Preparation of Iron Carbides Formed by Iron Oxalate Carburization for Fischer–Tropsch Synthesis
Abstract
:1. Introduction
2. Results
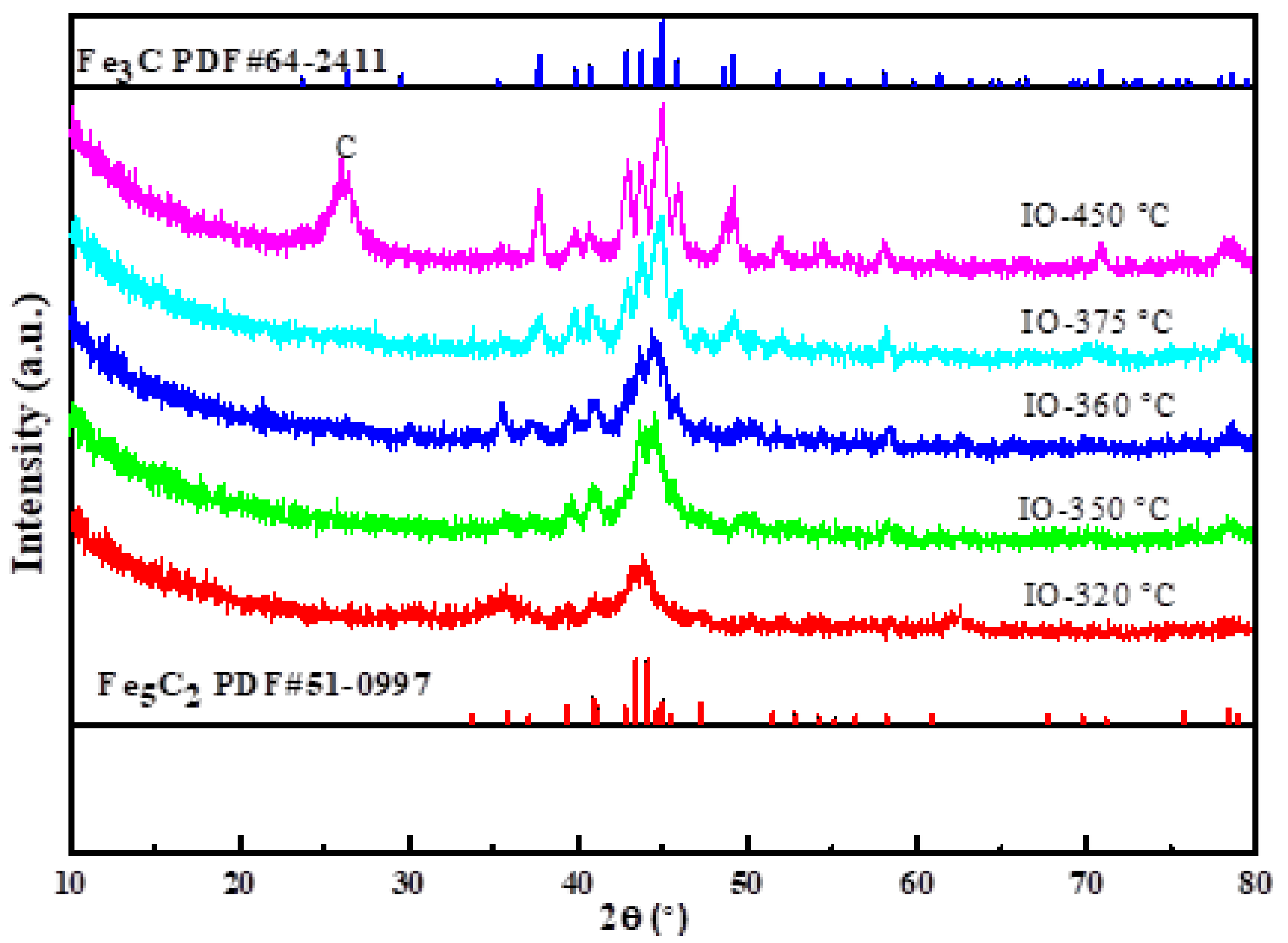
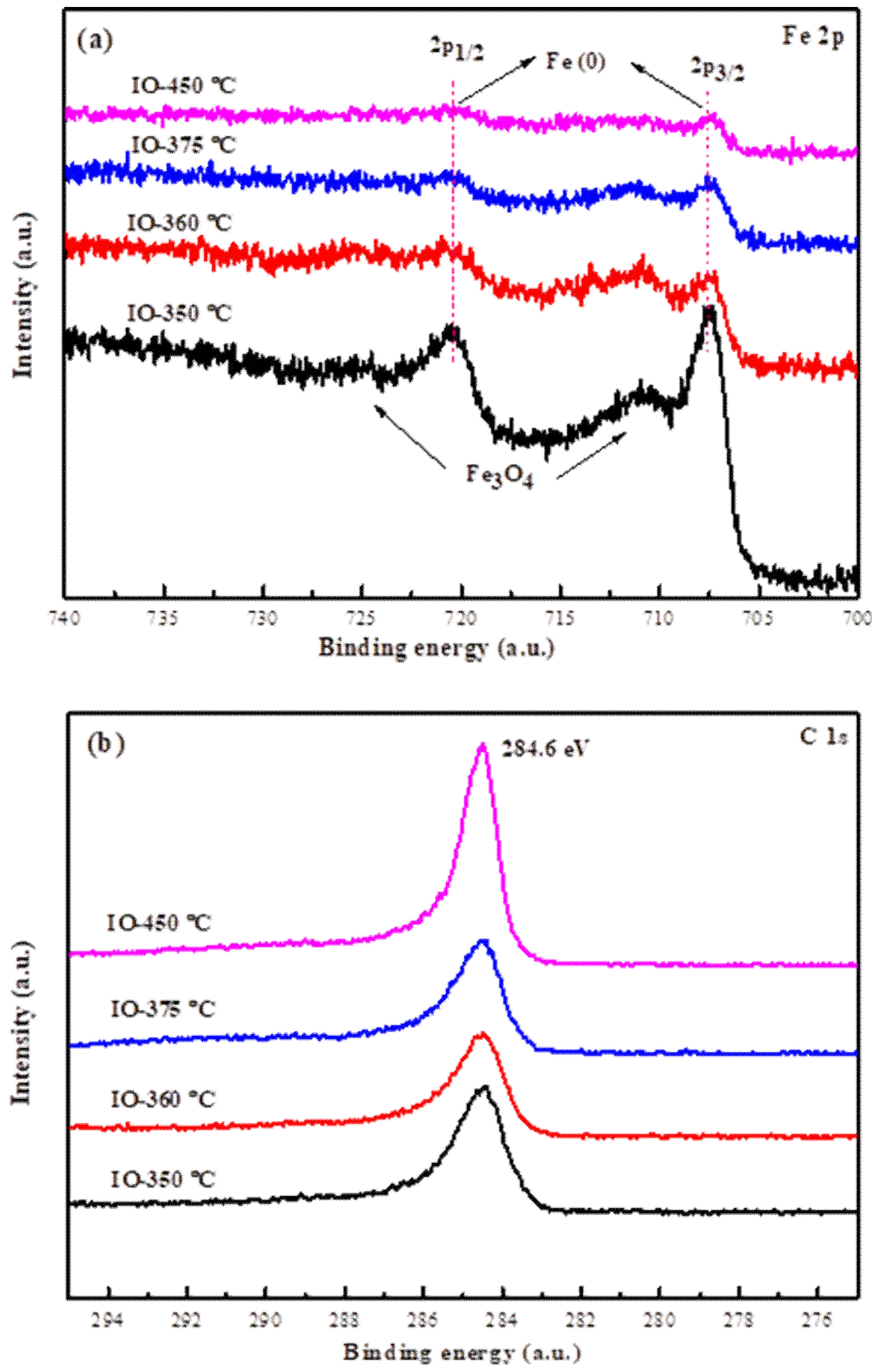
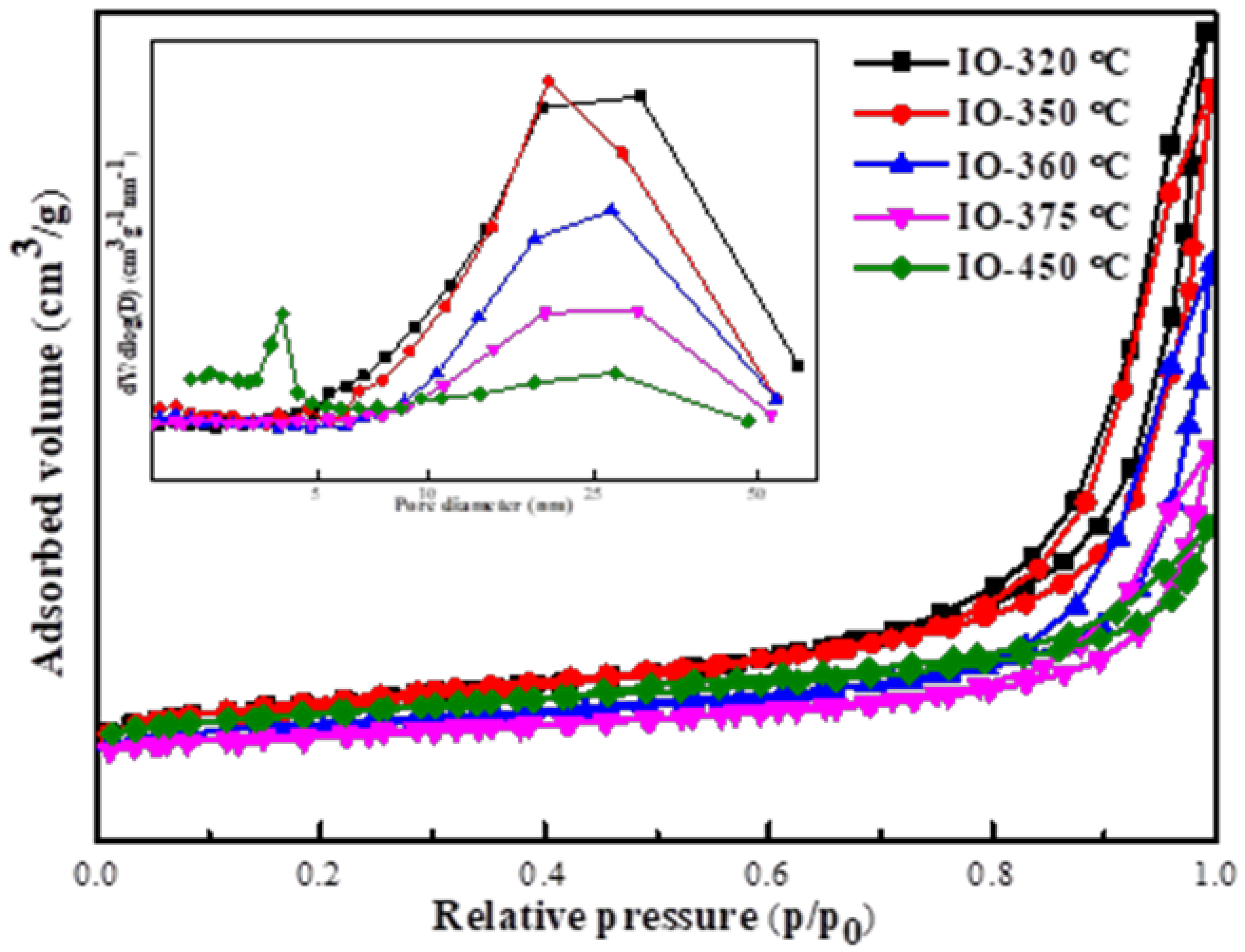
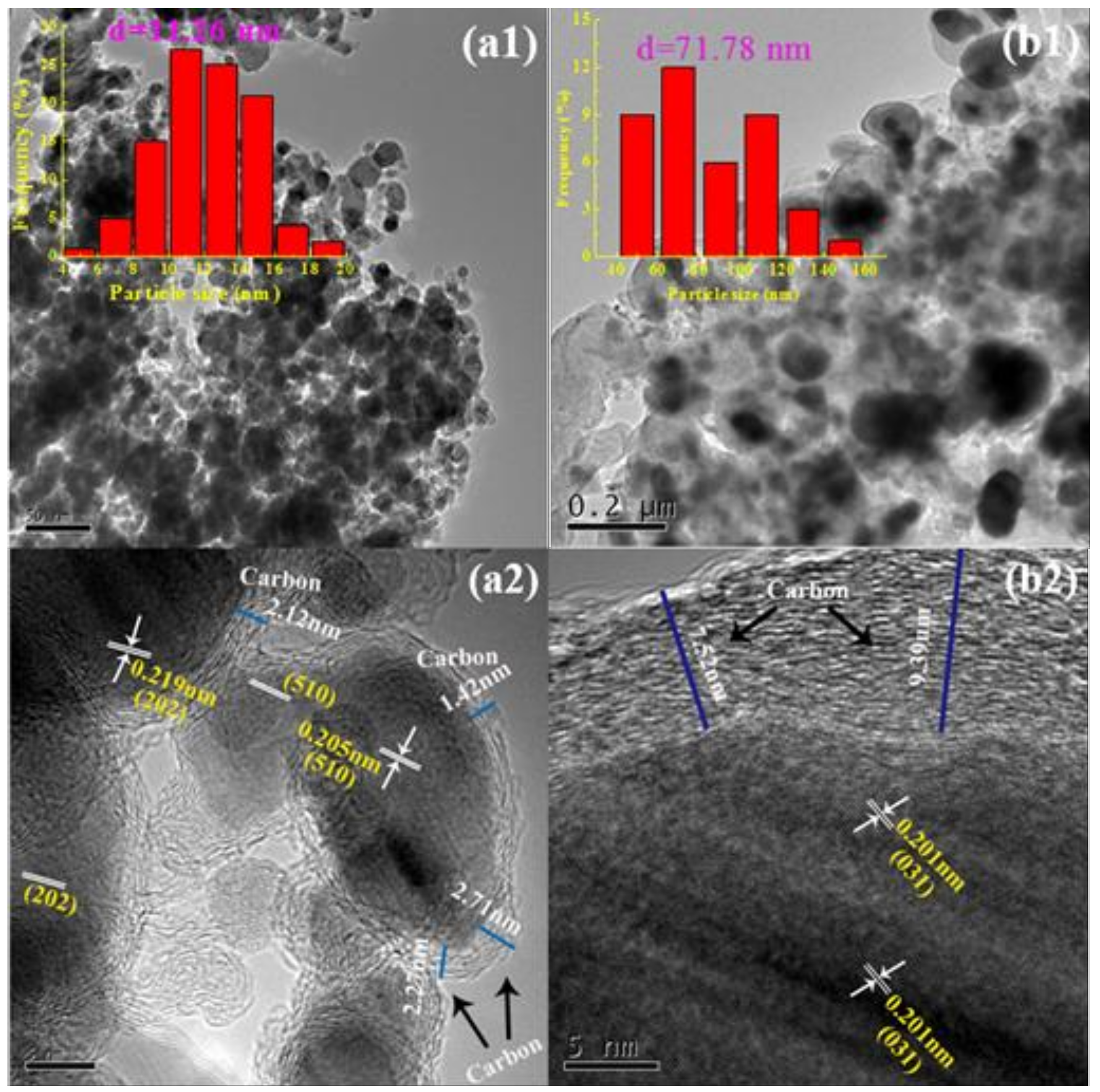
2.1. Characterization Catalysts
Characterization of the As-Prepared Iron Carbides
2.2. Fischer–Tropsch Synthesis (FTS) Performance
3. Discussion
3.1. Effect of Surface Adsorption Properties on FTS Performance
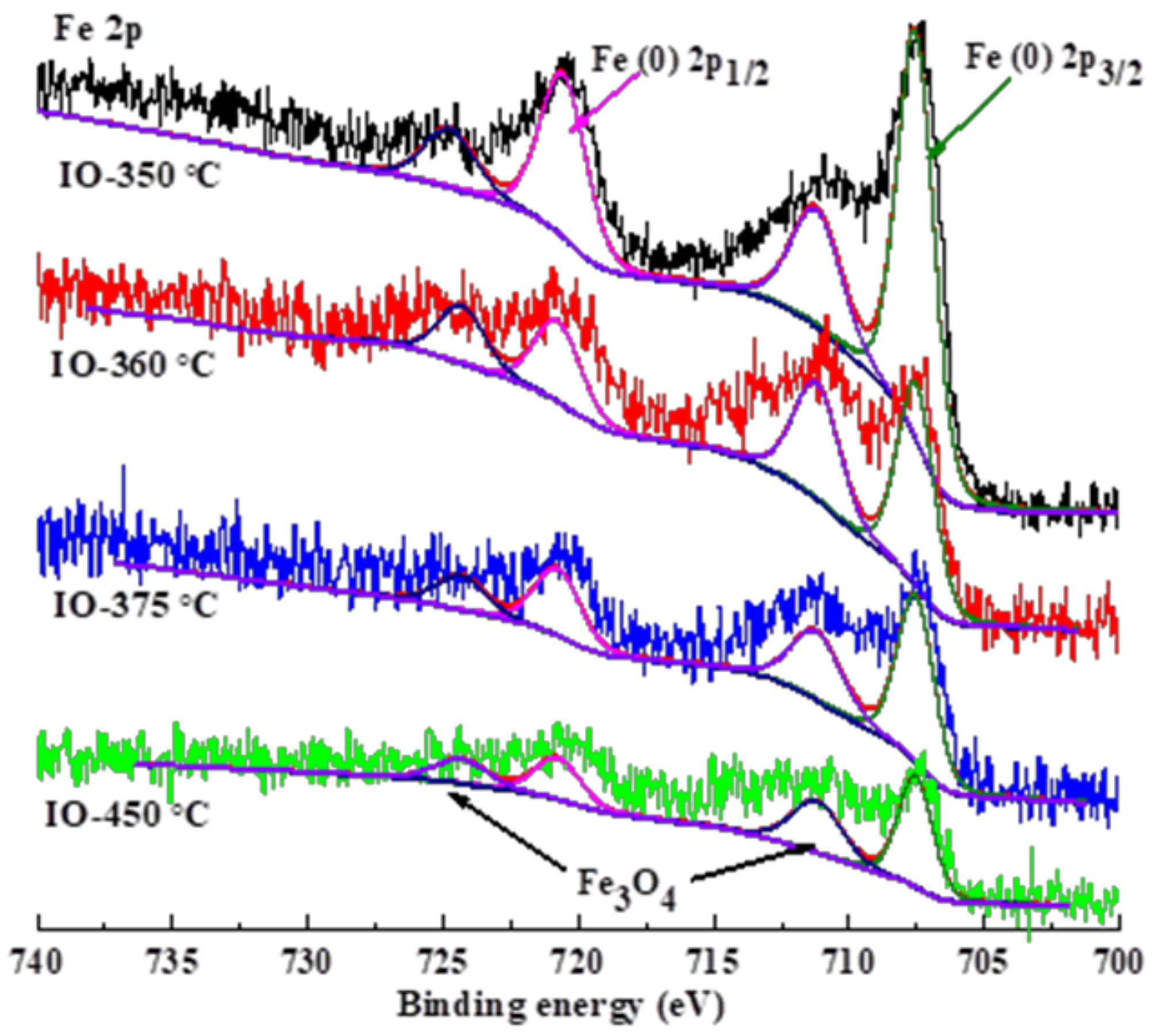
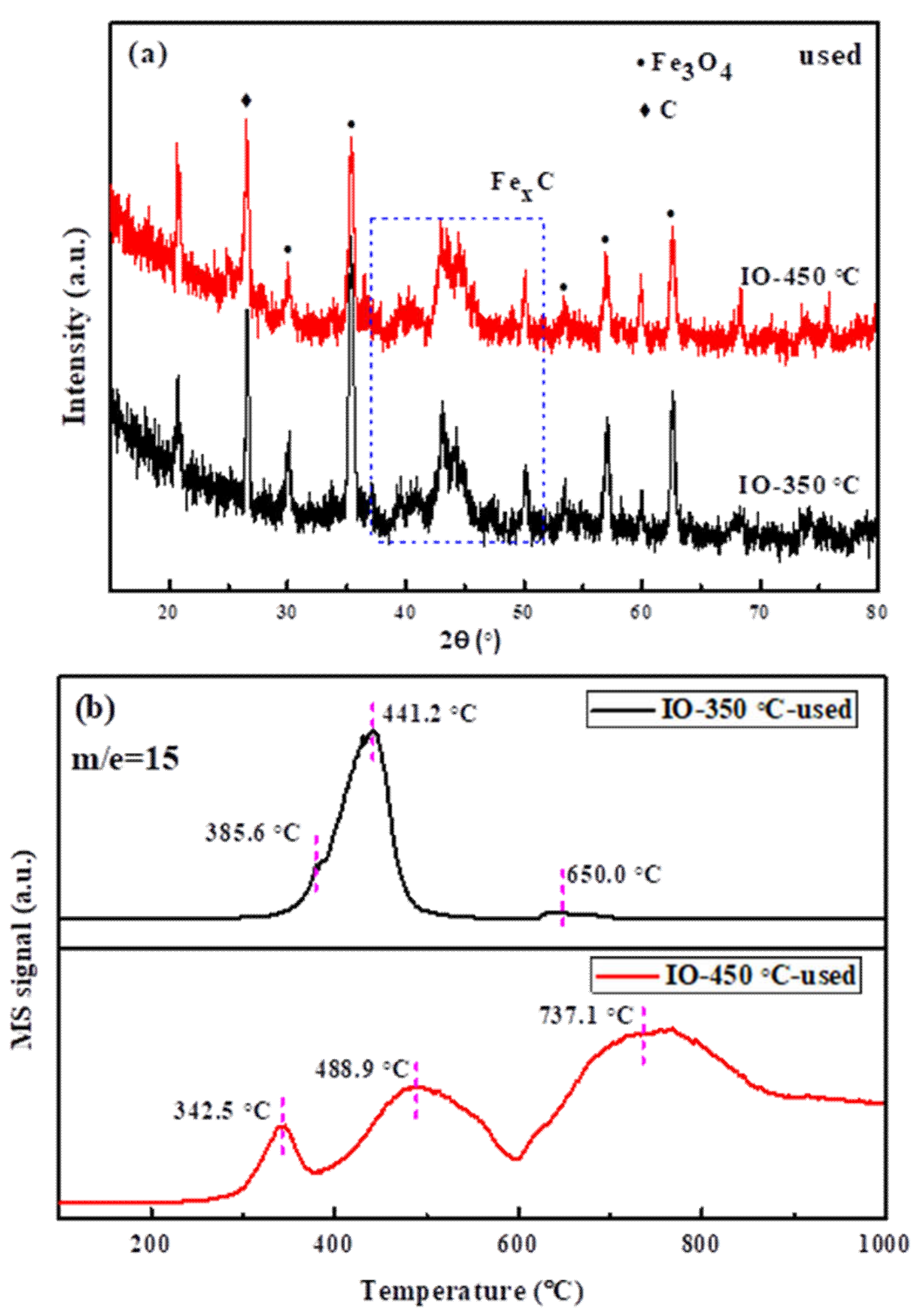
3.2. Characterization of the Used Iron Carbide Catalysts
3.3. Effect of Carburization Temperature on FTS Activity and Selectivity
4. Materials and Methods
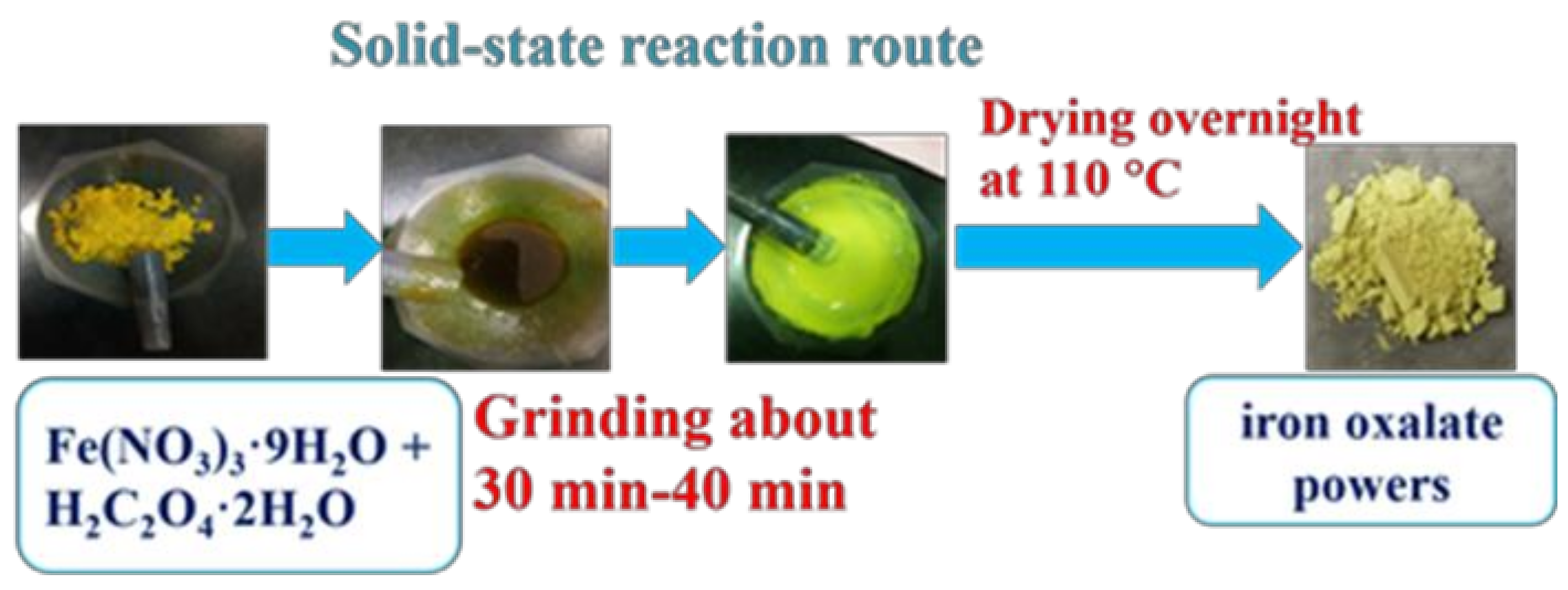
4.1. Catalyst Preparation
4.2. Characterization
4.3. Fischer–Tropsch Synthesis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casavola, M.; Hermannsdorfer, J.; de Jonge, N.; Dugulan, A.I.; de Jong, K.P. Fabrication of Fischer-ropsch Catalysts by Deposition of Iron Nanocrystals on Carbon Nanotubes. Adv. Funct. Mater. 2015, 25, 5309–5319. [Google Scholar] [CrossRef]
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M.; et al. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Galvis, H.M.T.; de Jong, K.P. Catalysts for Production of Lower Olefins from Synthesis Gas: A Review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Koeken, A.C.J.; Bitter, J.H.; Davidian, T.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Effect of precursor on the catalytic performance of supported iron catalysts for the Fischer–Tropsch synthesis of lower olefins. Catal. Today 2013, 215, 95–102. [Google Scholar] [CrossRef]
- Lu, Y.; Yan, Q.; Han, J.; Cao, B.; Street, J.; Yu, F. Fischer-Tropsch synthesis of olefin-rich liquid hydrocarbons from biomass-derived syngas over carbon-encapsulated iron carbide/iron nanoparticles catalyst. Fuel 2017, 193, 369–384. [Google Scholar] [CrossRef]
- Sun, B.; Xu, K.; Nguyen, L.; Qiao, M.; Tao, F. Preparation and Catalysis of Carbon-Supported Iron Catalysts for Fischer-Tropsch Synthesis. ChemCatChem 2012, 4, 1498–1511. [Google Scholar] [CrossRef]
- Liu, K.; Suo, H.; Zhang, C.; Xu, J.; Yang, Y.; Xiang, H.-W.; Li, Y.-W. An active Fischer-Tropsch synthesis FeMo/SiO2 catalyst prepared by a modified sol-gel technique. Catal. Commun. 2010, 12, 137–141. [Google Scholar] [CrossRef]
- Galvis, H.M.T.; Koeken, A.C.J.; Bitter, J.H.; Davidian, T.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Effects of sodium and sulfur on catalytic performance of supported iron catalysts for the Fischer–Tropsch synthesis of lower olefins. J. Catal. 2013, 303, 22–30. [Google Scholar] [CrossRef]
- Zhai, P.; Xu, C.; Gao, R.; Liu, X.; Li, M.; Li, W.; Fu, X.; Jia, C.; Xie, J.; Zhao, M.; et al. Highly Tunable Selectivity for Syngas-Derived Alkenes over Zinc and Sodium-Modulated Fe5C2 Catalyst. Angew. Chem. Int. Ed. Engl. 2016, 55, 9902–9907. [Google Scholar] [CrossRef]
- Yu, G.; Sun, B.; Pei, Y.; Xie, S.; Yan, S.; Qiao, M.; Fan, K.; Zhang, X.; Zong, B. FexOy@C Spheres as an Excellent Catalyst for Fischer-Tropsch Synthesis. J. Am. Chem. Soc. 2010, 132, 935–937. [Google Scholar] [CrossRef]
- An, B.; Cheng, K.; Wang, C.; Wang, Y.; Lin, W. Pyrolysis of Metal-Organic Frameworks to Fe3O4@Fe5C2 Core-Shell Nanoparticles for Fischer-Tropsch Synthesis. ACS Catal. 2016, 6, 3610–3618. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.; Hou, Y.; Ma, D. Fe5C2 Nanoparticles: A Facile Bromide-Induced Synthesis and as an Active Phase for Fischer-Tropsch Synthesis. J. Am. Chem. Soc. 2012, 134, 15814–15821. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.-F.; Bao, J.; Zhang, Y. Manganese-Modified Fe3O4 Microsphere Catalyst with Effective Active Phase of Forming Light Olefins from Syngas. ACS Catal. 2015, 5, 3905–3909. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Chiang, F.-K.; Dugulan, A.I.; Song, Y.; Pestman, R.; Zhang, K.; Yao, J.; Feng, B.; Miao, P.; et al. Synthesis of stable and low-CO2 selective epsilon-iron carbide Fischer-Tropsch catalysts. Sci. Adv. 2018, 4, eaau2947. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Clifford, D.; El-Gendy, A.; Carpenter, E.E. Solvothermal synthesis of Fe7C3 and Fe3C nanostructures with phase and morphology control. J. Appl. Phys. 2016, 120, 033904. [Google Scholar] [CrossRef]
- Mansker, L.D.; Jin, Y.; Bukur, D.B.; Datye, A.K. Characterization of slurry phase iron catalysts for Fischer-Tropsch synthesis. Appl. Catal. A 1999, 186, 277–296. [Google Scholar] [CrossRef]
- Datye, A.K.; Jin, Y.; Mansker, L.; Motjope, R.T.; Dlamini, T.H.; Coville, N.J. The nature of the active phase in iron Fischer-Tropsch catalysts. Stud. Surf. Sci. Catal. 2000, 130, 1139–1144. [Google Scholar]
- Chang, Q.; Zhang, C.; Liu, C.; Wei, Y.; Cheruvathur, A.V.; Dugulan, A.I.; Niemantsverdriet, J.W.; Liu, X.; He, Y.; Qing, M.; et al. Relationship between Iron Carbide Phases (ε-Fe2C, Fe7C3, and χ-Fe5C2) and Catalytic Performances of Fe/SiO2 Fischer–Tropsch Catalysts. ACS Catal. 2018, 8, 3304–3316. [Google Scholar] [CrossRef]
- Bukur, D.B.; Okabe, K.; Rosynek, M.P.; Li, C.P.; Wang, D.J.; Rao, K.; Huffman, G.P. Activation Studies with a Precipitated Iron Catalyst for Fischer-Tropsch Synthesis: I. Characterization Studies. J. Catal. 1995, 155, 353–365. [Google Scholar] [CrossRef]
- Gnanamani, M.K.; Hamdeh, H.; Jacobs, G.; Shafer, W.E.; Sparks, D.; Davis, B. Fischer-Tropsch synthesis: Activity and Selectivity of Fe5C2 and Fe3C carbides. In Fischer-Tropsch Synthesis, Catalysts, and Catalysis; Davis, B.H., Occelli, M.L., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2016; pp. 15–30. [Google Scholar]
- De Smit, E.; Cinquini, F.; Beale, A.M.; Safonova, O.V.; van Beek, W.; Sautet, P.; Weckhuysen, B.M. Stability and reactivity of ϵ-χ-θ iron carbide catalyst phases in Fischer-Tropsch synthesis: controlling μC. J. Am. Chem. Soc. 2010, 132, 14928–14941. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, D.; Liu, X.; Zhang, Z.; Zhang, C.; Shi, B.; Xu, J.; Han, Y.-F. Operando Spectroscopic Study of Dynamic Structure of Iron Oxide Catalysts during CO2 Hydrogenation. ChemCatChem 2018, 10, 1272–1276. [Google Scholar] [CrossRef]
- Hong, S.Y.; Chun, D.H.; Yang, J.-I.; Jung, H.; Lee, H.-T.; Hong, S.; Jang, S.; Lim, J.T.; Kim, C.S.; Park, J.C. A new synthesis of carbon encapsulated Fe5C2 nanoparticles for high-temperature Fischer-Tropsch synthesis. Nanoscale 2015, 7, 16616–16620. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, S.; Wang, L.; Li, L.; Wang, G.; Zhang, Y.; Li, J. Comparison of preparation methods of iron-based catalysts for enhancing Fischer-Tropsch synthesis performance. Mol. Catal. 2018, 449, 99–105. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, A.; Liu, M.; Hu, S.; Ding, F.; Song, C.; Guo, X. Fe-MOF-derived highly active catalysts for carbon dioxide hydrogenation to valuable hydrocarbons. J. CO2 Util. 2017, 21, 100–107. [Google Scholar] [CrossRef]
- Wang, R.; Wu, B.; Li, Y.-W. Synthesis of Single-Phase Iron Carbides and Their Adsorption Performance. Chinese J. Catal. 2012, 33, 863–869. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Wang, W.; Lei, X.; Yang, H. Fe3C and Mn doped Fe3C nanoparticles: synthesis, morphology and magnetic properties. RSC Advances 2015, 5, 57828–57832. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, L.; Fan, S.; Zhao, T.; Zhang, K. Effect of pretreatment of iron catalyst on light olefin selectivity in CO hydrogenation. J. Fuel Chem. Tech. 2012, 40, 1110–1114. [Google Scholar] [CrossRef]
- Xu, J.; Bartholomew, C.H. Temperature-programmed hydrogenation (TPH) and in situ Mossbauer spectroscopy studies of carbonaceous species on silica-supported iron Fischer-Tropsch catalysts. J. Phys. Chem. B 2005, 109, 2392–2403. [Google Scholar] [CrossRef] [PubMed]
- Peña, D.; Jensen, L.; Cognigni, A.; Myrstad, R.; Neumayer, T.; van Beek, W.; Ronning, M. The Effect of Copper Loading on Iron Carbide Formation and Surface Species in Iron-Based Fischer-Tropsch Synthesis Catalysts. ChemCatChem 2018, 10, 1300–1312. [Google Scholar] [CrossRef]
- De Smit, E.; Weckhuysen, B.M. The renaissance of iron-based Fischer-Tropsch synthesis: on the multifaceted catalyst deactivation behaviour. Chem. Soc. Rev. 2008, 37, 2758–2781. [Google Scholar] [CrossRef]
- Peña, D.; Cognigni, A.; Neumayer, T.; van Beek, W.; Jones, D.S.; Quijada, M.; Ronning, M. Identification of carbon species on iron-based catalysts during Fischer-Tropsch synthesis. Appl. Catal., A 2018, 554, 10–23. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, B.; Qiao, M.; Wei, J.; Yue, Q.; Wang, C.; Deng, Y.; Kaliaguine, S.; Zhao, D. A General Chelate-Assisted Co-Assembly to Metallic Nanoparticles-Incorporated Ordered Mesoporous Carbon Catalysts for Fischer-Tropsch Synthesis. J. Am. Chem. Soc. 2012, 134, 17653–17660. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, M.; Huang, C.; Zhong, L.; Fang, K. Carbon-encapsulated highly dispersed FeMn nanoparticles for Fischer-Tropsch synthesis to light olefins. New J. Chem. 2018, 42, 2413–2421. [Google Scholar] [CrossRef]
- Wezendonk, T.A.; Santos, V.P.; Nasalevich, M.A.; Warringa, Q.S.E.; Dugulan, A.I.; Chojecki, A.; Koeken, A.C.J.; Ruitenbeek, M.; Meima, G.; Islam, H.-U.; et al. Elucidating the Nature of Fe Species during Pyrolysis of the Fe-BTC MOF into Highly Active and Stable Fischer-Tropsch Catalysts. ACS Catal. 2016, 6, 3236–3247. [Google Scholar] [CrossRef]
- Herranz, T.; Rojas, S.; Perez-Alonso, F.J.; Ojeda, M.; Terreros, P.; Fierro, J.L.G. Genesis of iron carbides and their role in the synthesis of hydrocarbons from synthesis gas. J. Catal. 2006, 243, 199–211. [Google Scholar] [CrossRef]
- Li, L.; Han, W.-F.; Li, L.-H.; Liu, H.Z. Modification of Wustite Based Ammonia Synthesis Catalyst With Fine Fe particles. J. Mol. Catal. 2017, 31, 206–214. [Google Scholar]

| Samples | Surface Area (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| IO-320 °C | 92.79 | 18.24 | 0.42 |
| IO-350 °C | 95.08 | 16.44 | 0.39 |
| IO-360 °C | 64.61 | 17.99 | 0.29 |
| IO-375 °C | 50.02 | 14.77 | 0.18 |
| IO-450 °C | 76.12 | 7.38 | 0.14 |
| Catalysts | X(CO)/% | S(CO2)/% | O/Pb | Hydrocarbon Distribution/C mol% | |||
|---|---|---|---|---|---|---|---|
| CH4 | C2-C4 olefins | C2-C4 paraffins | C5+ | ||||
| IO-320 °C | 96.23 | 26.37 | 1.31 | 17.65 | 21.31 | 16.24 | 44.80 |
| IO-350 °C | 85.18 | 24.35 | 1.51 | 16.96 | 21.37 | 14.21 | 47.46 |
| IO-360 °C | 75.46 | 24.40 | 1.53 | 18.09 | 22.86 | 14.96 | 44.09 |
| IO-375 °C | 47.59 | 23.54 | 1.03 | 21.15 | 20.55 | 20.02 | 38.27 |
| IO-450 °C | 49.01 | 20.95 | 1.07 | 17.17 | 16.47 | 15.46 | 50.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Zhang, H.; Liu, Y.; Ning, W.; Han, W.; Liu, H.; Huo, C. Preparation of Iron Carbides Formed by Iron Oxalate Carburization for Fischer–Tropsch Synthesis. Catalysts 2019, 9, 347. https://doi.org/10.3390/catal9040347
Yang X, Zhang H, Liu Y, Ning W, Han W, Liu H, Huo C. Preparation of Iron Carbides Formed by Iron Oxalate Carburization for Fischer–Tropsch Synthesis. Catalysts. 2019; 9(4):347. https://doi.org/10.3390/catal9040347
Chicago/Turabian StyleYang, Xiazhen, Hong Zhang, Yingxin Liu, Wensheng Ning, Wenfeng Han, Huazhang Liu, and Chao Huo. 2019. "Preparation of Iron Carbides Formed by Iron Oxalate Carburization for Fischer–Tropsch Synthesis" Catalysts 9, no. 4: 347. https://doi.org/10.3390/catal9040347
APA StyleYang, X., Zhang, H., Liu, Y., Ning, W., Han, W., Liu, H., & Huo, C. (2019). Preparation of Iron Carbides Formed by Iron Oxalate Carburization for Fischer–Tropsch Synthesis. Catalysts, 9(4), 347. https://doi.org/10.3390/catal9040347






