Versatile Sulfathiazole-Functionalized Magnetic Nanoparticles as Catalyst in Oxidation and Alkylation Reactions
Abstract
:1. Introduction
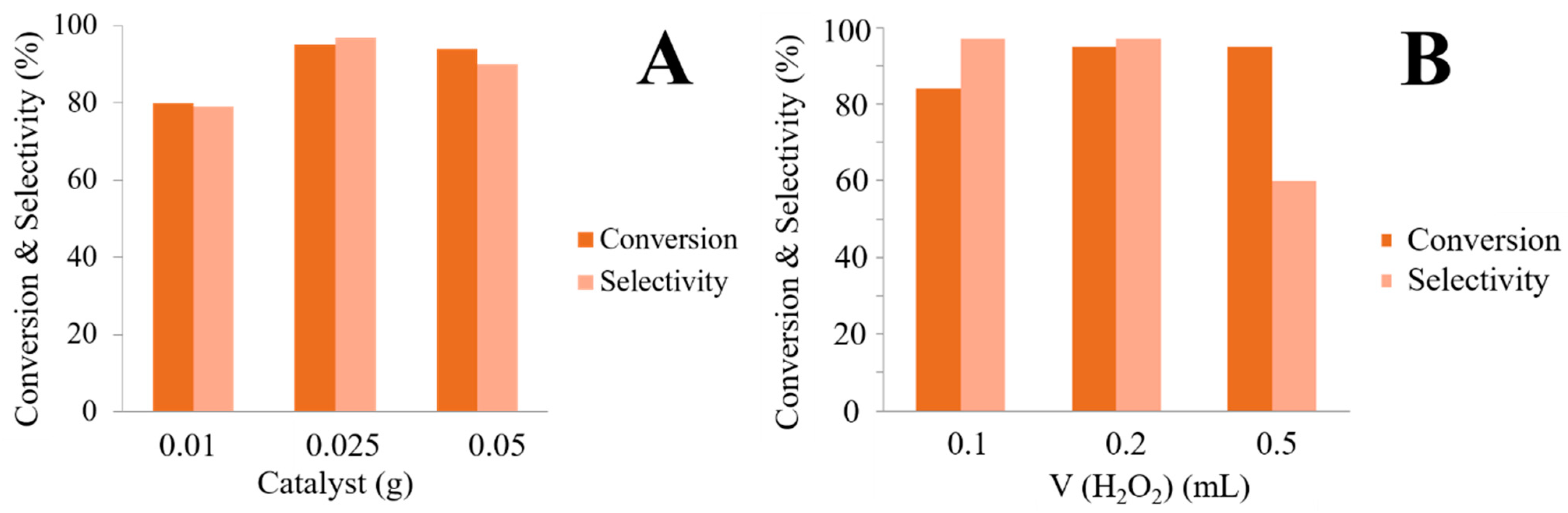
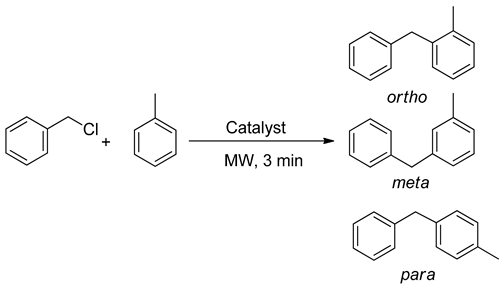
2. Results and Discussion
3. Experimental
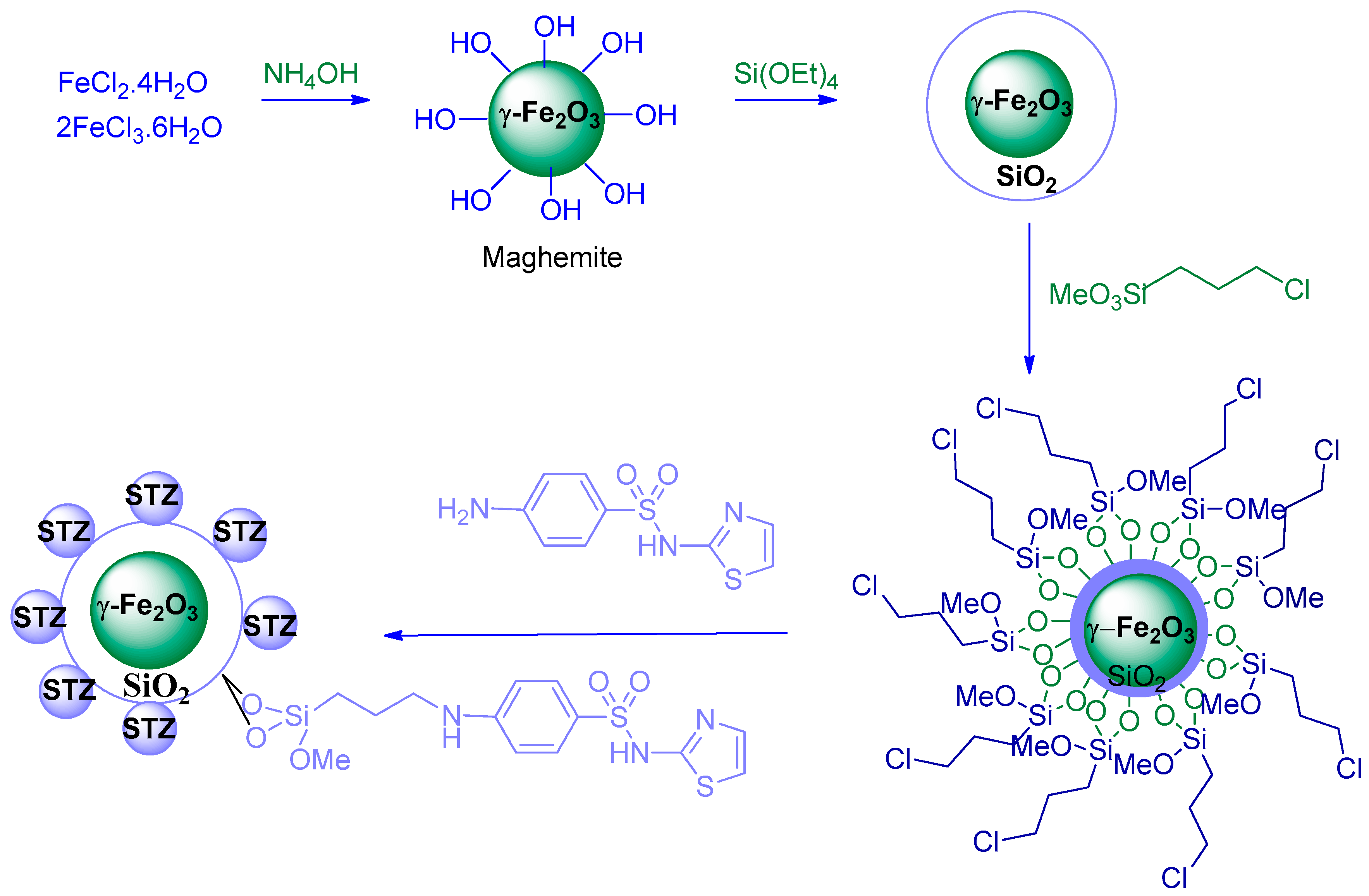
3.1. Preparation of γ-Fe2O3
3.2. Preparation of γ-Fe2O3/SiO2
3.3. Preparation of 3-Chloropropyl Trimethoxysilane-γ-Fe2O3/SiO2
3.4. Preparation of γ-Fe2O3/SiO2-STZ
3.5. Materials Characterization
3.6. General Procedure for the Oxidation of Benzyl Alcohol to Benzaldehyde
3.7. General Procedure for the Alkylation Reaction of Toluene with Benzyl Chloride
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zeng, T.; Chen, W.W.; Cirtiu, C.M.; Moores, A.; Song, G.; Li, C.J. Fe3O4 nanoparticles: A robust and magnetically recoverable catalyst for three-component coupling of aldehyde, alkyne and amine. Green Chem. 2010, 12, 570–573. [Google Scholar] [CrossRef]
- Zhu, Y.; Stubbs, L.P.; Ho, F.; Liu, R.; Ship, C.P.; Maguire, J.A.; Hosmane, N.S. Magnetic nanocomposites: A new perspective in catalysis. ChemCatChem 2010, 2, 365–374. [Google Scholar] [CrossRef]
- Amali, A.J.; Rana, R.K. Stabilisation of Pd (0) on surface functionalised Fe3O4 nanoparticles: Magnetically recoverable and stable recyclable catalyst for hydrogenation and Suzuki–Miyaura reactions. Green Chem. 2009, 11, 1781–1786. [Google Scholar] [CrossRef]
- Wu, L.; Tian, S. Immobilization of 1, 5, 7-Triazabicyclo [4.4.0] dec-5-ene on Magnetic γ-Fe2O3 Nanoparticles: A Highly Recyclable and Efficient Nanocatalyst for the Synthesis of Organic Carbonates. Eur. J. Inorg. Chem. 2014, 2014, 2080–2087. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Cui, L.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of iron oxide nanomaterials in wastewater treatment: A review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, F.; Karimi, N.; Saidi, M.R.; Primo, A.; Varma, R.S.; Luque, R. Unprecedented selective oxidation of styrene derivatives using a supported iron oxide nanocatalyst in aqueous medium. Adv. Synth. Catal. 2012, 354, 1707–1711. [Google Scholar] [CrossRef]
- Kitamura, H.; Zhao, L.; Hang, B.T.; Okada, S.; Yamaki, J.I. Effect of binder materials on cycling performance of Fe2O3 electrodes in alkaline solution. J. Power Sources 2012, 208, 391–396. [Google Scholar] [CrossRef]
- Figuerola, A.; Di Corato, R.; Manna, L.; Pellegrino, T. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol. Res. 2010, 62, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 117, 2842–2845. [Google Scholar] [CrossRef]
- Wei, S.; Wang, Q.; Zhu, J.; Sun, L.; Lin, H.; Guo, Z. Multifunctional composite core–shell nanoparticles. Nanoscale 2011, 3, 4474–4502. [Google Scholar] [CrossRef]
- Shylesh, S.; Schünemann, V.; Thiel, W.R. Magnetically separable nanocatalysts: Bridges between homogeneous and heterogeneous catalysis. Angew. Chem. Int. Ed. 2010, 49, 3428–3459. [Google Scholar] [CrossRef]
- Ostovar, S.; Prinsen, P.; Yepez, A.; Shaterian, H.R.; Luque, R. Catalytic Versatility of Novel Sulfonamide Functionalized Magnetic Composites. ACS Sustain. Chem. Eng. 2018, 6, 4586–4593. [Google Scholar] [CrossRef]
- Richter, M.K.; Sander, M.; Krauss, M.; Christl, I.; Dahinden, M.G.; Schneider, M.K.; Schwarzenbach, R.P. Cation binding of antimicrobial sulfathiazole to leonardite humic acid. Environ. Sci. Technol. 2009, 43, 6632–6638. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Khan, R. Environmental pharmacology: A new discipline. Indian J. Pharmacol. 2006, 38. [Google Scholar] [CrossRef]
- Cao, Q.; Dornan, L.M.; Rogan, L.; Hughes, N.L.; Muldoon, M.J. Aerobic oxidation catalysis with stable radicals. Chem. Commun. 2014, 50, 4524–4543. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, N.H. Immobilisation of Catalysts for Applications in Organic Reactions. Ph.D. Thesis, University of Limerick, Limerick, Ireland, 2016. [Google Scholar]
- Rodríguez-Padrón, D.; Balu, A.M.; Romero, A.A.; Luque, R. New bio-nanocomposites based on iron oxides and polysaccharides applied to oxidation and alkylation reactions. Beilstein J. Org. Chem. 2017, 13, 1982–1993. [Google Scholar] [CrossRef]
- Abednatanzi, S.; Abbasi, A.; Masteri-Farahani, M. Immobilization of catalytically active polyoxotungstate into ionic liquid-modified MIL-100 (Fe): A recyclable catalyst for selective oxidation of benzyl alcohol. Catal. Commun. 2017, 96, 6–10. [Google Scholar] [CrossRef]
- Ryland, B.L.; Stahl, S.S. Practical aerobic oxidations of alcohols and amines with homogeneous copper/TEMPO and related catalyst systems. Angew. Chem. Int. Ed. 2014, 53, 8824–8838. [Google Scholar] [CrossRef]
- Reddy, C.; Reddy, S.R.; Naidu, S. Chemoselective Oxidation of Benzyl, Amino, and Propargyl Alcohols to Aldehydes and Ketones under Mild Reaction Conditions. ChemistryOpen 2015, 4, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Yang, K.; Fu, X.; Yuan, R. Photocatalytic oxidation of benzyl alcohol by homogeneous CuCl2/solvent: A model system to explore the role of molecular oxygen. ACS Catal. 2015, 5, 3760–3766. [Google Scholar] [CrossRef]
- Prebil, R.; Stavber, G.; Stavber, S. Aerobic Oxidation of Alcohols by Using a Completely Metal-Free Catalytic System. Eur. J. Org. Chem. 2014, 2014, 395–402. [Google Scholar] [CrossRef]
- Verma, S.; Baig, R.N.; Nadagouda, M.N.; Varma, R.S. Selective oxidation of alcohols using photoactive VO@ g-C3N4. ACS Sustain. Chem. Eng. 2016, 4, 1094–1098. [Google Scholar] [CrossRef]
- Hosseinpour, R.; Pineda, A.; Ojeda, M.; Garcia, A.; Romero, A.A.; Luque, R. Microwave-assisted oxidation of benzyl alcohols using supported cobalt based nanomaterials under mild reaction conditions. Green Process. Synth. 2014, 3, 133–139. [Google Scholar] [CrossRef]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Na, H.B. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J. Am. Chem. Soc. 2001, 123, 12798–12801. [Google Scholar] [CrossRef]
- Yepez, A.; Hidalgo, J.M.; Pineda, A.; Černý, R.; Jíša, P.; Garcia, A.; Luque, R. Mechanistic insights into the hydroconversion of cinnamaldehyde using mechanochemically-synthesized Pd/Al-SBA-15 catalysts. Green Chem. 2015, 17, 565–572. [Google Scholar] [CrossRef]
- Luque, R.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. NH4F effect in post-synthesis treatment of Al-MCM-41 mesoporous materials. Microporous Mesoporous Mater. 2005, 84, 11–20. [Google Scholar] [CrossRef]
- Rajpure, K.Y. Exploring structural and magnetic properties of nanocrystalline iron oxide synthesized by autocombustion method. Superlattices Microstruct. 2015, 77, 181–195. [Google Scholar] [CrossRef]
- Cheon, J.; Kang, N.J.; Lee, S.M.; Lee, J.H.; Yoon, J.H.; Oh, S.J. Shape evolution of single-crystalline iron oxide nanocrystals. J. Am. Chem. Soc. 2004, 126, 1950–1951. [Google Scholar] [CrossRef]
- Shi, J.; Ai, Z.; Zhang, L. Fe@ Fe2O3 core-shell nanowires enhanced Fenton oxidation by accelerating the Fe (III)/Fe (II) cycles. Water Res. 2014, 59, 145–153. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, C.; Chen, R.; Chen, F. Selective oxidation of benzyl alcohol to benzaldehyde with H2O2 in water on epichlorohydrin-modified Fe3O4 microspheres. New J. Chem. 2015, 39, 4924–4932. [Google Scholar] [CrossRef]
- Bachari, K.; Millet, J.M.M.; Benaıchouba, B.; Cherifi, O.; Figueras, F. Benzylation of benzene by benzyl chloride over iron mesoporous molecular sieves materials. J. Catal. 2004, 221, 55–61. [Google Scholar] [CrossRef]
- Kalita, P.; Gupta, N.M.; Kumar, R. Synergistic role of acid sites in the Ce-enhanced activity of mesoporous Ce–Al-MCM-41 catalysts in alkylation reactions: FTIR and TPD-ammonia studies. J. Catal. 2007, 245, 338–347. [Google Scholar] [CrossRef]
- Gracia, M.J.; Losada, E.; Luque, R.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Activity of Gallium and Aluminum SBA-15 materials in the Friedel–Crafts alkylation of toluene with benzyl chloride and benzyl alcohol. Appl. Catal. A General 2008, 349, 148–155. [Google Scholar] [CrossRef]
- Ziarati, A.; Badiei, A.; Luque, R.; Ouyang, W. Designer hydrogenated wrinkled yolk@ shell TiO2 architectures towards advanced visible light photocatalysts for selective alcohol oxidation. J. Mater. Chem. A 2018, 6, 8962–8968. [Google Scholar] [CrossRef]

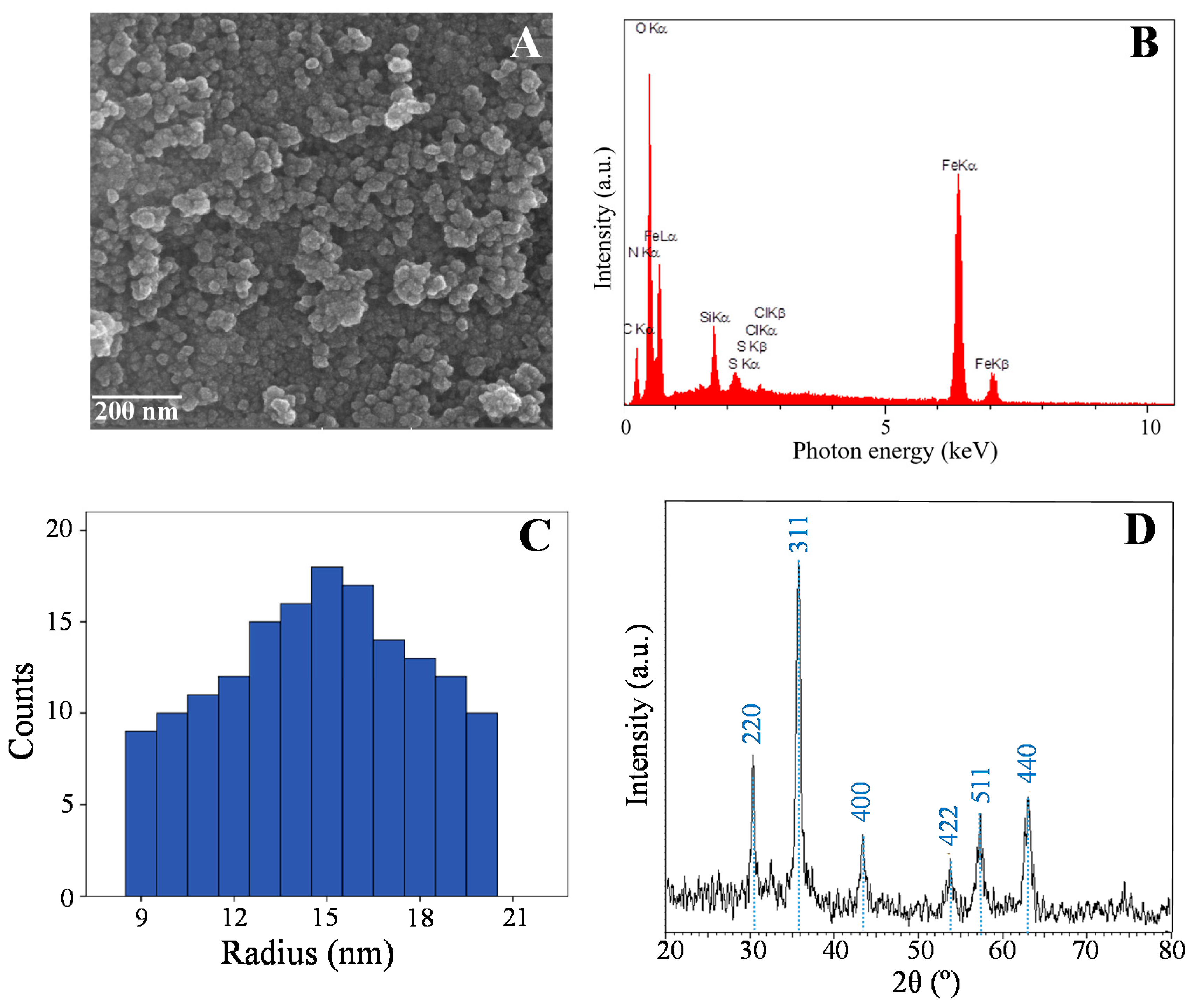
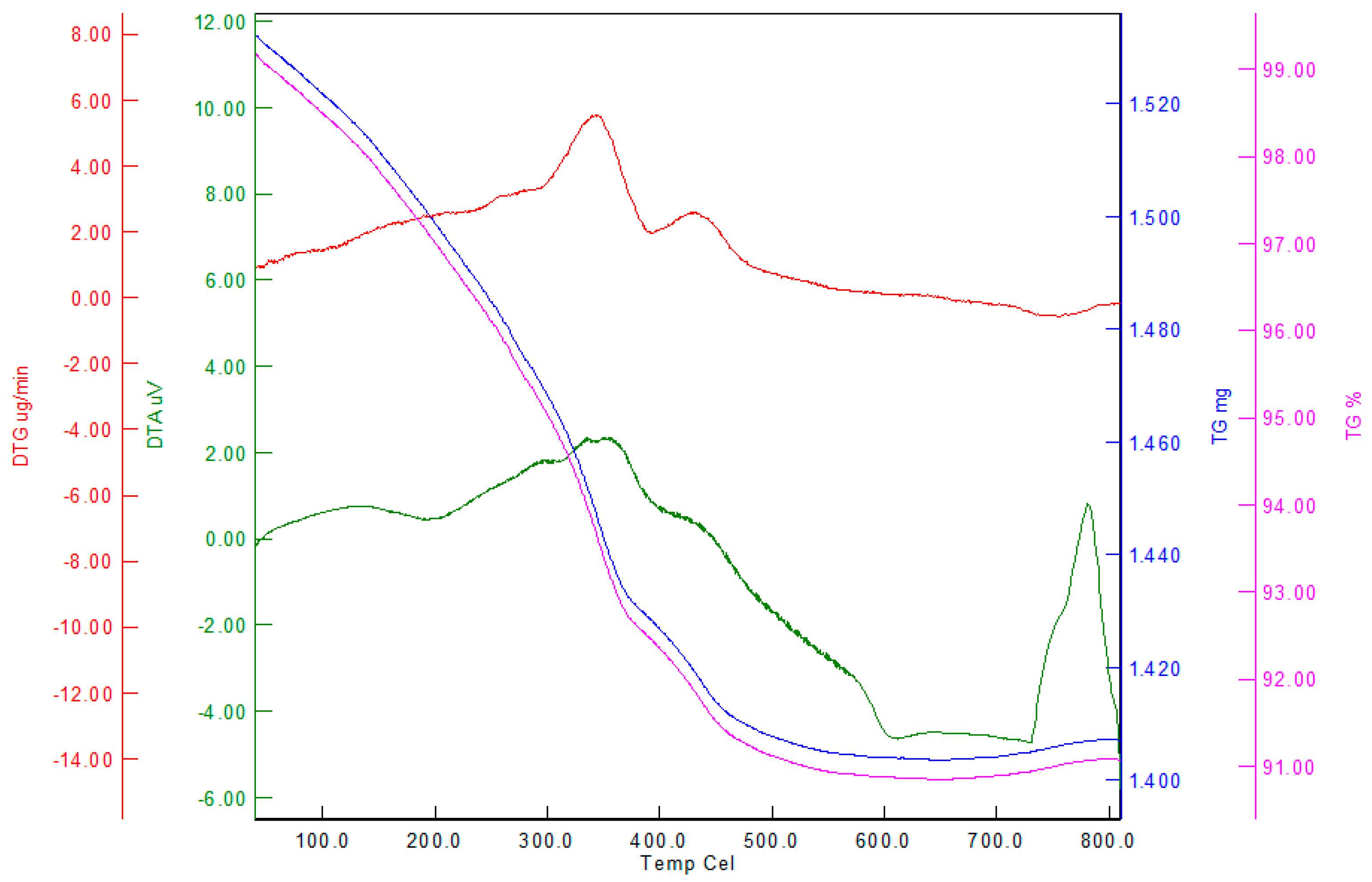
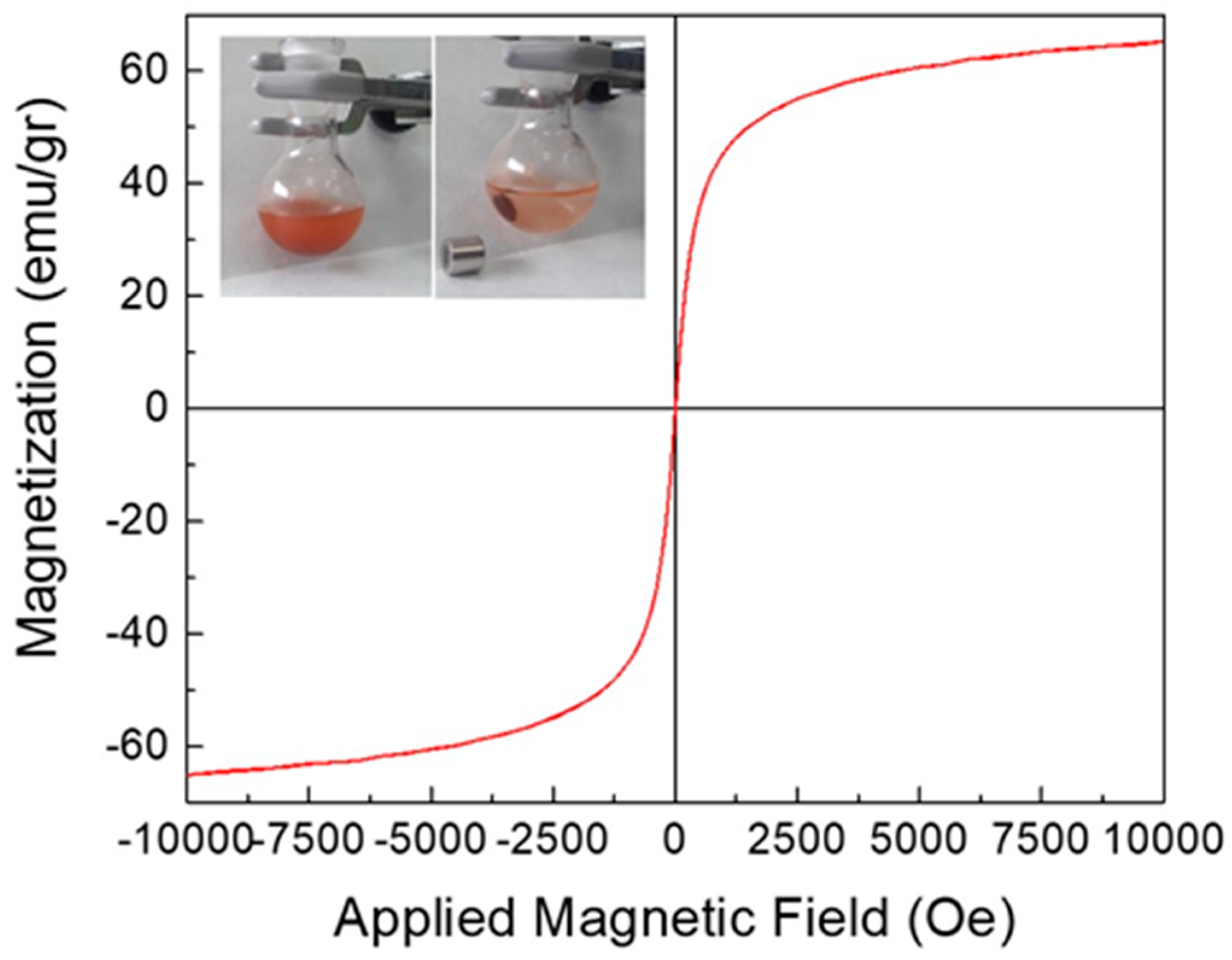

| Sample | Fe | O | Si | C | Cl | S | N | Total |
|---|---|---|---|---|---|---|---|---|
| γ-Fe2O3/SiO2-STZ | 42.1 | 36.6 | 1.78 | 16.88 | 0.3 | 0.64 | 1.65 | 100 |
| Entry | Catalyst | Conversion (mol%) | Selectivity (mol%) |
|---|---|---|---|
| 1 | Blank (no catalyst) | <10 | <10 |
| 2 | γ-Fe2O3 | 37 | >99 |
| 3 | γ-Fe2O3/SiO2 | 39 | >99 |
| 4 | γ-Fe2O3/SiO2-STZ | 95 | 97 |
 | ||||
|---|---|---|---|---|
| Entry | Substrate | Product | Conversion (mol%) | Selectivity (mol%) |
| 1 | benzyl alcohol | benzaldehyde | 95 | 97 |
| 2 | 4-chlorobenzyl alcohol | 4-chlorobenzaldehyde | 98 | 96 |
| 3 | 4-methylbenzyl alcohol | 4-methylbenzaldehyde | 90 | 95 |
| 4 | 4-methoxybenzyl alcohol | 4-methoxybenzaldehyde | 97 | 97 |
| 5 | 4-nitrobenzyl alcohol | 4-nitrobenzaldehyde | >99 | 96 |
 | ||||
|---|---|---|---|---|
| Material | Conversion (mol%) | Selectivity (mol%) | ||
| Meta | Ortho | Para | ||
| γ-Fe2O3 | 70 | 29 | 34 | 37 |
| γ-Fe2O3/SiO2 | 75 | 27 | 33 | 40 |
| γ-Fe2O3/SiO2-STZ | >99 | 5 | 45 | 50 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostovar, S.; Rodríguez-Padrón, D.; Saberi, F.; Balu, A.M.; Luque, R. Versatile Sulfathiazole-Functionalized Magnetic Nanoparticles as Catalyst in Oxidation and Alkylation Reactions. Catalysts 2019, 9, 348. https://doi.org/10.3390/catal9040348
Ostovar S, Rodríguez-Padrón D, Saberi F, Balu AM, Luque R. Versatile Sulfathiazole-Functionalized Magnetic Nanoparticles as Catalyst in Oxidation and Alkylation Reactions. Catalysts. 2019; 9(4):348. https://doi.org/10.3390/catal9040348
Chicago/Turabian StyleOstovar, Somayeh, Daily Rodríguez-Padrón, Farveh Saberi, Alina M. Balu, and Rafael Luque. 2019. "Versatile Sulfathiazole-Functionalized Magnetic Nanoparticles as Catalyst in Oxidation and Alkylation Reactions" Catalysts 9, no. 4: 348. https://doi.org/10.3390/catal9040348
APA StyleOstovar, S., Rodríguez-Padrón, D., Saberi, F., Balu, A. M., & Luque, R. (2019). Versatile Sulfathiazole-Functionalized Magnetic Nanoparticles as Catalyst in Oxidation and Alkylation Reactions. Catalysts, 9(4), 348. https://doi.org/10.3390/catal9040348







