Efficient Biodiesel Conversion from Microalgae Oil of Schizochytrium sp.
Abstract
1. Introduction
2. Results and Discussion
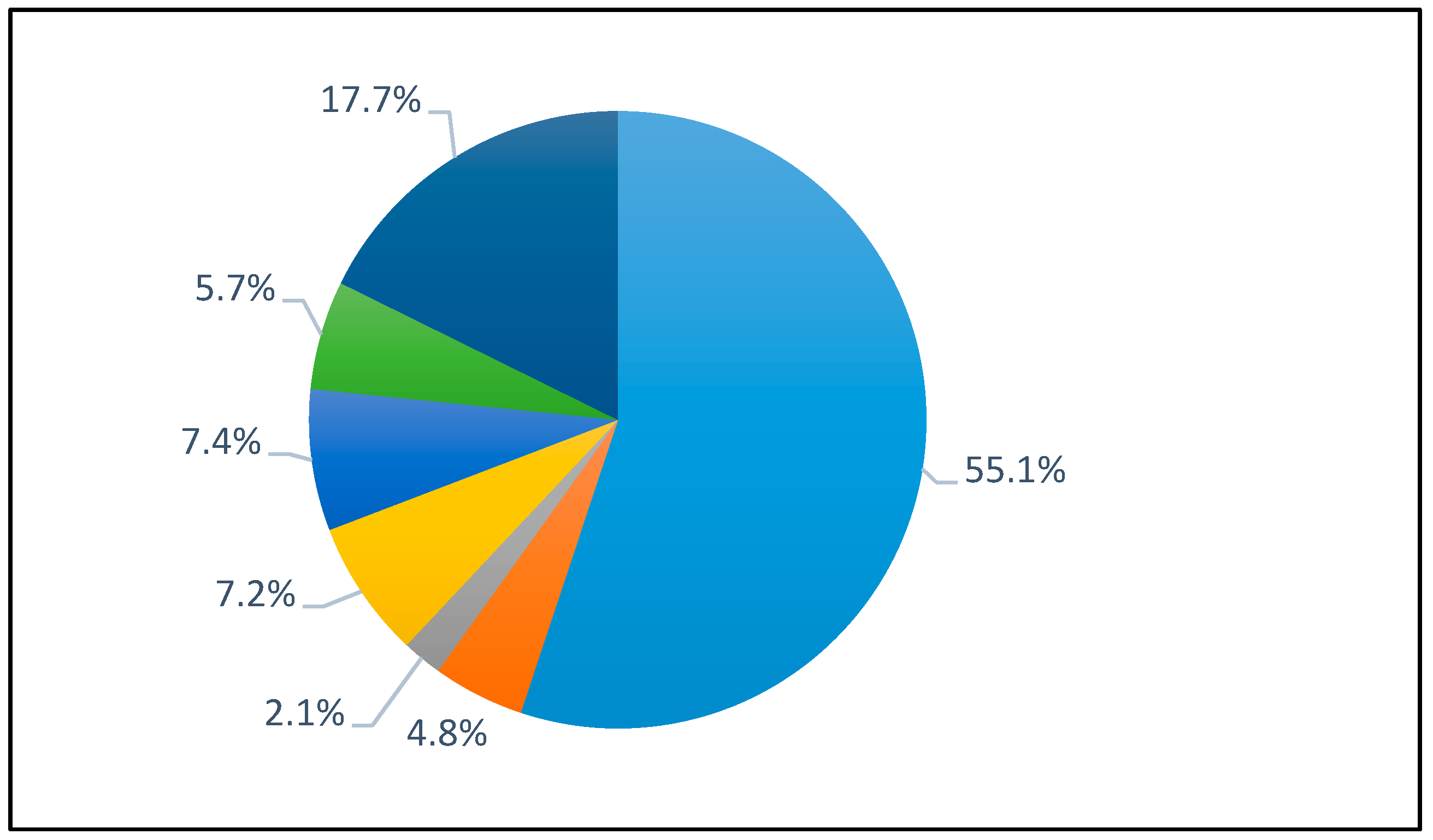
2.1. Analysis of FFAs Constitution of Schizochytrium sp. Oil
2.2. Hydrolysis of Microalgae Oil from Schizochytrium sp. at Non-Catalytic Condition
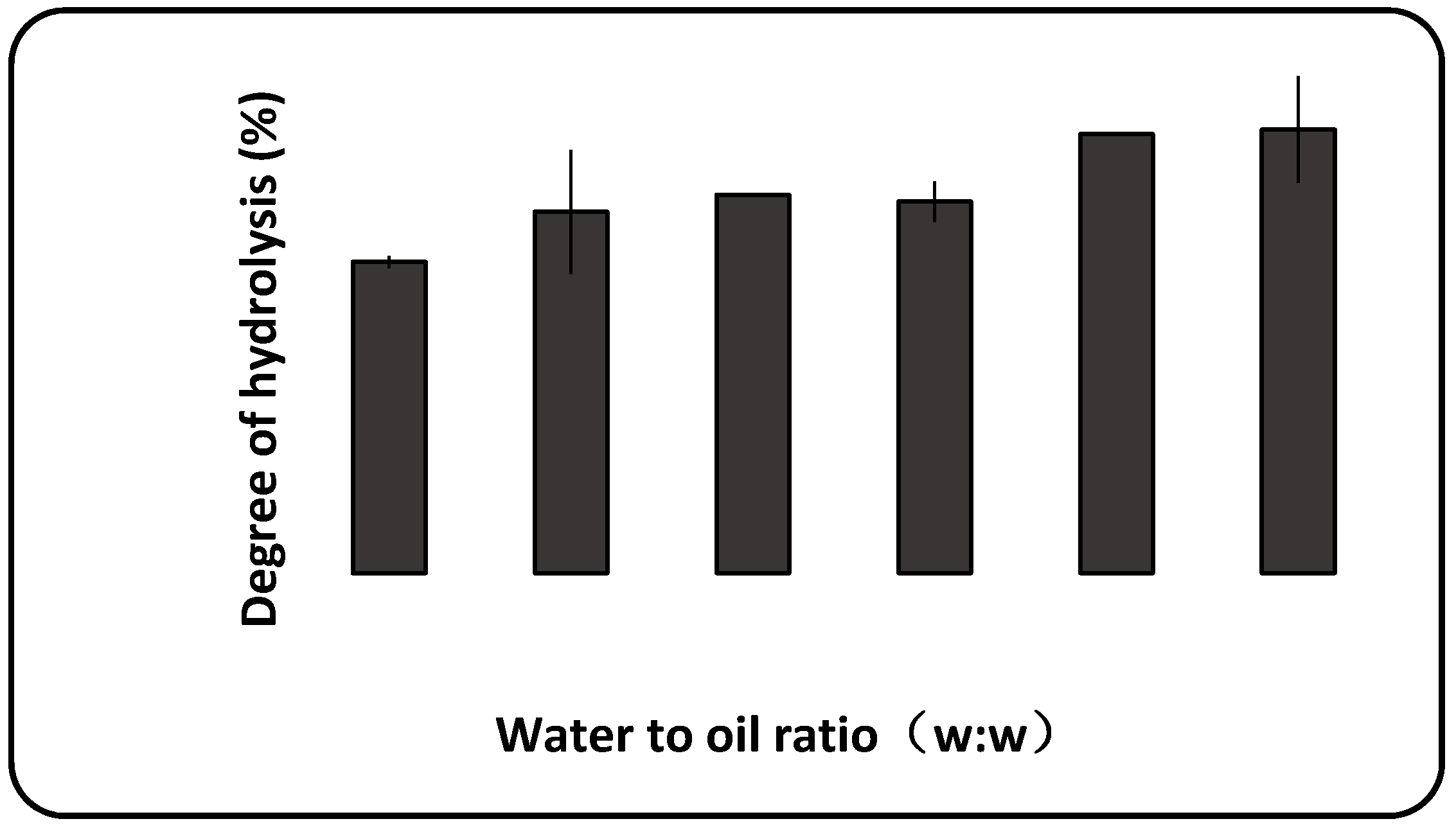
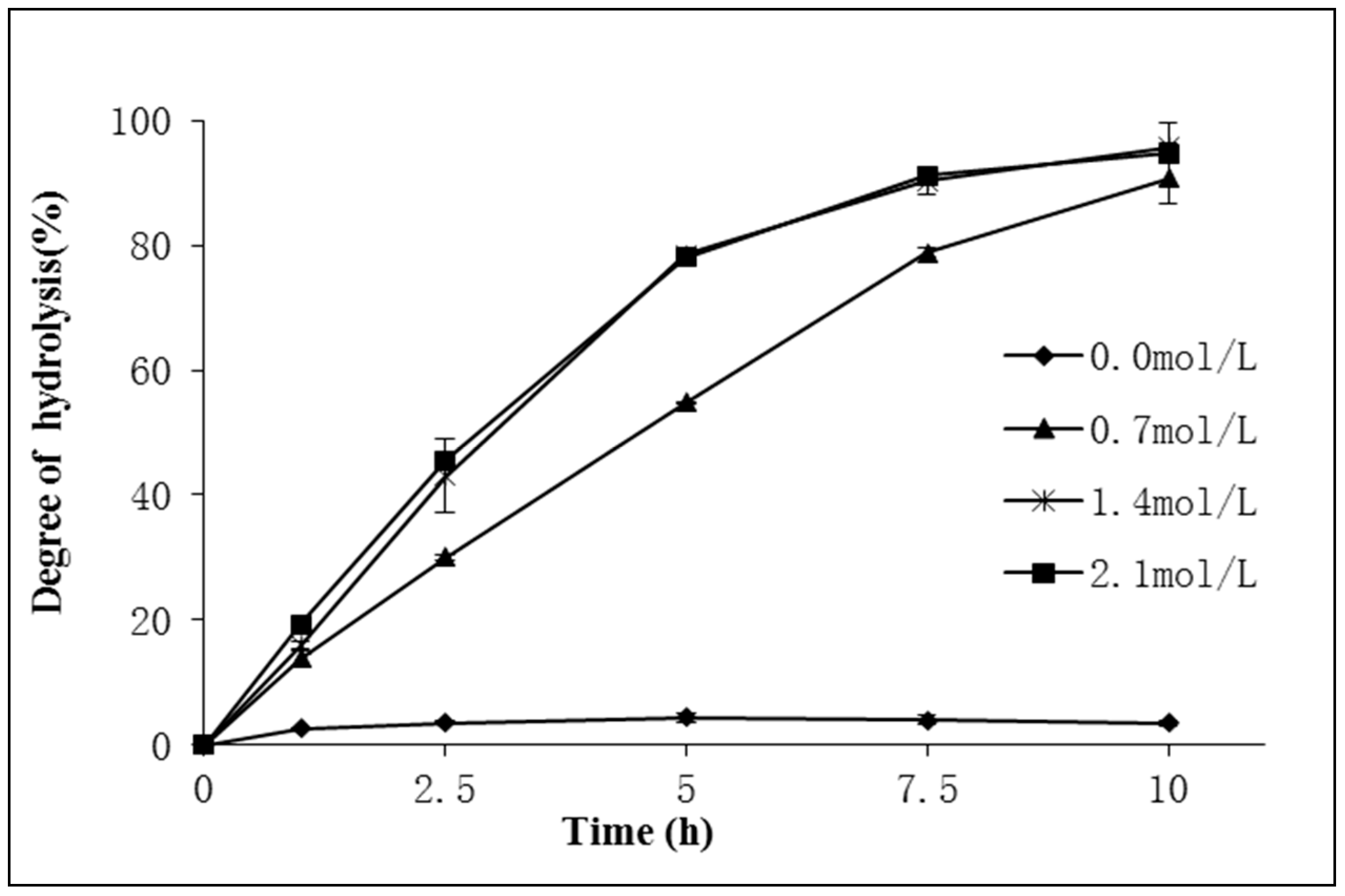
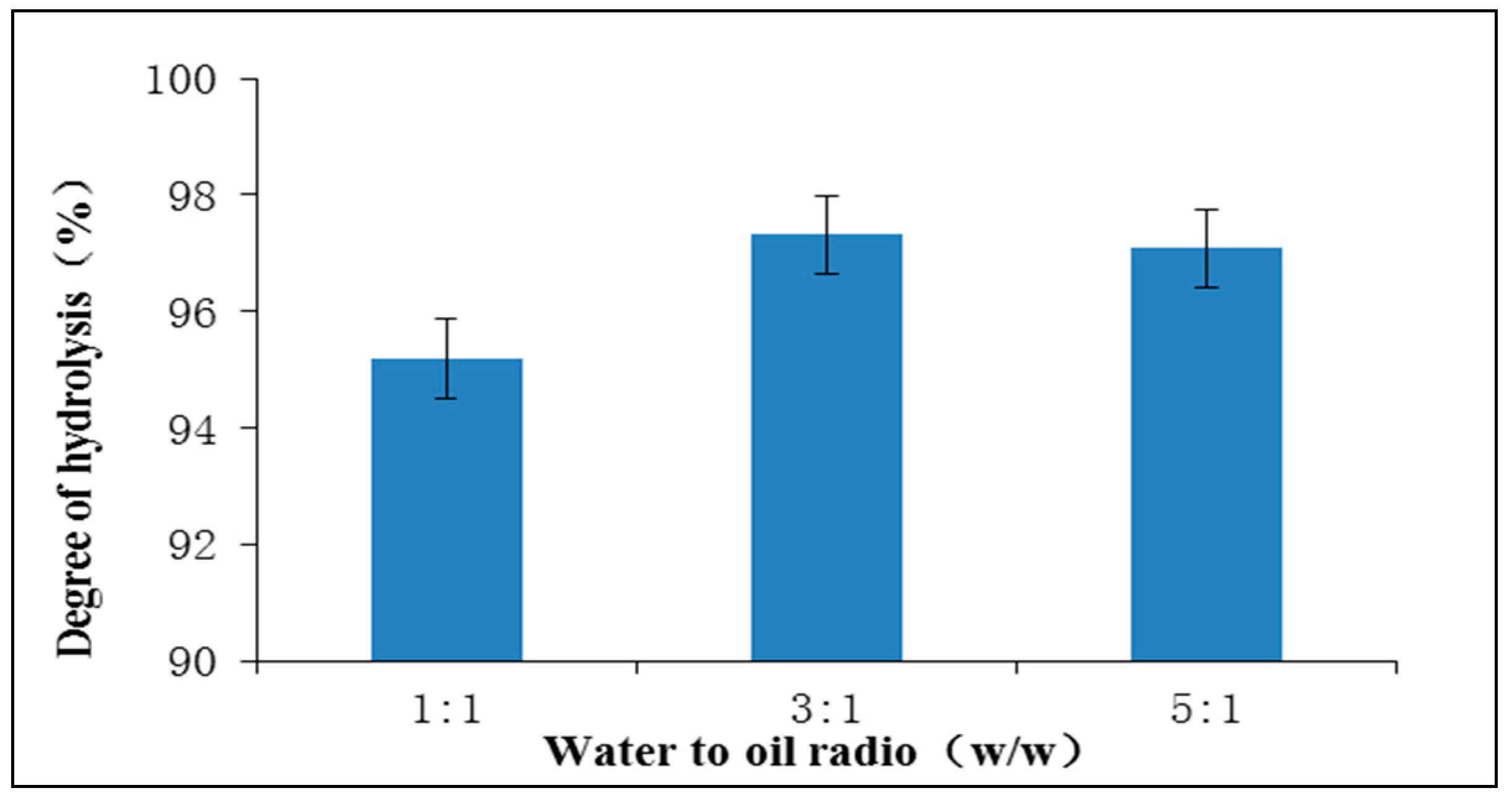
2.2.1. The Effect of Water to Oil Ratio on the Hydrolysis of Schizochytrium sp. Oil
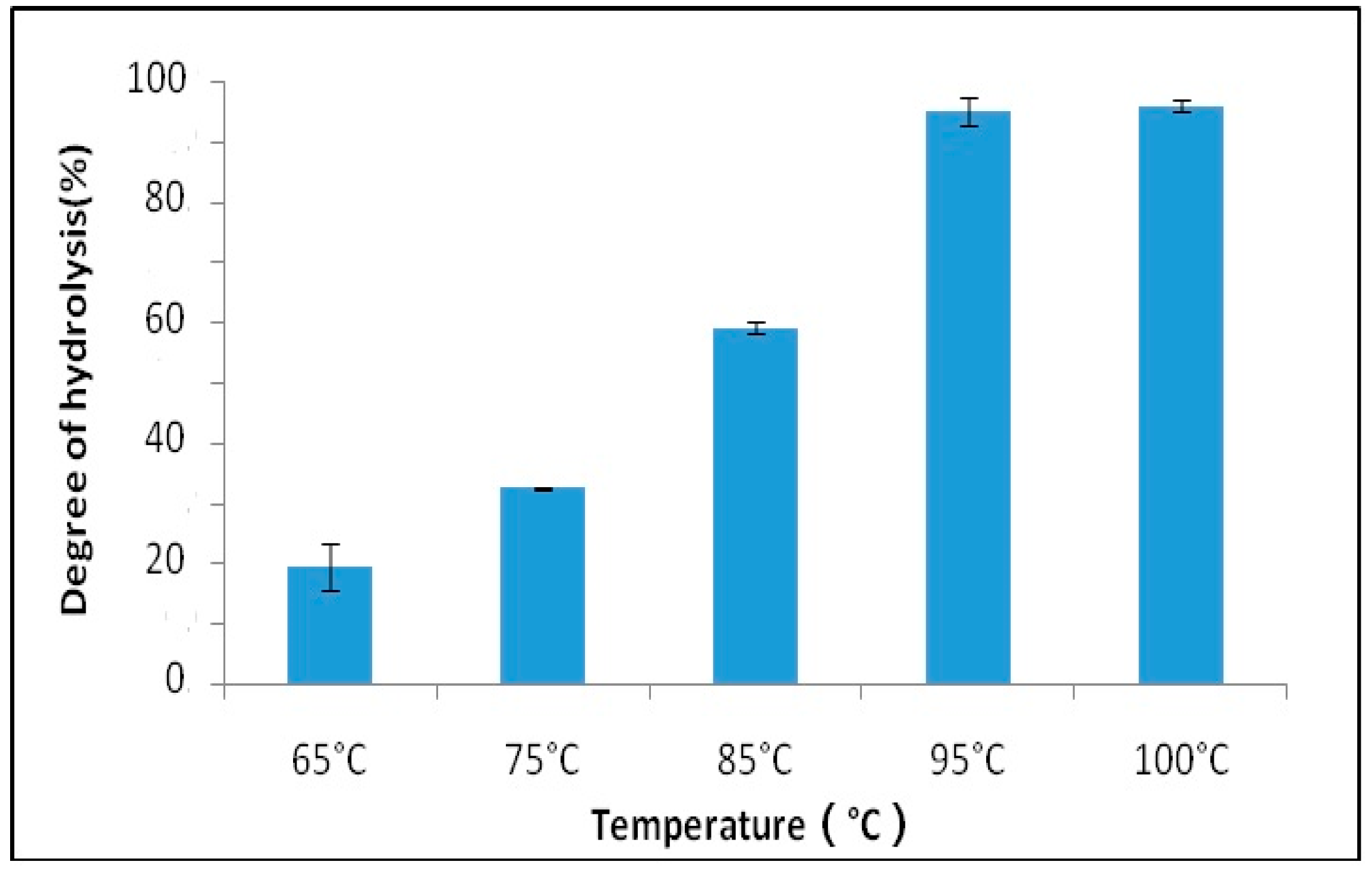
2.2.2. The Effect of Temperature on the Hydrolysis of Schizochytrium sp. Oil
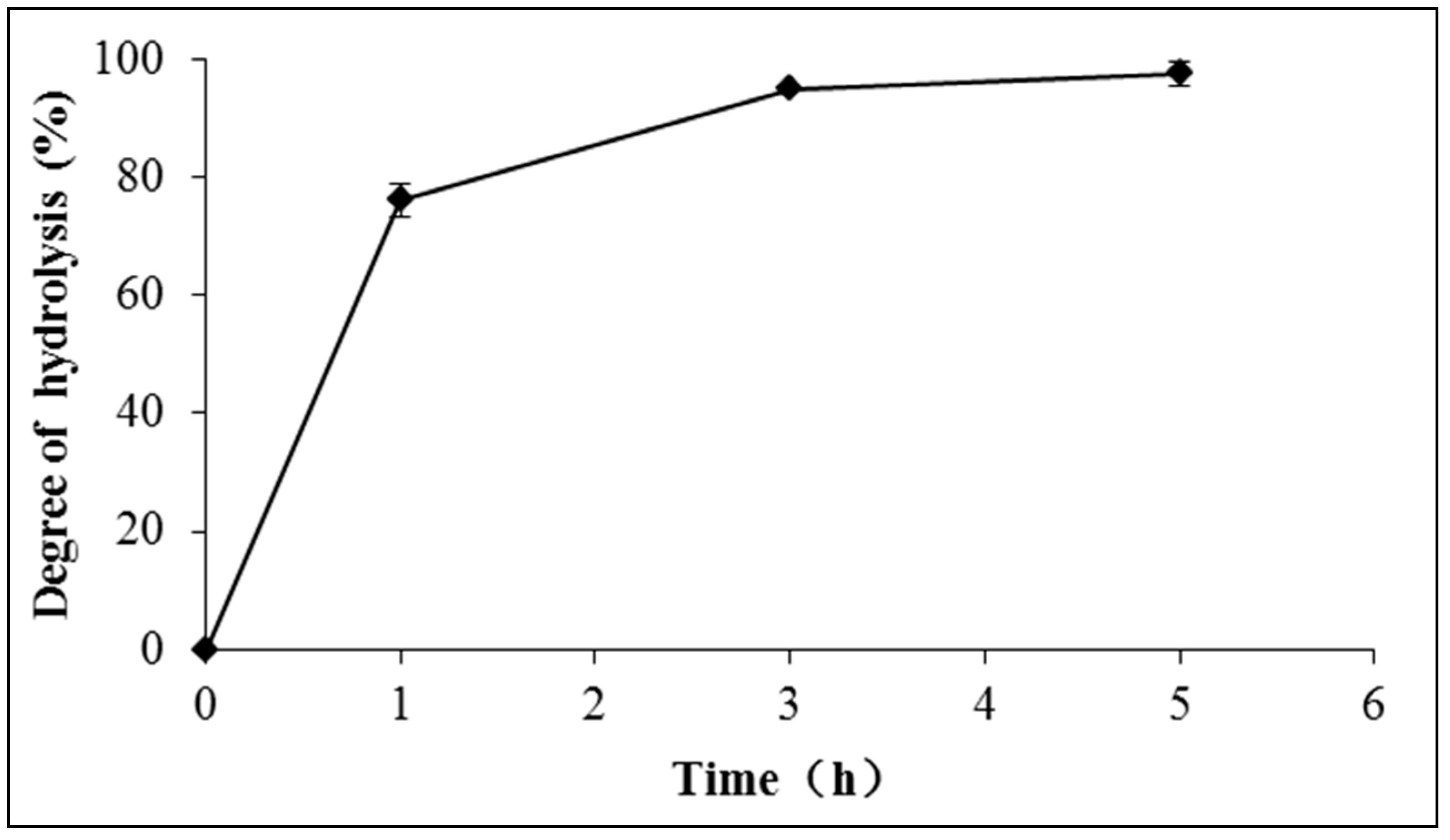
2.2.3. The Effect of Reaction Time on the Hydrolysis of Schizochytrium sp. Oil
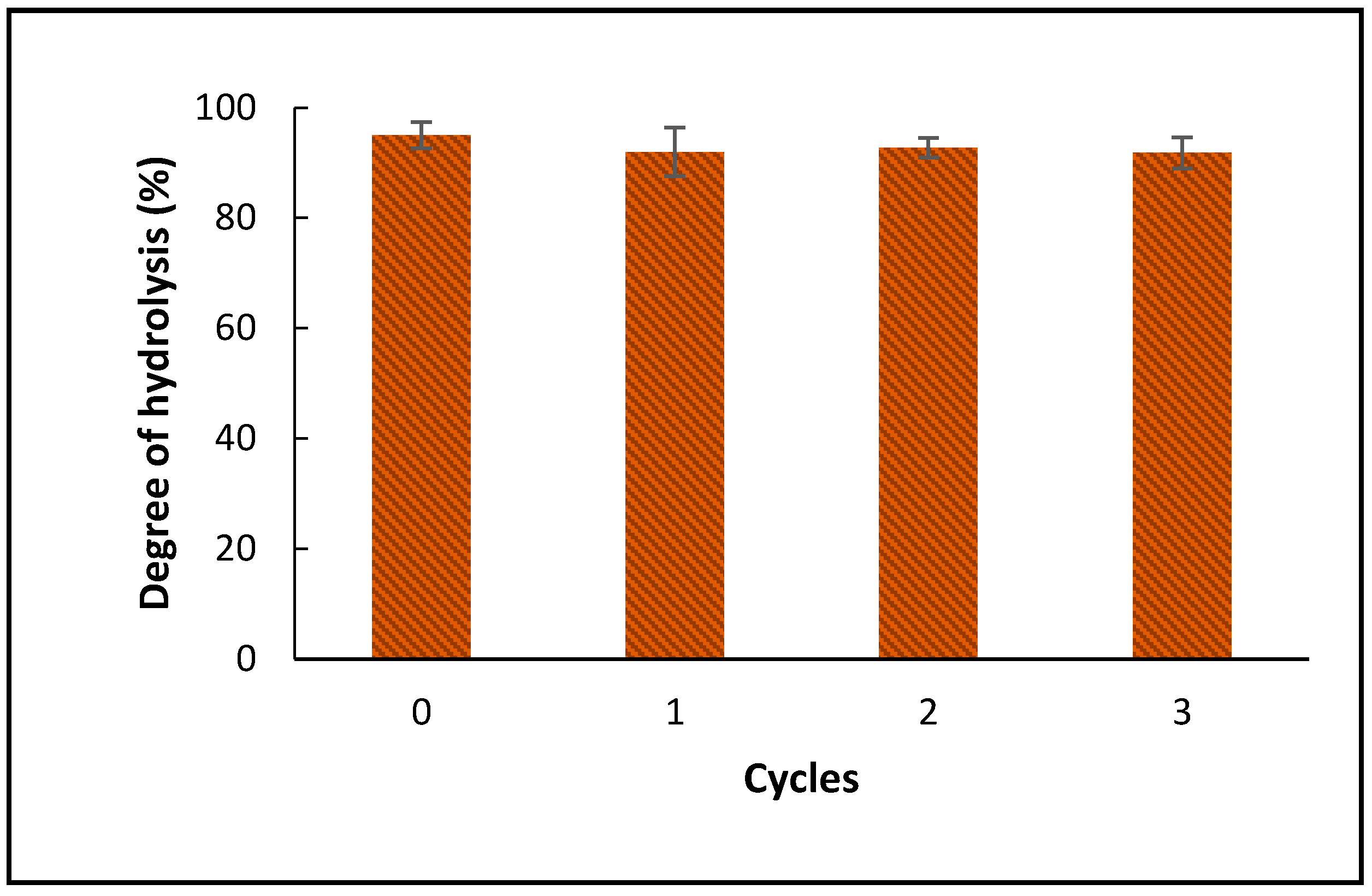
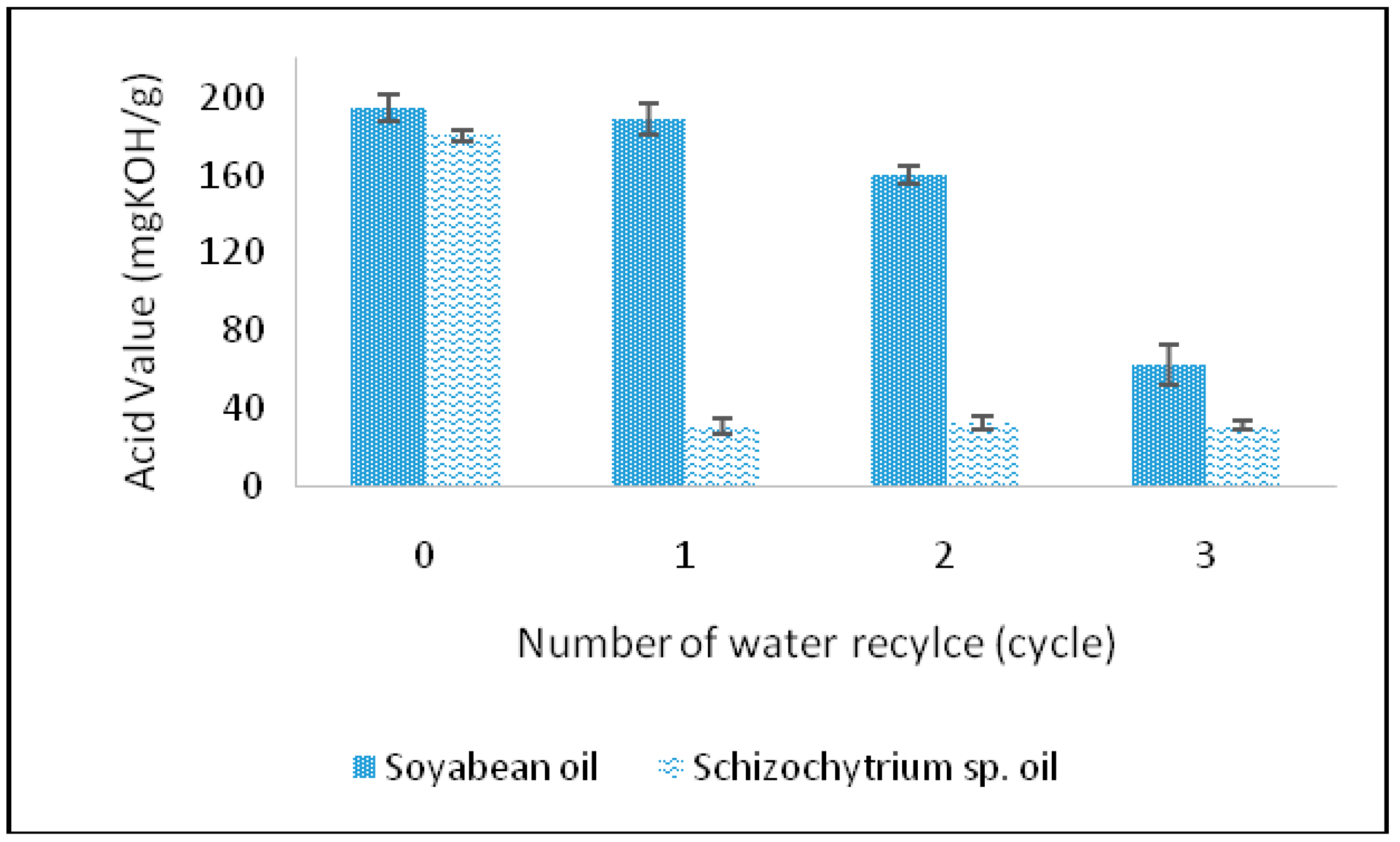
2.2.4. The Effect of Water Recycle on the Hydrolysis of Schizochytrium sp. Oil
2.3. Hydrolysis of Microalgae Oil from Schizochytrium sp. with Acid Catalysis
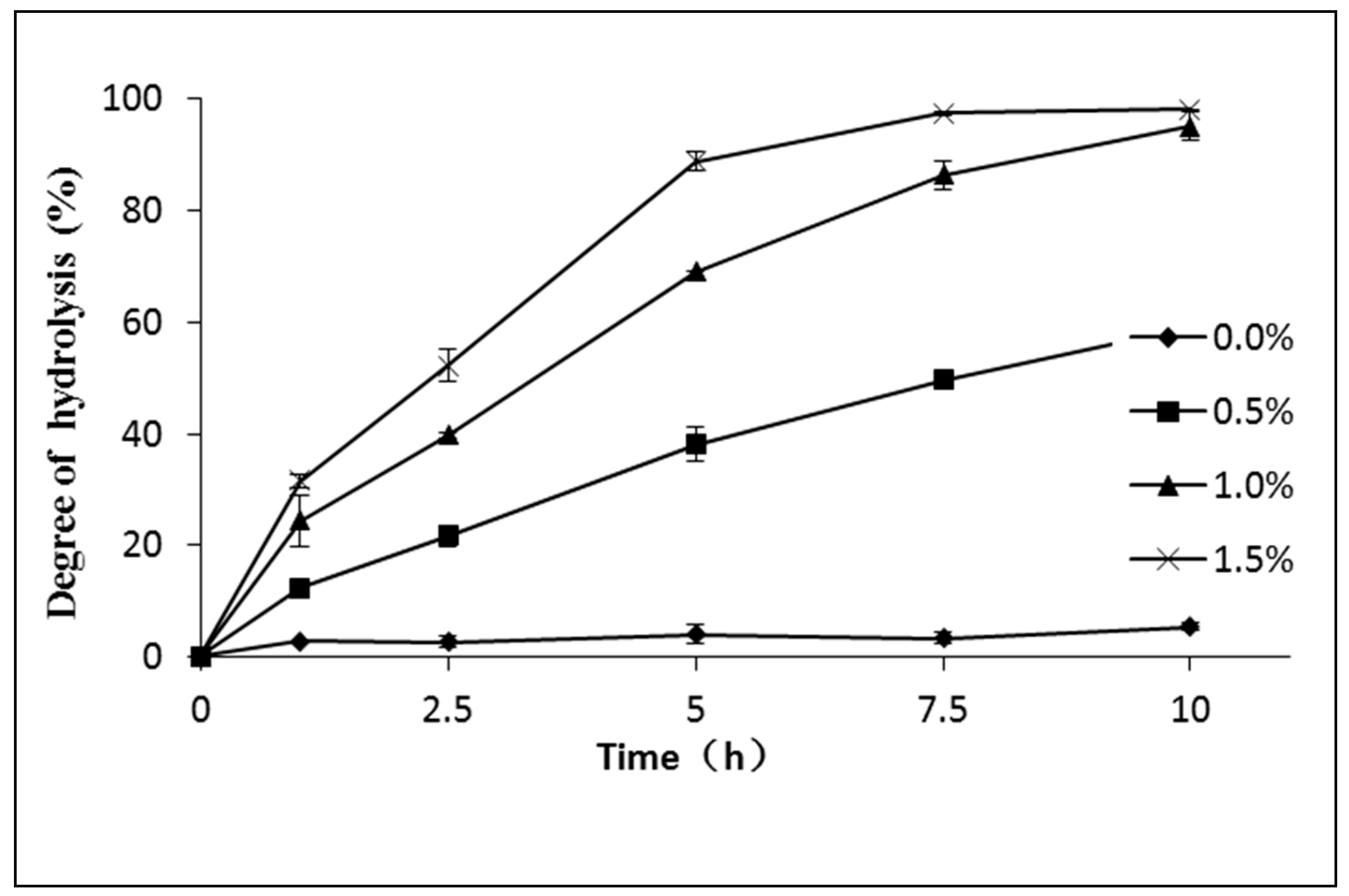
2.3.1. The Effect of the Acid Catalysis—Sulphuric Acid on the Hydrolysis of Schizochytrium sp. Oil
2.3.2. The Effect of Sodium Dodecylsulfate Addition on the Hydrolysis of Schizochytrium sp. Oil
2.3.3. The Effect of Water to Oil Ratio on Schizochytrium sp. Oil Hydrolysis
2.3.4. The Effect of Temperature on the Hydrolysis of Schizochytrium sp. Oil
2.3.5. The Effect of Stirring Speed on the Hydrolysis of Schizochytrium sp. Oil
2.3.6. The Effect of Water Recycle on the Hydrolysis of Schizochytrium sp. Oil
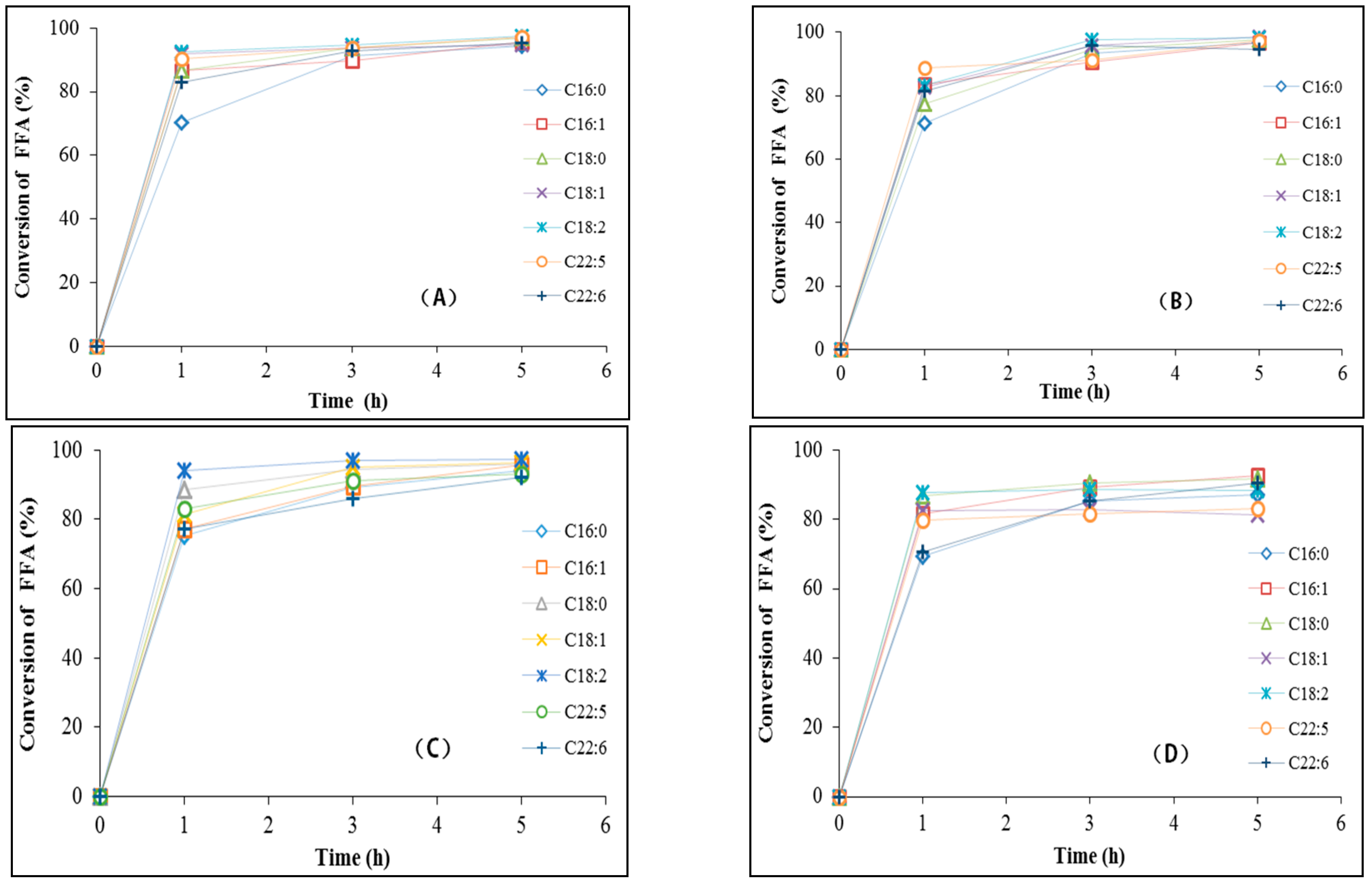
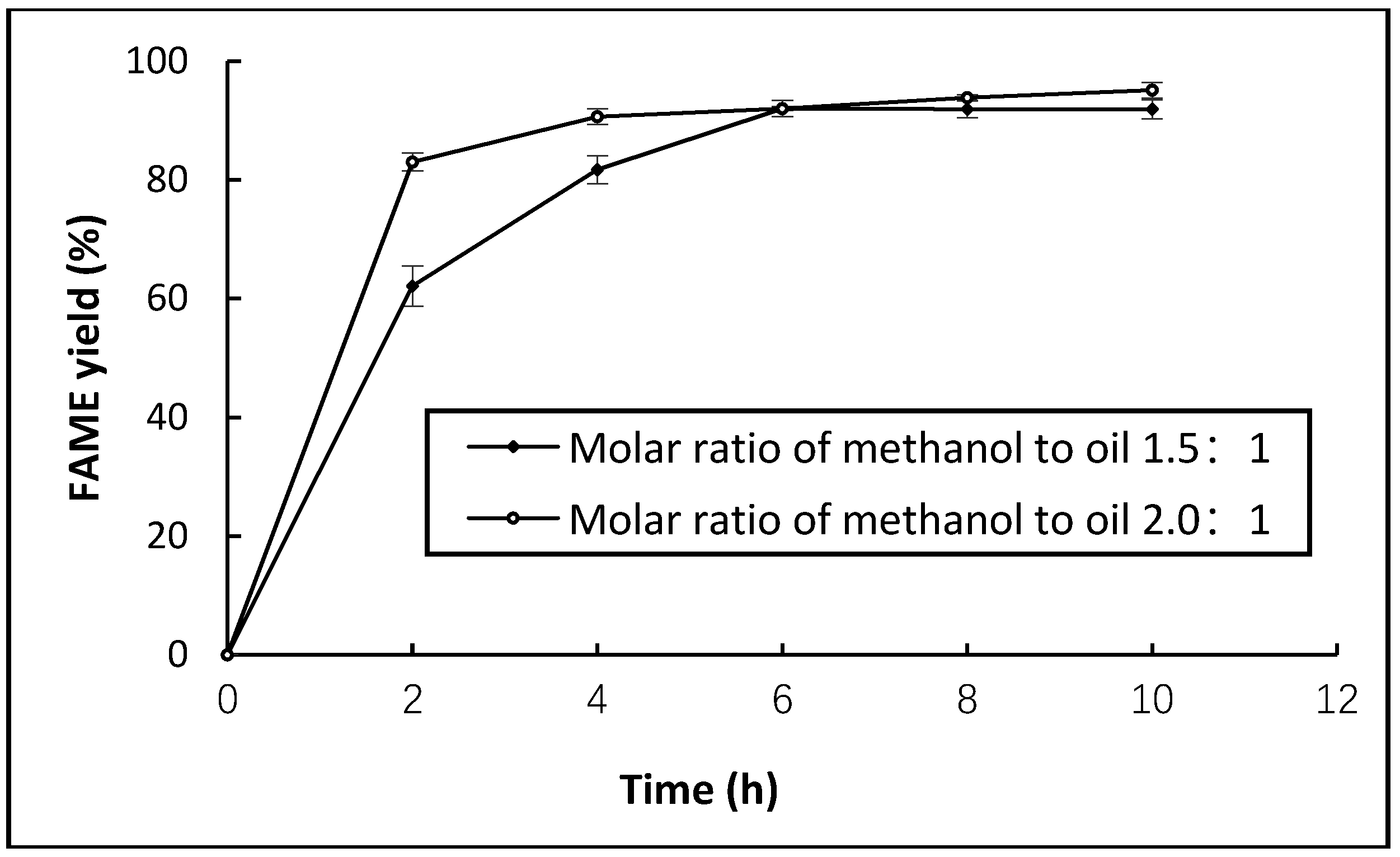
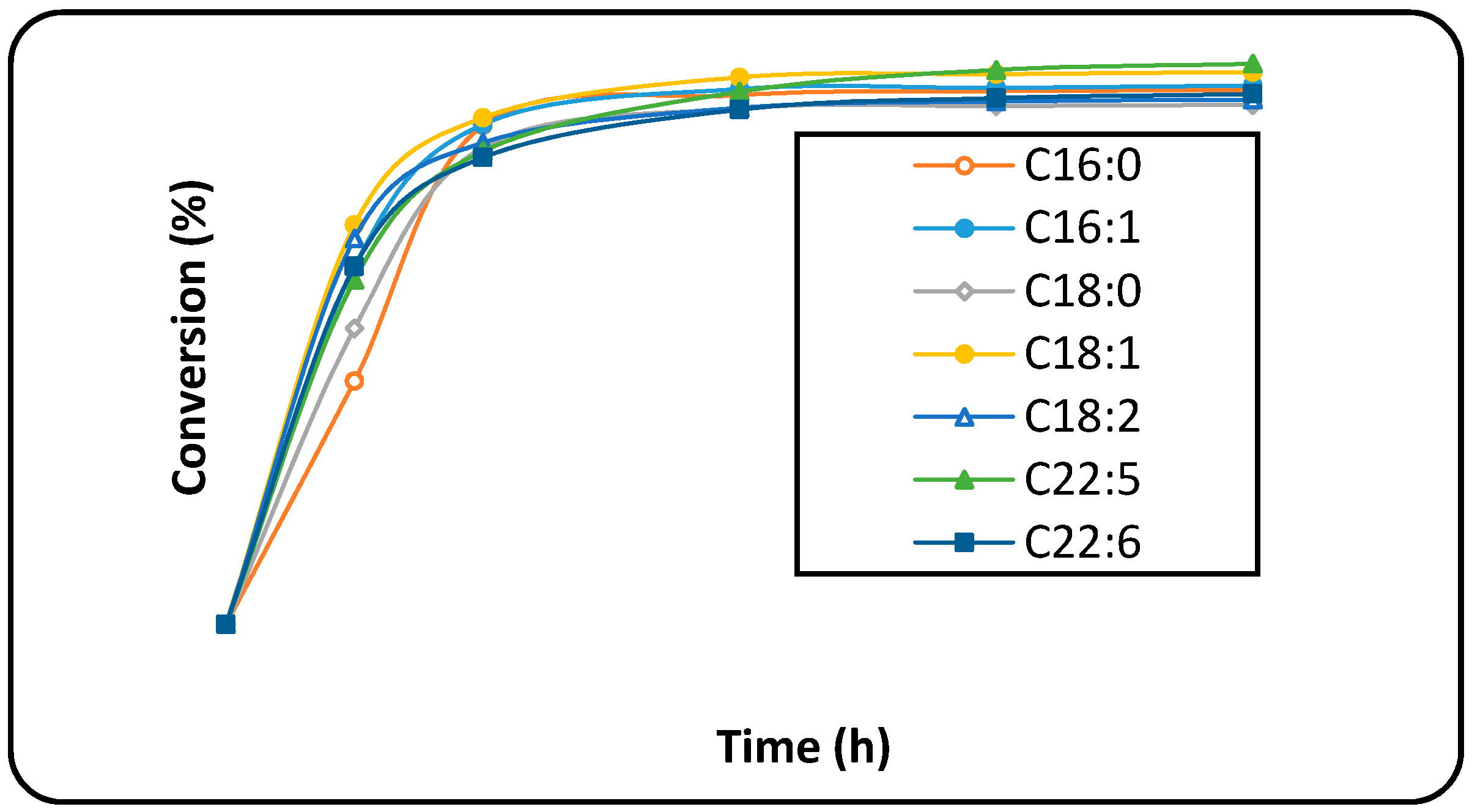
2.4. Lipase-Mediate Reaction of Hydrolysed Microalgae Oil for Biodiesel Production
3. Materials and Methods
3.1. Materials
3.2. Experimental Procedure
3.2.1. Non-Catalytic Hydrolysis Process
3.2.2. Catalytic Hydrolysis Process with Sulphuric Acid
3.2.3. Enzymatic Esterification
3.3. Analytical Methods
3.3.1. Determination of Acid Value and Degree of Hydrolysis
3.3.2. Fatty Acid Methyl Ester (FAME) Analysis
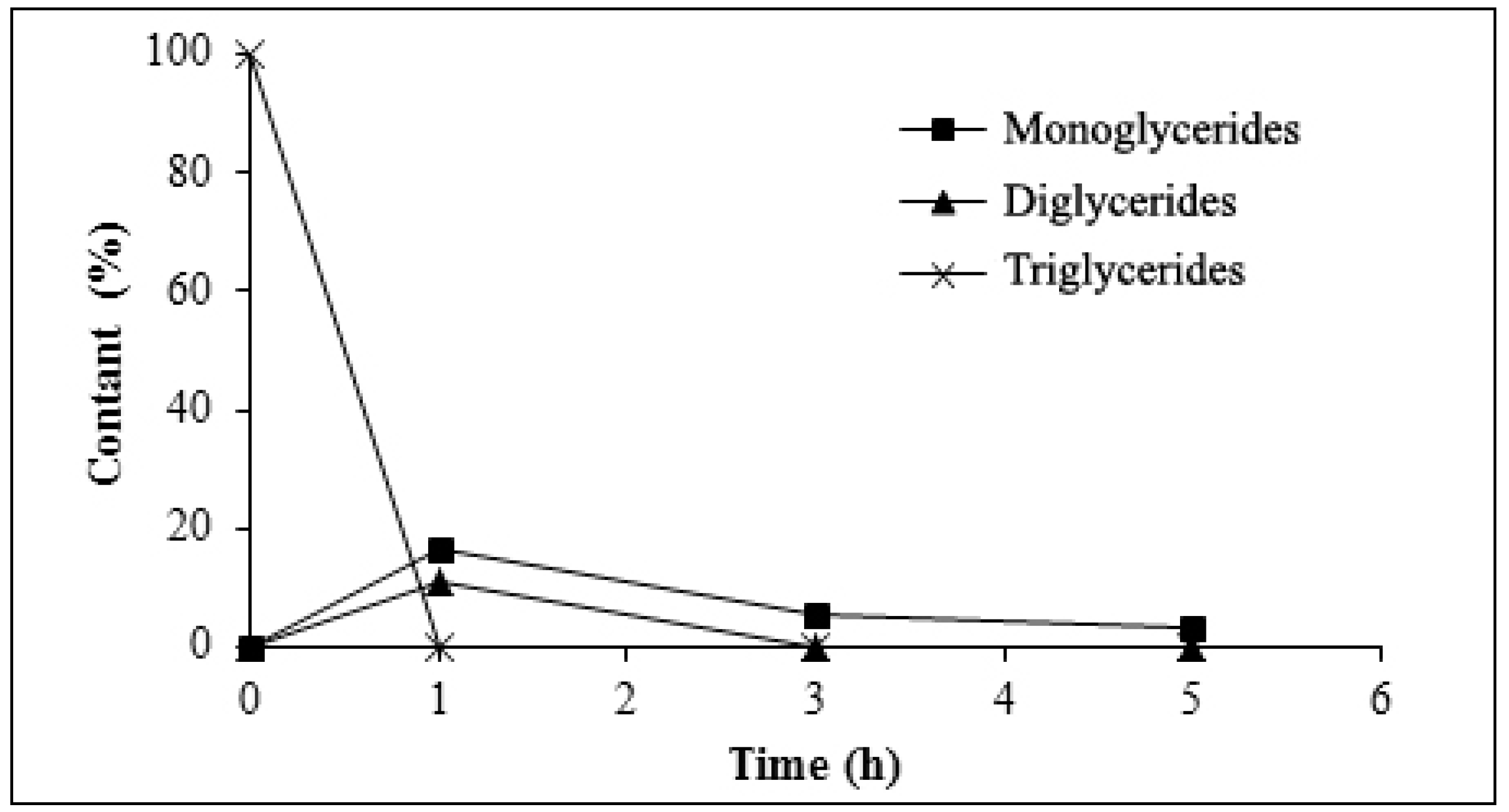
3.3.3. Analysis of Glyceride
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Enamala, M.K.; Enamala, S.; Chavali, M.; Donepudi, J.; Yadavalli, R.; Kolapalli, B.; Aradhyula, T.V.; Velpuri, J.; Kuppam, C. Production of biofuels from microalgae-A review on cultivation, harvesting, lipid extraction, and numerous applications of microalgae. Renew. Sustain. Energy. Rev. 2018, 94, 49–68. [Google Scholar] [CrossRef]
- Raslavičiusa, L.; Striūgasb, N.; Felneris, M. New insights into algae factories of the future. Renew. Sustain. Energy Rev. 2018, 81, 643–654. [Google Scholar] [CrossRef]
- Collet, P.; Hélias, A.; Lardon, L.; Steyer, J.P.; Bernard, O. Recommendations for Life Cycle Assessment of algal fuels. Appl. Energy. 2015, 154, 1089–1102. [Google Scholar] [CrossRef]
- Bux, F.; Chisti, Y. (Eds.) Algae Biotechnology: Products and Processes; Springer: New York, NY, USA, 2016. [Google Scholar]
- Law, S.Q.K.; Halim, R.; Scales, P.J.; Martin, G.J.O. Conversion and recovery of saponifiable lipids from microalgae using a nonpolar solvent via lipase-assisted extraction. Bioresour. Technol. 2018, 260, 338–347. [Google Scholar] [CrossRef] [PubMed]
- López, E.N.; Medina, A.R.; Cerdán, L.E.; Moreno, P.A.G.; Sánchez, M.D.G.; Grima, E.M. Fatty acid methyl ester production from wet microalgal biomass by lipasecatalyzed direct transesterification. Biomass Bioenerg. 2016, 93, 6–12. [Google Scholar] [CrossRef]
- Torres, S.; Acien, G.; García-Cuadra, F.; Navia, R. Direct transesterification of microalgae biomass and biodiesel refining with vacuum distillation. Algal. Res. 2017, 28, 30–38. [Google Scholar] [CrossRef]
- Kochepka, D.M.; Dill, L.P.; Couto, G.H.; Krieger, N.; Ramos, L.P. Production of fatty acid ethyl esters from waste cooking oil using Novozym435 in a solvent-free system. Energy. Fuel 2015, 29, 8074–8081. [Google Scholar] [CrossRef]
- Chen, C.L.; Huang, C.C.; Ho, K.C.; Hsiao, P.X.; Wu, M.S.; Chang, J.S. Biodiesel production from wet microalgae feedstock using sequential wet extraction/transesterification and direct transesterification processes. Bioresour. Technol. 2015, 194, 179–186. [Google Scholar] [PubMed]
- Guldhe, A.; Singh, B.; Mutanda, T.; Permaul, K.; Bux, F. Advances in synthesis of biodiesel via enzyme catalysis: Novel and sustainable approaches. Renew. Sust. Energ. Rev. 2015, 41, 1447–1464. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, M.S.; Lee, Y.C.; Yang, J.W. Advances in direct transesterification of algal oils from wet biomass. Bioresour. Technol. 2015, 184, 267–275. [Google Scholar] [CrossRef]
- Duan, Z.Q.; Du, W.; Liu, D.H. Novozym435-catalyzed 1,3-diacylglycerol preparation via esterification in t-butanol system. Process. Biochem. 2010, 45, 1923–1927. [Google Scholar] [CrossRef]
- Ma, G.J.; Dai, L.M.; Liu, D.H.; Du, W. A Robust Two-Step Process for the Efficient Conversion of Acidic Soybean Oil for Biodiesel Production. Catalysts 2018, 8, 527. [Google Scholar] [CrossRef]
- Ullah, F.; Dong, L.; Bano, A.; Peng, Q.; Huang, J. Current advances in catalysis toward sustainable biodiesel production. J. Energy Inst. 2016, 89, 282–292. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, O.K.; Kim, C.H.; Seo, J.W.; Oh, B.R.; Lee, E.Y. Lipase-catalyzed in-situ biosynthesis of glycerol-free biodiesel from heterotrophic microalgae, Aurantiochytrium sp KRS101 biomass. Bioresour. Technol. 2016, 211, 472–477. [Google Scholar] [CrossRef]
- IGo, A.R.; Lee, Y.; Kim, Y.H.; Park, S.; Choi, J.; Lee, J.; Han, S.O.; Kim, S.W.; Park, C. Enzymatic coproduction of biodiesel and glycerol carbonate from soybean oil in solvent-free system. Enzyme. Microb. Technol. 2013, 53, 154–158. [Google Scholar]
- Shimada, Y.; Watanabe, Y.; Sugihara, A.; Tominaga, Y. Enzymatic alcoholysis for biodiesel fuel production and application of the reaction to oil processing. J. Mol. Catal. B Enzym. 2002, 17, 133–142. [Google Scholar] [CrossRef]
- Tian, X.G.; Dai, L.M.; Liu, D.H.; Du, W. Improved lipase-catalyzed methanolysis for biodiesel production by combining in-situ removal of by-product glycerol. Fuel 2018, 232, 45–50. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Cao, X.J. Enzymatic synthesis of fatty acid ethyl esters by utilizing camellia oil soapstocks and diethyl carbonate. Bioresour. Technol. 2011, 102, 10173–10179. [Google Scholar] [CrossRef]
- Min, J.Y.; Lee, E.Y. Lipase-catalyzed simultaneous biosynthesis of biodiesel and glycerol carbonate from corn oil in dimethyl carbonate. Biotechnol. Lett. 2011, 33, 1789–1796. [Google Scholar] [CrossRef]
- Tirla, C.; Smith, B.R.; Dooling, T.; Kingsbury, N.; McKee, C.; Panter, R.; Allen, V.A. A Study of Alternative Catalysts and Analysis Methods for Biodiesel Production. Eur. Sci. J. 2017, 13. [Google Scholar] [CrossRef][Green Version]
- Gomez-Castro, F.I.; Segovia-Hernandez, J.G.; Hernandez, S.; Rico-Ramirez, V.; Gutierrez-Antonio, C.; Briones-Ramirez, A.; Cano-Rodriguez, I.; Gamino-Arroyo, Z. Analysis of alternative non-catalytic processes for the production of biodiesel fuel. Clean Technol. Environ. Policy. 2015, 17, 2041–2054. [Google Scholar] [CrossRef]
- Chen, C.; Chen, W.; Chang, C.J.; Setsu, I.; Tu, C.; Shieh, C. Subcritical hydrolysis and supercritical methylation of supercritical carbon dioxide extraction of Jatropha oil. Sep. Purif. Technol. 2010, 74, 7–13. [Google Scholar] [CrossRef]
- Kabbashi, N.A.; Mohammed, N.I.; Alam, M.Z.; Mirghani, M.E.S. Hydrolysis of Jatropha curcas oil for biodiesel synthesis using immobilized Candida cylindracea lipase. J. Mol. Catal. B Enzym. 2015, 116, 95–100. [Google Scholar] [CrossRef]
- Tian, X.G.; Dai, L.M.; Liu, M.S.; Liu, D.H.; Du, W.; Wu, H. Lipase-catalyzed methanolysis of microalgae oil for biodiesel production and PUFAs concentration. Catal. Commun. 2016, 84, 44–47. [Google Scholar] [CrossRef]
- Kumar, B.R.; Deviram, G.; Mathimani, T.; Duc, P.A.; Pugazhendhi, A. Microalgae as rich source of polyunsaturated fatty acids. Biocatal. Agri. Biotechnol. 2019, 17, 583–588. [Google Scholar] [CrossRef]
- Rohit, M.V.; Mohan, S.V. Quantum Yield and Fatty Acid Profile Variations with Nutritional Mode during Microalgae Cultivation. Front. Bioeng. Biotechnol. 2018, 6, 111. [Google Scholar] [CrossRef] [PubMed]
- Vassilev, S.V.; Vassileva, C.G. Composition, properties and challenges of algae biomass for biofuel application: An overview. Fuel 2016, 181. [Google Scholar] [CrossRef]
- Valverde, L.M.; Moreno, P.A.G.; Cerdán, L.E.; López, E.N.; López, B.C.; Medina, A.R. Concentration of docosahexaenoic and eicosapentaenoic acids by enzymatic alcoholysis with different acyl-acceptors. Biochem. Eng. J. 2014, 91, 163–173. [Google Scholar] [CrossRef]
- Tian, X.G.; Du, W.; Dai, L.M.; Liu, D.H. The development of enzymatic enrichment and separation of ω-3PUFAs. J. Chem. Eng. Chin. Univ. 2015, 29, 1285–1292. [Google Scholar]
- Chauton, M.S.; Reitan, K.I.; Norsker, N.H.; Tveterås, R.; Kleivdal, H.T. A technoeconomic analysis of industrial production of marine microalgae as a source of EPA and DHA-rich raw material for aquafeed: research challenges and possibilities. Aquaculture. 2015, 436, 95–103. [Google Scholar] [CrossRef]
- Lee, J.H.; O’Keefe, J.H.; Lavie, C.J.; Harris, W.S. Omega-3 fatty acids: Cardiovascular benefits, sources and sustainability. Nat. Rev. Cardiol. 2009, 6, 753–758. [Google Scholar] [CrossRef]
- Imai, Y. Role of omega-3 PUFA-derived mediators, the protectins, in influenza virus infection. Biochim et Biophys. Acta Mol. Cell. Biol. Lipids. 2015, 1851, 496–502. [Google Scholar] [CrossRef] [PubMed]
- La Rovere, M.T.; Christensen, J.H. The autonomic nervous system and cardiovascular disease: Role of n-3 PUFAs. Vasc. Pharmacol. 2015, 71. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Shamair, Z.; Ali, S.; Ahmad, N.; Hafeez, A.; Fazal, T.; Saif Ur Rehman, M.; Zimmerman, W.B.; Rehman, F. “Pushing and pulling” the equilibrium through bubble mediated reactive separation for ethyl acetate production. React. Chem. Eng. 2019, 4, 705–714. [Google Scholar] [CrossRef]
- Zimmerman, W.B.; Kokoo, R. Esterification for biodiesel production with a phantom catalyst: Bubble mediated reactive distillation. Appl. Energy. 2018, 221, 28–40. [Google Scholar] [CrossRef]
- IX-ISO. Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. In ISO 660-2009; International Organization for Standardization: Geneva, Switzerland, 2009; p. 9. [Google Scholar]
- Standard Test Method for Determination of the Saponification Value of Fats and Oils, ASTM D5558-1995(2006); ASTM: West Conshohocken, PA, USA, 1995; p. 2.
- Wang, S.M.; Kuan, D.Y.; Du, W.; Zhao, X.B.; Liu, D.H. Effect of organic acid treatment on microalgae lipid extraction. Chem. J. Chin. Univ. 2015, 36, 499–504. [Google Scholar]
- Wang, Z.T.; Du, W.; Dai, L.M.; Liu, D.H. Study on Lipozyme TL IM-catalyzed esterification of oleic acid and glycerol for 1,3-diolein preparation. J. Mol. Catal. B Enzym. 2016, 127, 11–17. [Google Scholar] [CrossRef]


| Time (min) | Flow Rate (mL/min) | Dichloromethane (V/V, %) | Acetonitrile with 0.15% Acetic Acid (V/V, %) |
|---|---|---|---|
| 0 | 1.50 | 0 | 100 |
| 4 | 1.50 | 0 | 100 |
| 12 | 1.50 | 10 | 90 |
| 25 | 1.50 | 10 | 90 |
| 30 | 1.50 | 30 | 70 |
| 35 | 1.50 | 30 | 70 |
| 45 | 1.50 | 80 | 20 |
| 55 | 1.50 | 80 | 20 |
| 60 | 1.50 | 0 | 100 |
| 65 | 1.50 | 0 | 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuan, D.; Dai, L.; Liu, D.; Liu, H.; Du, W. Efficient Biodiesel Conversion from Microalgae Oil of Schizochytrium sp. Catalysts 2019, 9, 341. https://doi.org/10.3390/catal9040341
Kuan D, Dai L, Liu D, Liu H, Du W. Efficient Biodiesel Conversion from Microalgae Oil of Schizochytrium sp. Catalysts. 2019; 9(4):341. https://doi.org/10.3390/catal9040341
Chicago/Turabian StyleKuan, Dingyaw, Lingmei Dai, Dehua Liu, Hongjuan Liu, and Wei Du. 2019. "Efficient Biodiesel Conversion from Microalgae Oil of Schizochytrium sp." Catalysts 9, no. 4: 341. https://doi.org/10.3390/catal9040341
APA StyleKuan, D., Dai, L., Liu, D., Liu, H., & Du, W. (2019). Efficient Biodiesel Conversion from Microalgae Oil of Schizochytrium sp. Catalysts, 9(4), 341. https://doi.org/10.3390/catal9040341







