumentation upgrades and experimental results. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2009, 603, 95–98. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. Data analysis for X-ray absorption spectroscopy using ifeffit. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef]
| Catalyst | Max. Achieved CO Conversion, % | Temperature of max. CO Conversion, °C | Reference |
|---|---|---|---|
| 5 wt% Pd/CeO2 | 100 | 108 | [2] |
| 5 wt% Pd/ZrO2 | 98 | 150 | |
| 5 wt% Pd/SiO2 | 92 | 240 | |
| 5 wt% Pd/CeO2 | 100 | 151 | [3] |
| 10 wt% Pd/CeO2 | 100 | 173 | |
| 5 wt% Pd/ZnO | 98 | 200 | |
| 10 wt% Pd/ZnO | 99 | 194 | |
| 5 wt% Pd/CuO | 98 | 186 | |
| 10 wt% Pd/CuO | 97 | 192 | |
| 0.7% Pd-CeO2/Al2O3 (PCA-R) | 100 | 180 | [4] |
| 2 wt% Pd/CeO2 | 100 | 125 | [5] |
| 2 wt% Pd/CeO2 (T) | 143 | ||
| 2 wt% Pd/CeO2 (HT) | 75 | ||
| 2 wt% Pd/CeO2 (H2O) | 105 | ||
| 0.74 wt% Pd/CeO2 (450 °C) | 100 | 190 | [6] |
| 0.74 wt% Pd/CeO2 (600 °C) | 190 | ||
| 0.74 wt% Pd/CeO2 (800 °C) | 190 | ||
| 3.8 wt% Pd/CeO2 (450 °C) | 145 | ||
| 3.8 wt% Pd/CeO2 (600 °C) | 90 | ||
| 3.8 wt% Pd/CeO2 (800 °C) | 175 | ||
| 7.7 wt% Pd/CeO2 (450 °C) | 115 | ||
| 7.7 wt% Pd/CeO2 (600 °C) | 70 | ||
| 7.7 wt% Pd/CeO2 (800 °C) | 170 | ||
| 1 wt% Pd/CeO2-rod | 100 | 150 | [7] |
| 1 wt% Pd/CeO2-cube | 200 | ||
| 5 wt% Pd/SiO2 | 60 | 300 | |
| 1 wt% Pd/CeO2 | 100 | 204 | [8] |
| 1 wt% Pd/TiO2 | 120 | ||
| 1 wt% Pd/CeO2–TiO2 | 97 | ||
| 1 wt% Pd/CeO2 (LTR) | 97 | ||
| 1 wt% Pd/TiO2 (LTR) | 70 | ||
| 1 wt% Pd/CeO2–TiO2 (LTR) | 54 | ||
| 3 wt% Pd/CeO2 | 100 | 50 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tereshchenko, A.; Polyakov, V.; Guda, A.; Lastovina, T.; Pimonova, Y.; Bulgakov, A.; Tarasov, A.; Kustov, L.; Butova, V.; Trigub, A.; et al. Ultra-Small Pd Nanoparticles on Ceria as an Advanced Catalyst for CO Oxidation. Catalysts 2019, 9, 385. https://doi.org/10.3390/catal9040385
Tereshchenko A, Polyakov V, Guda A, Lastovina T, Pimonova Y, Bulgakov A, Tarasov A, Kustov L, Butova V, Trigub A, et al. Ultra-Small Pd Nanoparticles on Ceria as an Advanced Catalyst for CO Oxidation. Catalysts. 2019; 9(4):385. https://doi.org/10.3390/catal9040385
Chicago/Turabian StyleTereshchenko, Andrei, Vladimir Polyakov, Alexander Guda, Tatiana Lastovina, Yulia Pimonova, Alexey Bulgakov, Andrey Tarasov, Leonid Kustov, Vera Butova, Alexander Trigub, and et al. 2019. "Ultra-Small Pd Nanoparticles on Ceria as an Advanced Catalyst for CO Oxidation" Catalysts 9, no. 4: 385. https://doi.org/10.3390/catal9040385
APA StyleTereshchenko, A., Polyakov, V., Guda, A., Lastovina, T., Pimonova, Y., Bulgakov, A., Tarasov, A., Kustov, L., Butova, V., Trigub, A., & Soldatov, A. (2019). Ultra-Small Pd Nanoparticles on Ceria as an Advanced Catalyst for CO Oxidation. Catalysts, 9(4), 385. https://doi.org/10.3390/catal9040385








