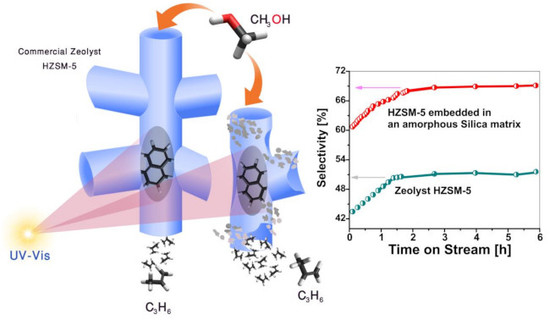
H-ZSM-5 Materials Embedded in an Amorphous Silica Matrix: Highly Selective Catalysts for Propylene in Methanol-to-Olefin Process
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure, Morphology, and Porosity of Synthesized Materials
2.2. Acidity Measurnments
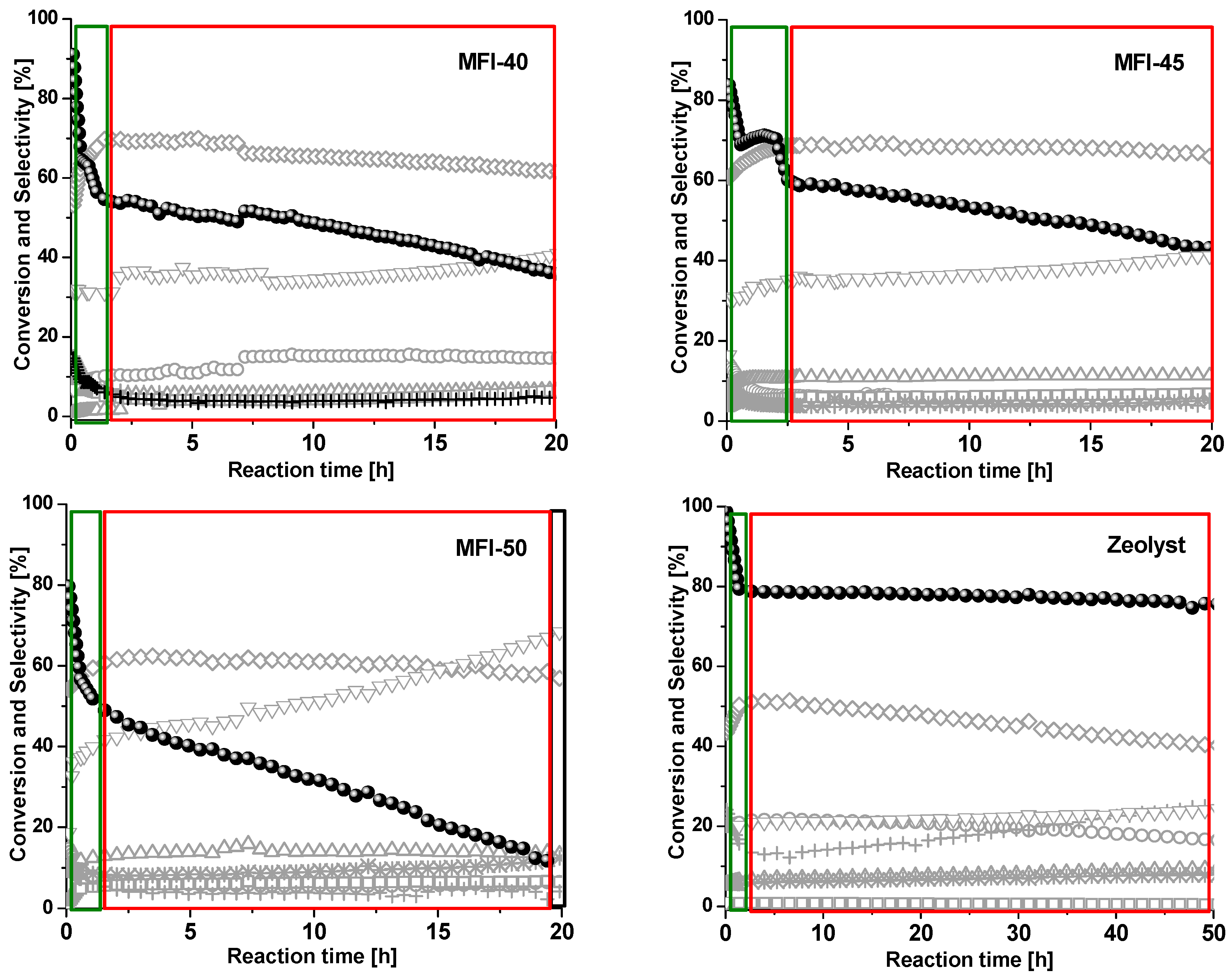
2.3. Catalytic Performance in the MTO Reaction
3. Experimental
3.1. Chemicals and Materials
3.2. Catalyst Preparation
3.3. Characterization of Materials
3.4. Catalytic MTO Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mei, C.; Wen, P.; Liu, Z.; Liu, H.; Wang, Y.; Yang, W.; Xie, Z.; Hua, W.; Gao, Z. Selective production of propylene from methanol: Mesoporosity development in high silica HZSM-5. J. Catal. 2008, 258, 243–249. [Google Scholar] [CrossRef]
- Corma, A.; Mengual, J.; Miguel, P. IM-5 zeolite for steam catalytic cracking of naptha to produce propene and ethane. An alternative to ZSM-5 zeolite. J. Appl. Catal. A Gen. 2013, 460–461, 106–115. [Google Scholar] [CrossRef]
- Meng, T.; Mao, D.; Guo, Q.; Lu, G. The effect of crystal sizes of HZSM-5 zeolites in ethanol conversion to propylene. Catal. Commun. 2012, 21, 52–57. [Google Scholar] [CrossRef]
- Yang, G.; Wei, Y.; Xu, S.; Chen, J.; Li, J.; Liu, Z.; Yu, J.; Xu, R. Nanosize-Enhaned Lifetime of SAPO-34 Catalysts in Methanol-to-Olefin Reaction. J. Phys. Chem. C 2013, 117, 8214–8222. [Google Scholar] [CrossRef]
- Jo, C.; Jung, J.; Shin, H.S.; Kim, J.; Ryoo, R. Capping with Multivalent Surfactants for Zeolite Nanocrystal Synthesis. Angew. Chem. Int. Ed. 2013, 52, 10014–10017. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to Olefins (MTO): From Fundamentals to Commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Hong, Y.; Gruver, V.; Fripiat, J.J. Role of Lewis Acidity in the Isomerization of n-Pentane and o-Xylene on Dealuminated H-Mordenites. J. Catal. 1994, 150, 421–429. [Google Scholar] [CrossRef]
- Goetze, J.; Meirer, F.; Yarulina, I.; Gascon, I.; Kapteijn, F.; Ruiz-Martínez, J.; Weckhuysen, B.M. Insights into the Activity and Deactivation of the Methanol-to-Olefins Process over Different Small-Pore Zeolites As Studied with Operando UV–vis Spectroscopy. ACS Catal. 2017, 7, 4033–4046. [Google Scholar] [CrossRef]
- Catizzone, E.; Cirelli, Z.; Aloise, A.; Lanzafame, P.; Migliori, M.; Giordano, G. Methanol conversion over ZSM-12, ZSM-22 and EU-1 zeolites: From DME to hydrocarbons production. Catal. Today 2018, 304, 39–50. [Google Scholar] [CrossRef]
- Palčić, A.; Ordomsky, V.V.; Qin, Z.; Georgieva, V.; Valtchev, V. Tuning Zeolite Properties for a Highly Efficient Synthesis of Propylene from Methanol. Chem. Eur. J. 2018, 24, 13136–13149. [Google Scholar] [CrossRef]
- Losch, P.; Boltz, M.; Bernardon, C.; Louis, B.; Palčić, A.; Valtchev, V. Impact of external surface passivation of nano-ZSM-5 zeolites in the methanol-to-olefins reaction. Appl. Catal. A Gen. 2016, 509, 30–37. [Google Scholar] [CrossRef]
- Ibáñez, M.; Epelde, E.; Aguayo, A.T.; Gayubo, A.G.; Bilbao, J.; Castaño, P. Selective dealumination of HZSM-5 zeolite boosts propylene by modifying 1-butene cracking pathway. Appl. Catal. A Gen. 2017, 543, 1–9. [Google Scholar] [CrossRef]
- Mirodatos, C.; Barthomeuf, D. Superacid sites in zeolites. J. Chem. Soc. Chem. Commun. 1981, 39–40. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Rey, F. Extraction of extra-framework aluminium in ultrastable Y zeolites by (NH4)2SiF6 treatments: I. Physicochemical Characterization. Appl. Catal. 1990, 59, 267–274. [Google Scholar] [CrossRef]
- Däumer, D.; Räuchle, K.; Reschetilowski, W. Experimental and Computational Investigations of the Deactivation of H-ZSM-5 Zeolite by Coking in the Conversion of Ethanol into Hydrocarbons. ChemCatChem 2012, 4, 802–814. [Google Scholar] [CrossRef]
- Hadi, N.; Niaei, A.; Nabavi, S.R.; Farzi, A.; Shirazia, M.N. Development of a New Kinetic Model for Methanol to Propylene Process on Mn/H-ZSM-5 Catalyst. Chem. Biochem. Eng. Q. 2014, 28, 53. [Google Scholar]
- Bleken, F.L.; Chavan, S.; Olsbye, U.; Boltz, M.; Ocampo, F.; Louis, B. Conversion of methanol into light olefins over ZSM-5 zeolite: Strategy to enhance propene selectivity. Appl. Catal. A Gen. 2012, 447–448, 178–185. [Google Scholar] [CrossRef]
- Xi, D.; Sun, Q.; Xu, J.; Cho, M.; Cho, H.S.; Asahina, S.; Li, Y.; Deng, F.; Terasaki, O.; Yu, J. In situ growth-etching approach to the preparation of hierarchically macroporous zeolites with high MTO catalytic activity and selectivity. J. Mater. Chem. A 2014, 2, 17994–18004. [Google Scholar] [CrossRef]
- Perez-Ramirez, J.; Christensen, C.H.; Egeblad, K.; Christensen, C.H.; Groen, J.C. Hierarchical zeolites: Enhanced utilisation of microporous crystals in catalysis by advances in materials design. Chem. Soc. Rev. 2008, 37, 2530–2542. [Google Scholar] [CrossRef]
- Li, L.; Cui, X.; Li, J.; Wang, J. Synthesis of SAPO-34/ZSM-5 Composite and Its Catalytic Performance in the Conversion of Methanol to Hydrocarbonsc. J. Braz. Chem. Soc. 2014, 26, 290–296. [Google Scholar] [CrossRef]
- Conte, M.; Xu, B.; Davies, T.E.; Bartley, J.K.; Carley, A.F.; Taylor, S.H.; Khalid, K.; Hutchings, G.J. Enhanced selectivity to propene in the methanol to hydrocarbons reaction by use of ZSM-5/11 intergrowth zeolite. Microporous Mesoporous Mater. 2012, 164, 207–213. [Google Scholar] [CrossRef]
- Lonstad Bleken, B.-T.; Mino, L.; Giordanino, F.; Beato, P.; Svelle, S.; Lillerud, K.P.; Bordiga, S. Probing the surface of nanosheet H-ZSM-5 with FTIR spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 13363–13370. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Liu, Y.; Vishnuvarthan, M.; Sun, X.; van Veen, A.C.; Haller, G.L.; Sanchez-Sanchez, M.; Lercher, J.A. Coke formation and deactivation pathways on H-ZSM-5 in the conversion of methanol to olefins. J. Catal. 2015, 325, 48–59. [Google Scholar] [CrossRef]
- Zhang, S.; Gong, Y.; Zhang, L.; Liu, Y.; Dou, T.; Xu, J.; Deng, F. Hydrothermal treatment on ZSM-5 extrudates catalyst for methanol to propylene reaction: Finely tuning the acidic property. Fuel Process. Technol. 2015, 129, 130–138. [Google Scholar] [CrossRef]
- Wu, W.; Guo, W.; Xiao, W.; Luo, M. Dominant reaction pathway for methanol conversion to propene over high silicon H-ZSM-5. Chem. Eng. Sci. 2011, 66, 4722–4732. [Google Scholar] [CrossRef]
- Yarulina, I.; Bailleul, S.; Pustovarenko, A.; Martinez, J.R.; De Wispelaere, K.; Hajek, J.; Weckhuysen, B.M.; Houben, K.; Baldus, M.; Van Speybroeck, V.; et al. Suppression of the Aromatic Cycle in Methanol-to-Olefins Reaction over ZSM-5 by Post-Synthetic Modification Using Calcium. ChemCatChem 2016, 8, 3057–3063. [Google Scholar] [CrossRef]
- Yarulina, I.; De Wispelaere, K.; Bailleul, S.; Goetze, J.; Radersma, M.; Abou-Hamad, E.; Vollmer, I.; Goesten, M.; Mezari, B.; Hensen, E.J.M.; et al. Structure–performance descriptors and the role of Lewis acidity in the methanol-to-propylene process. Nat. Chem. 2018, 10, 804–812. [Google Scholar]
- Jacobs, P.A.; Derouane, E.G.; Weitkamp, J. Evidence for X-ray-amorphous zeolites. Chem. Commun. 1981, 591–593. [Google Scholar] [CrossRef]
- Nicolaides, C. A novel family of solid acid catalysts: Substantially amorphous or partially crystalline zeolitic materials. Appl. Catal. A Gen. 1999, 185, 211–217. [Google Scholar] [CrossRef]
- Triantafyllidis, K.S.; Nalbandian, L.; Trikalitis, P.N.; Ladavos, A.K.; Mavromoustakos, T.; Nicolaides, C.P. Structural, compositional and acidic characteristics of nanosized amorphous or partially crystalline ZSM-5 zeolite-based materials. Microporous Mesoporous Mater. 2004, 75, 89–100. [Google Scholar] [CrossRef]
- Corma, A.; Díaz-Cabañas, M.J. Amorphous microporous molecular sieves with different pore dimensions and topologies: Synthesis, characterization and catalytic activity. Microporous Mesoporous Mater. 2006, 89, 39–46. [Google Scholar] [CrossRef]
- Tago, T.; Masuda, Y. Zeolite nanocrystals-synthesis and applications. In Nanocrystals; IntechOpen: London, UK, 2010; pp. 8–206. [Google Scholar]
- Kim, W.J.; Kim, S.D. Method of Preparing zsm-5 Using Variable Temperature without Organic Template. U.S. Patent 7,361,328, 22 April 2008. [Google Scholar]
- Yeong, Y.F.; Abdullah, A.Z.; Ahmad, A.L.; Bhatia, S. Propylsulfonic acid-functionalized partially crystalline silicalite-1 materials: Synthesis and characterization. J. Porous Mater. 2011, 18, 147–157. [Google Scholar] [CrossRef]
- Mostafa, M.M.M.; Rao, K.N.; Harun, H.S.; Basahel, S.N.; El-Maksod, I.H.A. Synthesis and characterization of partially crystalline nanosized ZSM-5 zeolites. Ceram. Int. 2013, 39, 683–689. [Google Scholar] [CrossRef]
- Haw, K.-G.; Gilson, J.-P.; Nesterenko, N.; Akouche, M.; El Siblani, H.; Goupil, J.-M.; Rigaud, B.; Minoux, D.; Dath, J.-P.; Valtchev, V. Supported Embryonic Zeolites and their Use to Process Bulky Molecules. ACS Catal. 2018, 8, 8199–8212. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, L.-J.; Li, J.-S.; Yang, Y.-C.; Sun, X.-Y. Preparation and characterization of nanosized ZSM-5 zeolites in the absence of organic template. Mater. Lett. 2005, 59, 3427–3430. [Google Scholar] [CrossRef]
- Barbera, K.; Bonino, F.; Bordiga, S.; Janssens, T.V.W.; Beato, P. Structure–deactivation relationship for ZSM-5 catalysts governed by framework defects. J. Catal. 2011, 280, 196–205. [Google Scholar] [CrossRef]
- Bjørgen, M.; Svelle, S.; Joensen, F.; Nerlov, J.; Kolboe, S.; Bonino, F.; Palumbo, L.; Bordiga, S.; Olsbye, U. Conversion of methanol to hydrocarbons over zeolite H-ZSM-5: On the origin of the olefinic species. J. Catal. 2007, 249, 195–207. [Google Scholar] [CrossRef]
- Robson, H.; Lillerud, K.P. Verified Synthesis of Zeolitic Materials; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Coster, D.; Blumenfeld, A.L.; Fripiat, J.J. Lewis Acid Sites and Surface Aluminum in Aluminas and Zeolites: A High-Resolution NMR Study. J. Phys. Chem. 1994, 98, 6201–6211. [Google Scholar] [CrossRef]
- Corma, A.; Fornés, V.; Martínez, A.; Sanz, J. Tetrahedral and Octahedral Extraframework Aluminum in Ultrastable Y Zeolites. In Fluid Catalytic Cracking; American Chemical Society: Washington, DC, USA, 1988; pp. 2–17. [Google Scholar]
- Kunkeler, P.J.; Zuurdeeg, B.J.; van der Waal, J.C.; van Bokhoven, J.A.; Koningsberger, D.C.; van Bekkum, H. Zeolite Beta: The Relationship between Calcination Procedure, Aluminum Configuration, and Lewis Acidity. J. Catal. 1998, 180, 234–244. [Google Scholar] [CrossRef]
- Thomas, J.M.; Klinowski, J. The Study of Aluminosilicate and Related Catalysts by High-Resolution Solid-State NMR Spectroscopy. Adv. Catal. 1985, 33, 199–374. [Google Scholar]
- Fyfe, C.A.; Gobbi, G.C.; Kennedy, G.J.; Graham, J.D.; Ozubko, R.S.; Murphy, W.J.; Bothner-By, A.; Dadok, J.; Chesnick, A.S. Detailed interpretation of the 29Si and 27Al high-field MAS n.m.r. spectra of zeolites offretite and omega. Zeolites 1985, 5, 179–183. [Google Scholar] [CrossRef]
- Zhu, L.; Yin, S.; Wang, X.; Liu, Y.; Wang, S. The catalytic properties evolution of HZSM-5 in the conversion of methanol to gasoline. RSC Adv. 2016, 6, 82515–82522. [Google Scholar] [CrossRef]
- Jacobsen, C.J.H.; Madsen, C.; Janssens, T.V.W.; Jakobsen, H.J.; Skibsted, J. Zeolites by confined space synthesis—Characterization of the acid sites in nanosized ZSM-5 by ammonia desorption and 27Al/29Si-MAS NMR spectroscopy. Microporous Mesoporous Mater. 2000, 39, 393–401. [Google Scholar] [CrossRef]
- Costa, C.; Dzikh, I.P.; Lopes, J.M.; Lemos, F.; Ribeiro, F.R. Activity–acidity relationship in zeolite ZSM-5. Application of Brönsted-type equations. J. Mol. Catal. A Chem. 2000, 154, 193–201. [Google Scholar] [CrossRef]
- Kondo, J.N.; Nishitani, R.; Yoda, E.; Yokoi, T.; Tatsumi, T.; Domen, K. A comparative IR characterization of acidic sites on HY zeolite by pyridine and CO probes with silica-alumina and [gamma]-alumina references. Phys. Chem. Chem. Phys. 2010, 12, 11576–11586. [Google Scholar] [CrossRef] [PubMed]
- Leydier, F.; Chizallet, C.; Costa, D.; Raybaud, P. CO adsorption on amorphous silica-alumina: Electrostatic or Bronsted acidity probe? Chem. Commun. 2012, 48, 4076–4078. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Ono, Y. Solid Acid Catalysis: From Fundamentals to Applications; Tylor & Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Tanabe, K.; Misono, M.; Ono, Y.; Hattori, H. New Solid Acids and Bases: Their Catalytic Properties (Studies in Surface Science and Catalysis); Elsevier Science Ltd.: Amsterdam, The Netherlands, 1989. [Google Scholar]
- Moreno-Piraján, J.C.; Garcia-Cuello, V.S.; Giraldo, L. Synthesis of HMOR and HZSM-5 and their Behaviour in the Catalytic Conversion of Methanol to Propylene (MTP). J. Thermodyn. Catal. 2010, 1, 101. [Google Scholar] [CrossRef]
- Lin, L.; Qiu, C.; Zhuo, Z.; Zhang, D.; Zhao, S.; Wu, H.; Liu, Y.; He, M. Acid strength controlled reaction pathways for the catalytic cracking of 1-butene to propene over ZSM-5. J. Catal. 2014, 309, 136–145. [Google Scholar] [CrossRef]
- Castaño, P.; Gayubo, A.G.; Pawelec, B.; Fierro, J.L.G.; Arandes, J.M. Kinetic modelling of methylcyclohexane ring-opening over a HZSM-5 zeolite catalyst. Chem. Eng. J. 2008, 140, 287–295. [Google Scholar] [CrossRef]
- Qian, Q.; Vogt, C.; Mokhtar, M.; Asiri, A.M.; Al-Thabaiti, S.A.; Basahel, S.N.; Ruiz-Martínez, J.; Weckhuysen, B.M. Combined Operando UV/Vis/IR Spectroscopy Reveals the Role of Methoxy and Aromatic Species during the Methanol-to-Olefins Reaction over H-SAPO-34. ChemCatChem 2014, 6, 3396–3408. [Google Scholar] [CrossRef]
- Borodina, E.; Meirer, F.; Lezcano-González, I.; Mokhtar, M.; Asiri, A.M.; Al-Thabaiti, S.A.; Basahel, S.N.; Ruiz-Martinez, J.; Weckhuysen, B.M. Influence of the reaction temperature on the nature of the active and deactivating species during methanol to olefins conversion over H-SSZ-13. ACS Catal. 2015, 5, 992–1003. [Google Scholar] [CrossRef]
- Borodina, E.; Kamaluddin, H.S.H.; Meirer, F.; Mokhtar, M.; Asiri, A.M.; Al-Thabaiti, S.A.; Basahel, S.N.; Ruiz-Martinez, J.; Weckhuysen, B.M. Influence of the Reaction Temperature on the Nature of the Active and Deactivating Species During Methanol-to-Olefins Conversion over H-SAPO-34. ACS Catal. 2017, 7, 5268–5281. [Google Scholar] [CrossRef]
- Sun, X.; Mueller, S.; Shi, H.; Haller, G.L.; Sanchez-Sanchez, M.; van Veen, A.C.; Lercher, J.A. On the impact of co-feeding aromatics and olefins for the methanol-to-olefins reaction on HZSM-5. J. Catal. 2014, 314, 21–31. [Google Scholar] [CrossRef]
- Bjørgen, M.; Joensen, F.; Lillerud, K.-P.; Olsbye, U.; Svelle, S. The mechanisms of ethene and propene formation from methanol over high silica H-ZSM-5 and H-beta. Catal. Today 2009, 142, 90–97. [Google Scholar] [CrossRef]
- Campbell, S.M.; Jiang, X.-Z.; Howe, R.F. Methanol to hydrocarbons: Spectroscopic studies and the significance of extra-framework aluminium. Microporous Mesoporous Mater. 1999, 29, 91–108. [Google Scholar] [CrossRef]
- Westgård Erichsen, M.; De Wispelaere, K.; Hemelsoet, K.; Moors, S.L.C.; Deconinck, T.; Waroquier, M.; Svelle, S.; Van Speybroeck, V.; Olsbye, U. How zeolitic acid strength and composition alter the reactivity of alkenes and aromatics towards methanol. J. Catal. 2015, 328, 186–196. [Google Scholar] [CrossRef]
- Song, C.; Wang, M.; Zhao, L.; Xue, N.; Peng, L.; Guo, X.; Ding, W.; Yang, W.; Xie, Z. Synergism between the Lewis and Brönsted acid sites on HZSM-5 zeolites in the conversion of methylcyclohexane. Chin. J. Catal. 2013, 34, 2153–2159. [Google Scholar] [CrossRef]








| Sample | Si/Al Ratio a | Average Crystal Size (nm) b | SBETc(m2/g) | Smicro (m2/g) | Smeso (m2/g) | VTotald (cm3/g) | Vmicroe (cm3/g) | Vmesof (cm3/g) | Hierarchy Factor g |
|---|---|---|---|---|---|---|---|---|---|
| MFI-40 | 42 | 89 | 383 | 358 | 45 | 0.901 | 0.039 | 0.862 | 0.005 |
| MFI-45 | 46 | 126 | 403 | 322 | 81 | 1.141 | 0.042 | 1.099 | 0.007 |
| MFI-50 | 53 | 140 | 420 | 347 | 73 | 1.057 | 0.049 | 1.008 | 0.008 |
| Zeolyst | 40 | 63 | 262 | 115 | 147 | 0.115 | 0.098 | 0.017 | 0.478 |
| Sample | Total Number of Acid Sites (mmoL g−1) c | ||
|---|---|---|---|
| MFI-40 | 90.44 | 9.56 | 0.25 |
| MFI-45 | 78.20 | 21.80 | 0.28 |
| MFI-50 | 67.34 | 32.34 | 0.16 |
| Zeolyst | 95.13 | 4.87 | 0.39 |
| Sample | Initiation Time for Active Species [h] | Initiation Time for Deactivating Species [h] | Average Rate for Growing of Active Species [cg/h−1] a |
|---|---|---|---|
| MFI-40 | 0.10 | 0.1 | 45.5 |
| MFI-45 | 0.14 | 0.3 | 61.5 |
| MFI-50 | 0.18 | 0.3 | 37.0 |
| Zeolyst | 0.10 | 1.3 | 20.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamaluddin, H.S.; Basahel, S.N.; Narasimharao, K.; Mokhtar, M. H-ZSM-5 Materials Embedded in an Amorphous Silica Matrix: Highly Selective Catalysts for Propylene in Methanol-to-Olefin Process. Catalysts 2019, 9, 364. https://doi.org/10.3390/catal9040364
Kamaluddin HS, Basahel SN, Narasimharao K, Mokhtar M. H-ZSM-5 Materials Embedded in an Amorphous Silica Matrix: Highly Selective Catalysts for Propylene in Methanol-to-Olefin Process. Catalysts. 2019; 9(4):364. https://doi.org/10.3390/catal9040364
Chicago/Turabian StyleKamaluddin, Huda Sharbini, Sulaiman Nassir Basahel, Katabathini Narasimharao, and Mohamed Mokhtar. 2019. "H-ZSM-5 Materials Embedded in an Amorphous Silica Matrix: Highly Selective Catalysts for Propylene in Methanol-to-Olefin Process" Catalysts 9, no. 4: 364. https://doi.org/10.3390/catal9040364
APA StyleKamaluddin, H. S., Basahel, S. N., Narasimharao, K., & Mokhtar, M. (2019). H-ZSM-5 Materials Embedded in an Amorphous Silica Matrix: Highly Selective Catalysts for Propylene in Methanol-to-Olefin Process. Catalysts, 9(4), 364. https://doi.org/10.3390/catal9040364