Characterization and Effect of Ag(0) vs. Ag(I) Species and Their Localized Plasmon Resonance on Photochemically Inactive TiO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Photocatalysts
2.2. Characterization of Photocatalysts
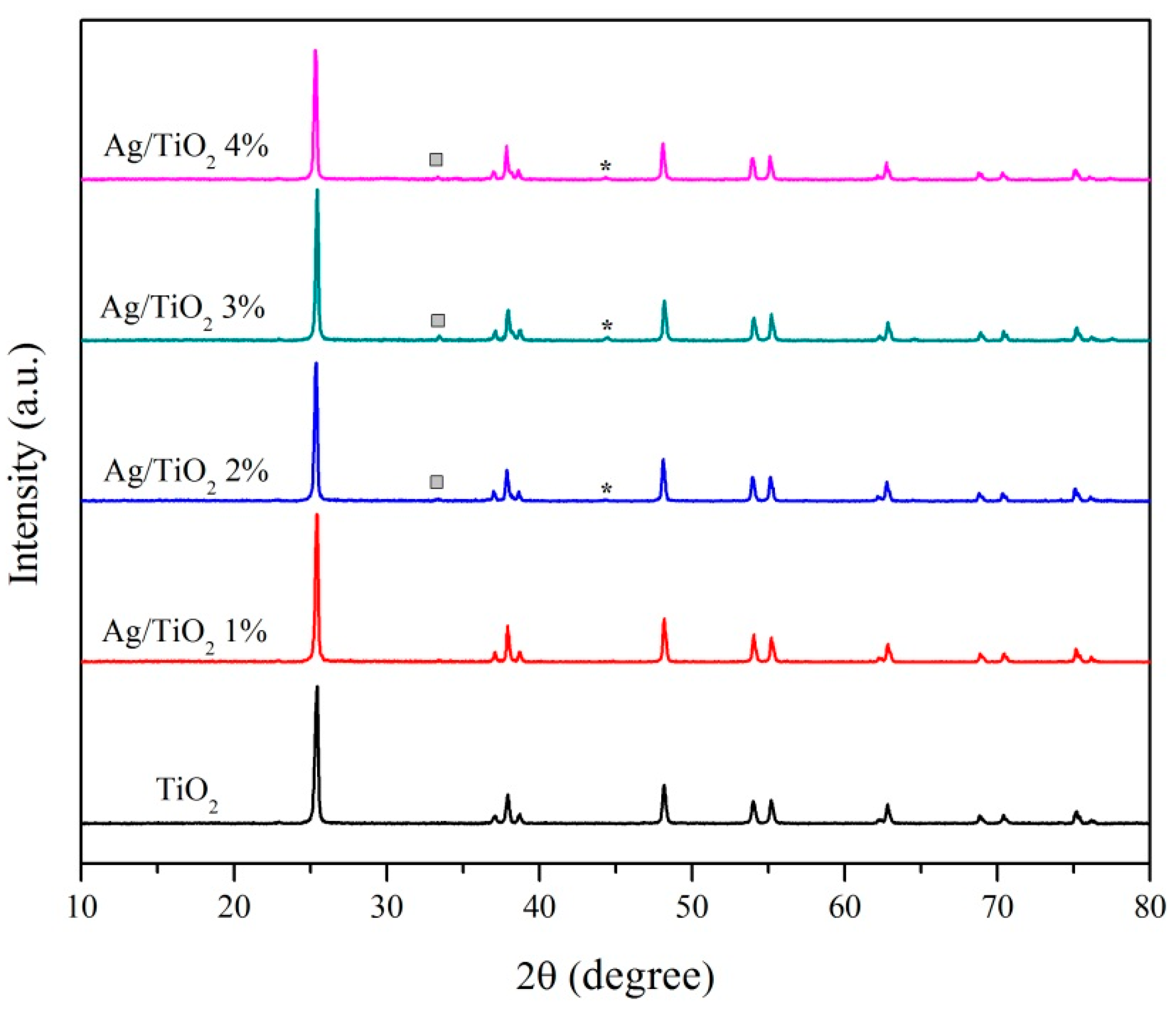
2.2.1. Powder X-ray Diffraction
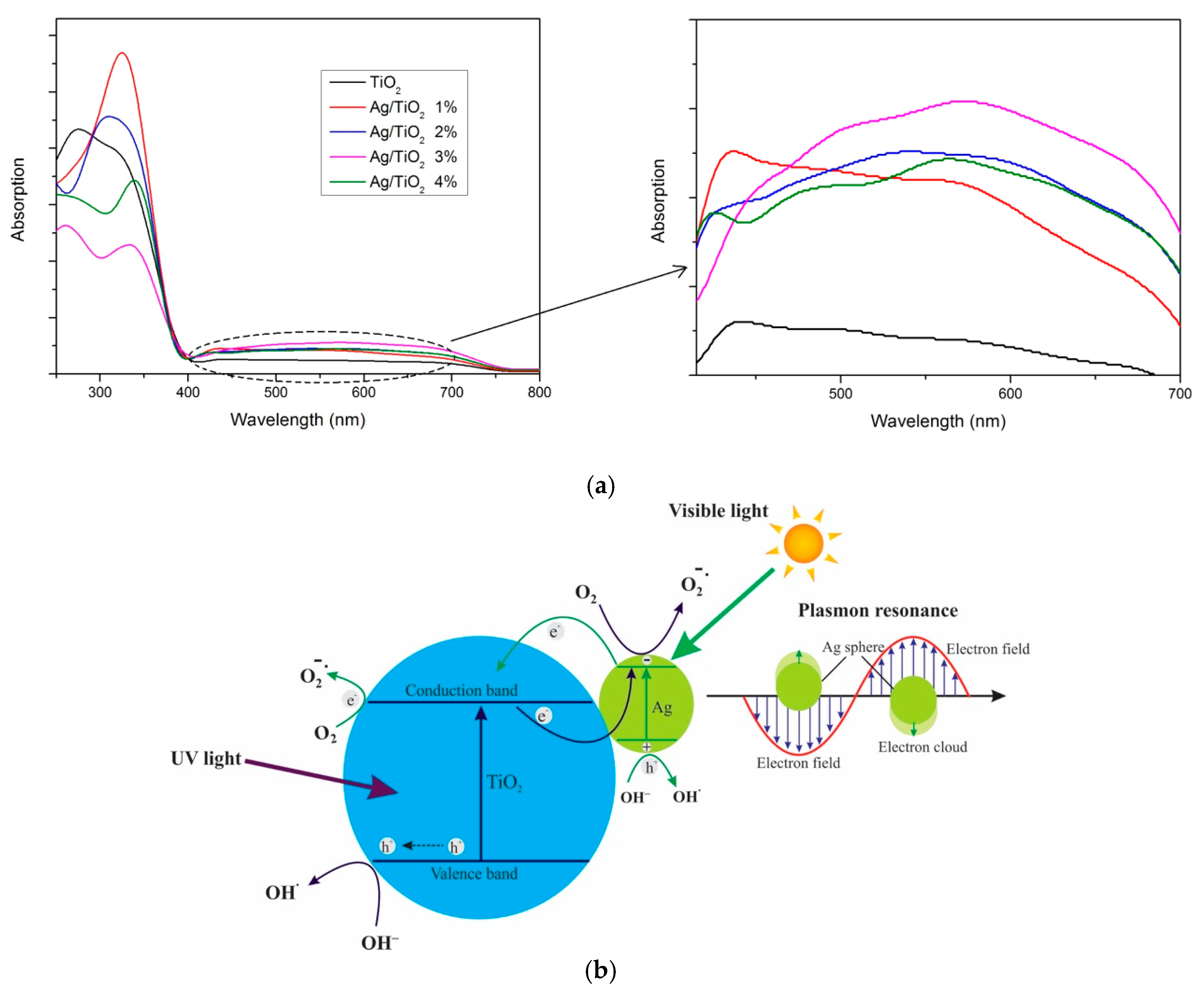
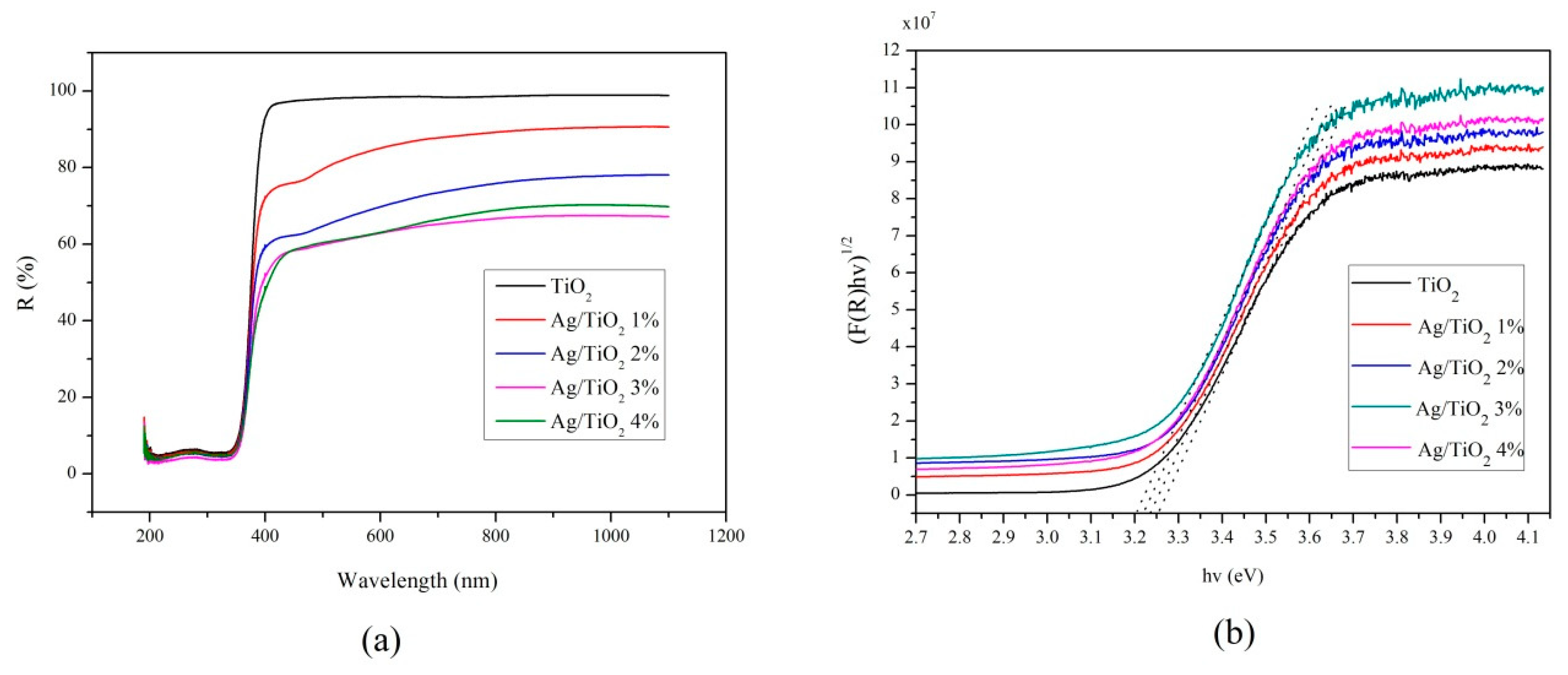
2.2.2. Electronic Spectra
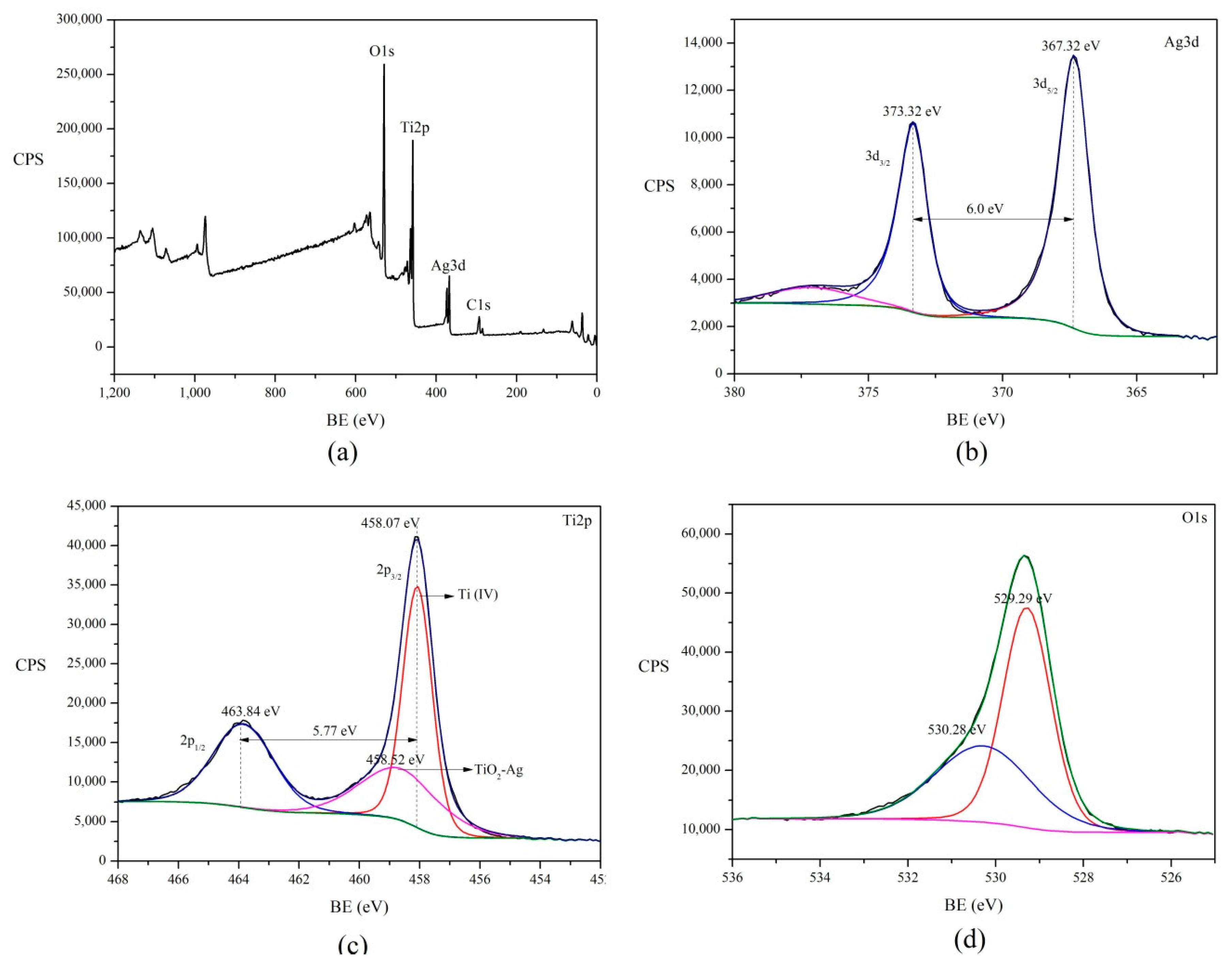
2.2.3. Determination and Characterization of Surface Ag on TiO2
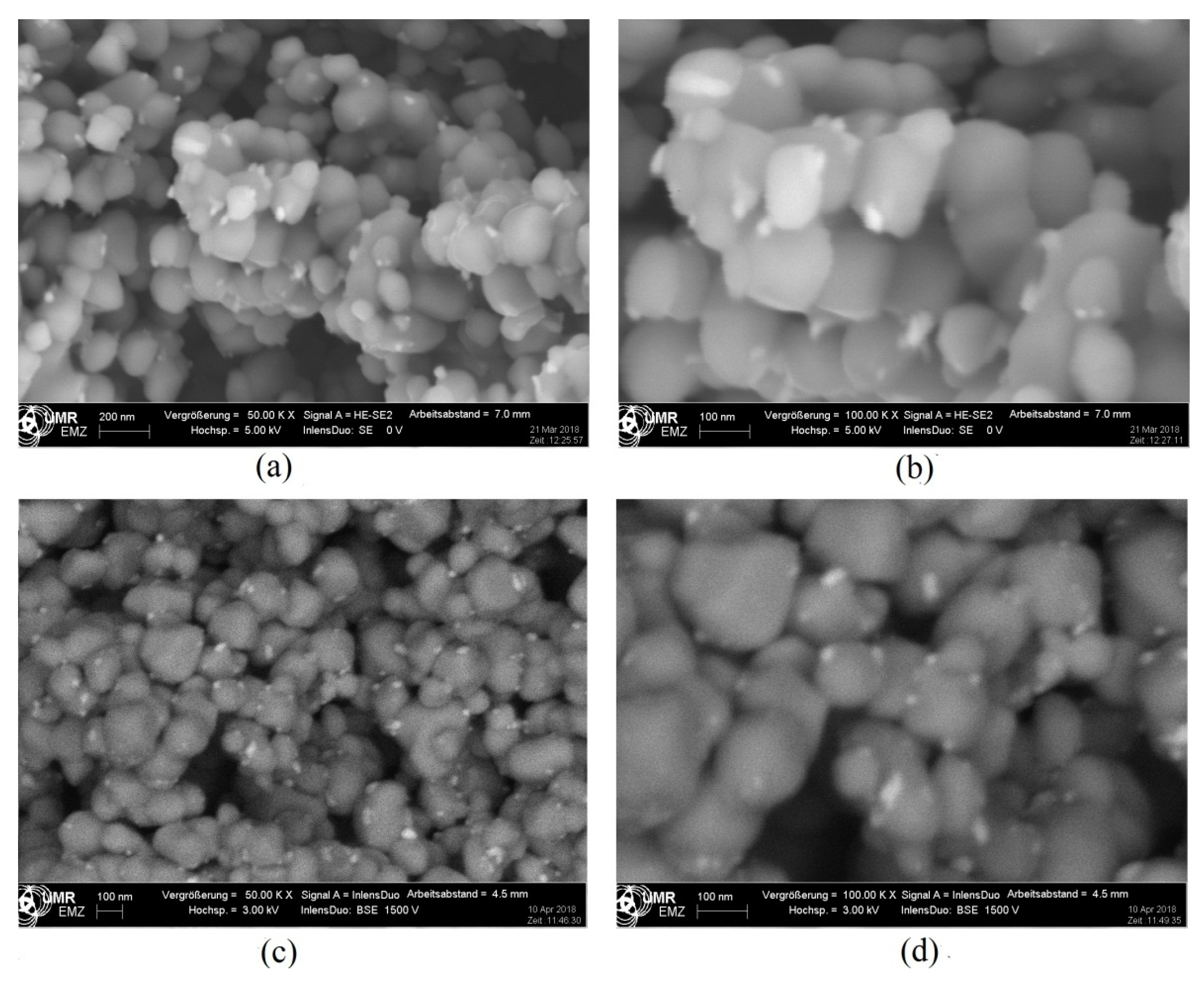
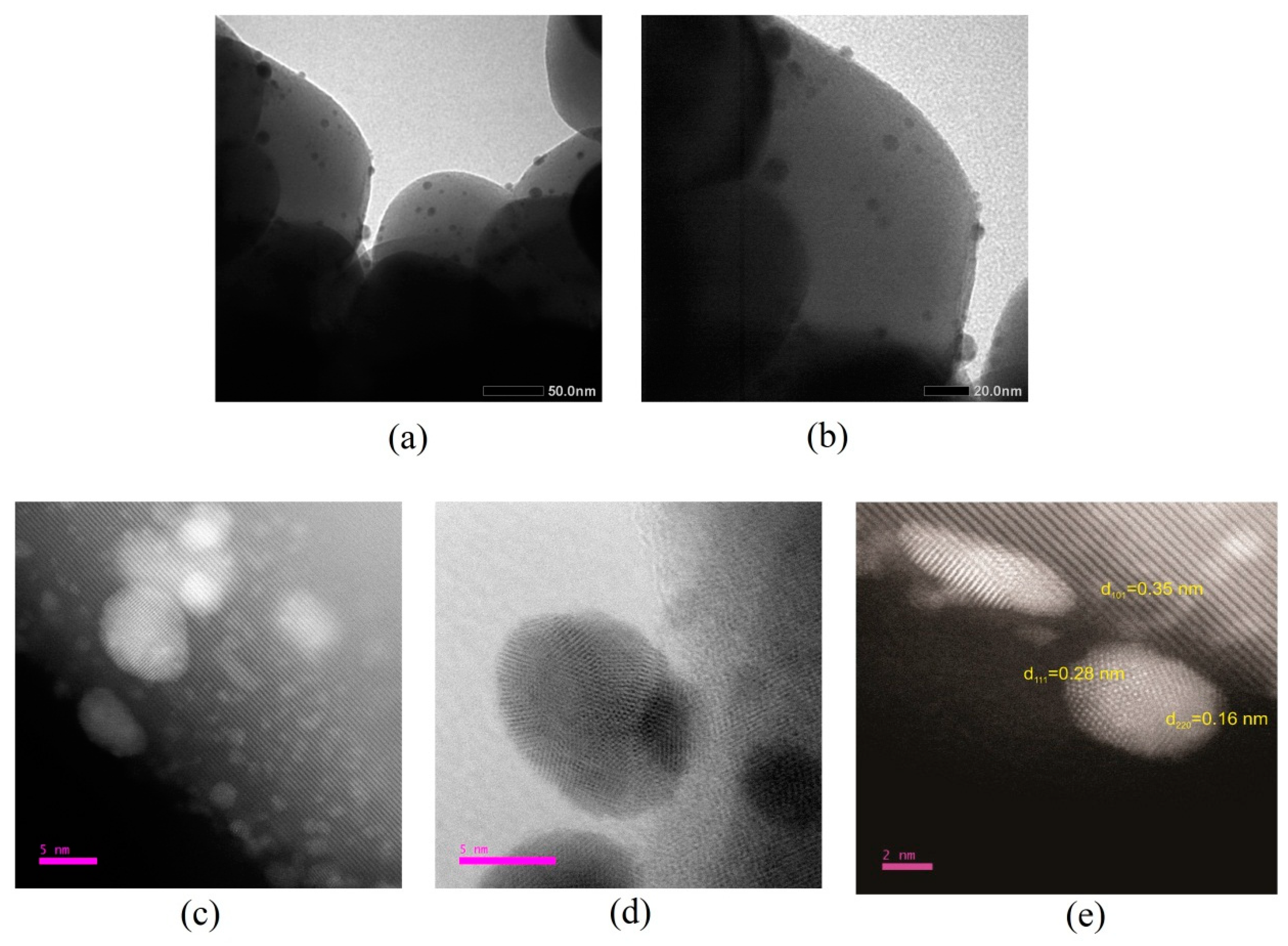
2.2.4. Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
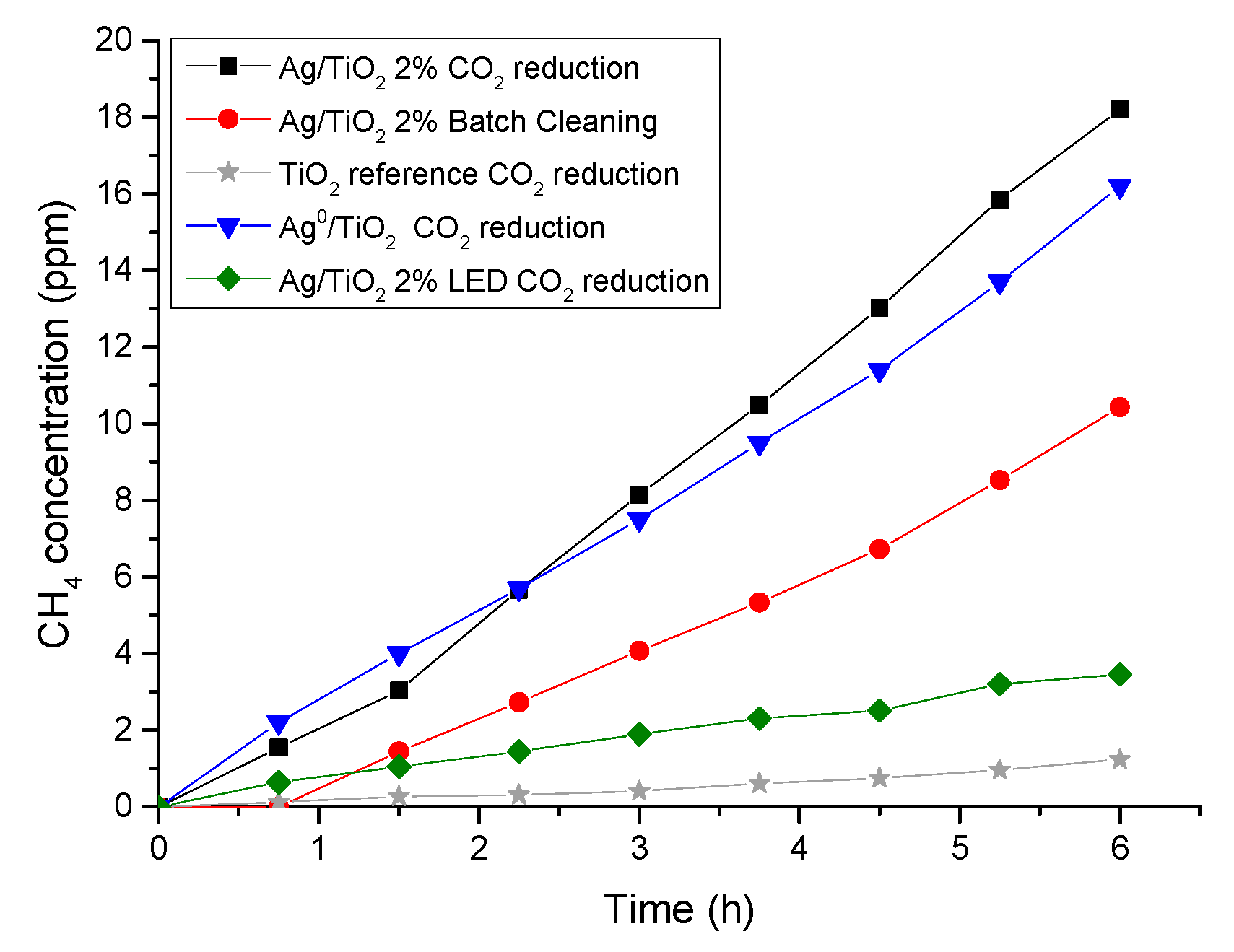
2.3. Photocatalytic Activity in CO2 Reduction
Sample Testing in Photocatalytic CO2 Reduction
3. Experimental Section
3.1. General Considerations
3.2. Materials
3.3. Preparation of Photocatalysts
3.4. Photocatalytic Setup
3.5. Sample Pretreatment
3.6. Photocatalytic CO2 Reduction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- NASA. Climate Change Vital Signs of the Planet: Carbon Dioxide. Available online: https://climate.nasa.gov/vital-signs/carbon-dioxide/ (accessed on 6 March 2018).
- NASA. Global Climate Change Vital Signs of the Planet: Global Temperature. Available online: https://climate.nasa.gov/vital-signs/global-temperature/ (accessed on 6 March 2018).
- Jiang, Z.; Xiao, T.; Kuznetsov, V.L.; Edwards, P.P. Turning carbon dioxide into fuel. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 3343–3364. [Google Scholar] [CrossRef] [Green Version]
- Ji, Y.; Luo, Y. New mechanism for photocatalytic reduction of CO2 on the anatase TiO2 (101) surface: The essential role of oxygen vacancy. J. Am. Chem. Soc. 2016, 138, 15896–15902. [Google Scholar] [CrossRef]
- Mei, B.; Pougin, A.; Strunk, J. Influence of photodeposited gold nanoparticles on the photocatalytic activity of titanate species in the reduction of CO2 to hydrocarbons. J. Catal. 2013, 306, 184–189. [Google Scholar] [CrossRef]
- Li, K.; An, X.; Park, K.H.; Khraisheh, M.; Tang, J. A critical review of CO2 photoconversion: Catalysts and reactors. Catal. Today 2014, 224, 3–12. [Google Scholar] [CrossRef]
- Handoko, A.D.; Li, K.; Tang, J. Recent progress in artificial photosynthesis: CO2 photoreduction to valuable chemicals in a heterogeneous system. Curr. Opin. Chem. Eng. 2012, 2, 200–206. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, Y.-J. Photocatalytic conversion of CO2 into value-added and renewable fuels. Appl. Surf. Sci. 2015, 342, 154–167. [Google Scholar] [CrossRef]
- Sato, S.; Arai, T.; Morikawa, T. Toward solar-driven photocatalytic CO2 reduction using water as an electron donor. Inorg. Chem. 2015, 54, 5105–5113. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Y.-W. Ag/TiO2 sol prepared by a sol–gel method and its photocatalytic activity. J. Phys. Chem. Solids 2011, 72, 1312–1318. [Google Scholar] [CrossRef]
- Lai, Y.; Chen, Y.; Zhuang, H.; Lin, C. A facile method for synthesis of Ag/TiO2 nanostructures. Mater. Lett. 2008, 62, 3688–3690. [Google Scholar] [CrossRef]
- Li, X.; Wen, J.; Low, J.; Fang, Y.; Yu, J. Design and fabrication of semiconductor photocatalyst for photocatalytic reduction of CO2 to solar fuel. Sci. China Mater. 2014, 57, 70–100. [Google Scholar] [CrossRef]
- Saha, S.; Wang, J.M.; Pal, A. Nano silver impregnation on commercial TiO2 and a comparative photocatalytic account to degrade malachite green. Sep. Purif. Technol. 2012, 89, 147–159. [Google Scholar] [CrossRef]
- Etacheri, V.; Valentin, C.D.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–83. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Ablat, A.; Wu, R.; Mamat, M.; Ghupur, Y.; Aimidula, A.; Bake, M.A.; Gholam, T.; Wang, J.; Qian, H.; Wu, R.; et al. Electronic structure and room temperature ferromagnetism of C doped TiO2. Solid State Commun. 2016, 243, 7–11. [Google Scholar] [CrossRef]
- Kočí, K.; Matějová, L.; Ambrožová, N.; Šihor, M.; Troppová, I.; Čapek, L.; Kotarba, A.; Kustrowski, P.; Hospodková, A.; Obalová, L. Optimization of cerium doping of TiO2 for photocatalytic reduction of CO2 and photocatalytic decomposition of N2O. J. Sol-Gel Sci. Technol. 2016, 78, 550–558. [Google Scholar] [CrossRef]
- Rho, W.-Y.; Jeon, H.; Kim, H.-S.; Chung, W.-J.; Suh, J.S.; Jun, B.-H. Recent progress in dye-sensitized solar cells for improving efficiency: TiO2 nanotube arrays in active layer. J. Nanomater. 2015, 2015, 1–17. [Google Scholar] [CrossRef]
- Wang, T.; Xia, M.; Kong, X. The pros and cons of polydopamine-sensitized titanium oxide for the photoreduction of CO2. Catalysts 2018, 8, 215. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Sasaki, T.; Koshizaki, N.; Traversa, E. Preparation and characterization of M/TiO2 (M = Ag, Au, Pt) nanocomposite thin films. Scripta Mater. 2001, 44, 1865–1868. [Google Scholar] [CrossRef]
- Kim, K.D.; Han, D.N.; Lee, J.B.; Kim, H.T. Formation and characterization of Ag-deposited TiO2 nanoparticles by chemical reduction method. Scripta Mater. 2006, 54, 143–146. [Google Scholar] [CrossRef]
- Tahir, K.; Ahmad, A.; Li, B.; Khan, A.U.; Nazir, S.; Khan, S.; Khan, Z.U.H.; Khan, S.U. Preparation, characterization and an efficient photocatalytic activity of Au/TiO2 nanocomposite prepared by green deposition method. Mater. Lett. 2016, 178, 56–59. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Brocato, S.; Marra, G.; Tozzola, G.; Meda, L.; Selli, E. Aqueous ammonia abatement on Pt- and Ru-modified TiO2: Selectivity effects of the metal nanoparticles deposition method. Catal. Today 2017, 287, 148–154. [Google Scholar] [CrossRef]
- Olivo, A.; Ghedini, E.; Pascalicchio, P.; Manzoli, M.; Cruciani, G.; Signoretto, M. Sustainable carbon dioxide photoreduction by a cooperative effect of reactor design and titania metal promotion. Catalysts 2018, 8, 41. [Google Scholar] [CrossRef]
- Mital, G.S.; Manoj, T. A review of TiO2 nanoparticles. Chin. Sci. Bull. 2011, 56, 1639–1657. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Badiei, A.; Behnajady, M.A.; Afshar, M. Enhanced photocatalytic removal of phenazopyridine by using silver-impregnated SiO2–TiO2 nanoparticles: Optimization of synthesis variables. Res. Chem. Intermediat. 2015, 41, 9929–9949. [Google Scholar] [CrossRef]
- Liu, E.; Kang, L.; Wu, F.; Sun, T.; Hu, X.; Yang, Y.; Liu, H.; Fan, J. Photocatalytic reduction of CO2 into methanol over Ag/TiO2 nanocomposites enhanced by surface plasmon resonance. Plasmonics 2014, 9, 61–70. [Google Scholar] [CrossRef]
- Kočí, K.; Zatloukalová, K.; Obalová, L.; Krejčíková, S.; Lacný, Z.; Čapek, L.; Hospodková, A.; Šolcová, O. Wavelength effect on photocatalytic reduction of CO2 by Ag/TiO2 catalyst. Chin. J. Catal. 2011, 32, 812–815. [Google Scholar] [CrossRef]
- Kulkarni, R.M.; Malladi, R.S.; Hanagadakar, M.S.; Doddamani, M.R.; Bhat, U.K. Ag-TiO2 nanoparticles for photocatalytic degradation of lomefloxacin. Desalin. Water Treat. 2016, 57, 1–8. [Google Scholar] [CrossRef]
- Kočí, K.; Matějů, K.; Obalová, L.; Krejčíková, S.; Lacný, Z.; Plachá, D.; Čapek, L.; Hospodková, A.; Šolcová, O. Effect of silver doping on the TiO2 for photocatalytic reduction of CO2. Appl. Catal. B-Environ. 2010, 96, 239–244. [Google Scholar] [CrossRef]
- Dilla, M.; Pougin, A.; Strunk, J. Evaluation of the plasmonic effect of Au and Ag on Ti-based photocatalysts in the reduction of CO2 to CH4. J. Energy Chem. 2017, 26, 277–283. [Google Scholar] [CrossRef]
- Yu, B.; Zhou, Y.; Li, P.; Tu, W.; Li, P.; Tang, L.; Ye, J.; Zou, Z. Photocatalytic reduction of CO2 over Ag/TiO2 nanocomposites prepared with a simple and rapid silver mirror method. Nanoscale 2016, 8, 11870–11874. [Google Scholar] [CrossRef]
- Pougin, A.; Dilla, M.; Strunk, J. Identification and exclusion of intermediates of photocatalytic CO2 reduction on TiO2 under conditions of highest purity. Phys. Chem. Chem. Phys. 2016, 18, 10809–10817. [Google Scholar] [CrossRef]
- Dilla, M.; Schlögl, R.; Strunk, J. Photocatalytic CO2 reduction under continuous flow high-purity conditions: Quantitative evaluation of CH4 formation in the steady state. ChemCatChem 2017, 9, 696–704. [Google Scholar] [CrossRef]
- Tampi, K.R.; Kiwi, J.; Grätzel, M. Methanation and photo-methanation of carbon dioxide at room temperature and atmospheric pressure. Nature 1987, 327, 506–508. [Google Scholar] [CrossRef]
- Zhao, C.; Krall, A.; Zhao, H.; Zhang, Q.; Li, Y. Ultrasonic spray pyrolysis synthesis of Ag/TiO2 nanocomposite photocatalysts for simultaneous H2 production and CO2 reduction. Int. J. Hydrog. Energy 2012, 37, 9967–9976. [Google Scholar] [CrossRef]
- Thein, M.T.; Chim, J.E.; Punga, S.-Y.; Pung, Y.-F. Highly UV light driven WOx@ZnO nanocomposites synthesized by liquid impregnation method. J. Ind. Eng. Chem. 2017, 46, 119–129. [Google Scholar] [CrossRef]
- Amadio, T.d.M.; Hotza, D.; Neto, J.B.R.; Blosi, M.; Costa, A.L.; Dondi, M. Bentonites functionalized by impregnation with TiO2, Ag, Pd and Au nanoparticles. Appl. Clay Sci. 2017, 146, 1–6. [Google Scholar] [CrossRef]
- Lee, K.-C.; Lin, S.-J.; Lin, C.-H.; Lin, C.-H.; Tsai, C.-S.; Lu, Y.-J. Size effect of Ag nanoparticles on surface plasmon resonance. Surf. Coat. Tech. 2008, 202, 5339–5342. [Google Scholar] [CrossRef]
- Leong, K.H.; Gan, B.L.; Ibrahim, S.; Saravanan, P. Synthesis of surface plasmon resonance (SPR) triggered Ag/TiO2 photocatalyst for degradation of endocrine disturbing compounds. Appl. Surf. Sci. 2014, 319, 128–135. [Google Scholar] [CrossRef]
- Handoko, C.T.; Huda, A.; Bustan, M.D.; Yudono, B.; Gulo, F. Green synthesis of silver nanoparticle and its antibacterial activity. Rasayan J. Chem. 2017, 10, 1137–1144. [Google Scholar] [CrossRef]
- Eom, H.; Jung, J.-Y.; Shin, Y.; Kim, S.; Choi, J.-H.; Lee, E.; Jeong, J.-H.; Park, I. Strong localized surface plasmon resonance effects of Ag/TiO2 core–shell nanowire arrays in UV and visible light for photocatalytic activity. Nanoscale 2014, 6, 226–234. [Google Scholar] [CrossRef]
- Handoko, C.T.; Huda, A.; Gulo, F. Synthesis pathway and powerful antimicrobial properties of silver nanoparticle: A critical review. Asian J. Sci. Res. 2019, 12, 1–17. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Boerigter, C.; Aslam, U.; Linic, S. Mechanism of charge transfer from plasmonic nanostructures to chemically attached materials. ACS Nano 2016, 10, 6108–6115. [Google Scholar] [CrossRef]
- Shi, L.; Liang, L.; Ma, J.; Wang, F.; Sun, J. Enhanced photocatalytic activity over the Ag2O–g-C3N4 composite under visible light. Catal. Sci. Technol. 2014, 4, 758–765. [Google Scholar] [CrossRef]
- Wu, F.; Hu, X.; Fan, J.; Liu, E.; Sun, T.; Kang, L.; Hou, W.; Zhu, C.; Liu, H. Photocatalytic activity of Ag/TiO2 nanotube arrays enhanced by surface plasmon resonance and application in hydrogen evolution by water splitting. Plasmonics 2012, 8, 501–508. [Google Scholar] [CrossRef]
- Jahan, F.; Islam, M.H.; Smith, B.E. Band gap and refractive index determination of Mo-black coatings using several techniques. Sol. Energy Mater. Sol. Cells 1995, 37, 283–293. [Google Scholar] [CrossRef]
- Uddin, M.T.; Nicolas, Y.; Olivier, C.; Jaegermann, W.; Rockstroh, N.; Junge, H.; Toupance, T. Band alignment investigations of heterostructure NiO/TiO2 nanomaterials used as efficient heterojunction earth-abundant metal oxide photocatalysts for hydrogen production. Phys. Chem. Chem. Phys. 2017, 19, 19279–19288. [Google Scholar] [CrossRef] [PubMed]
- Belver, C.; Hinojosa, M.; Bedia, J.; Tobajas, M.; Alvarez, M.A.; Rodríguez-González, V.; Rodriguez, J.J. Ag-coated heterostructures of ZnO-TiO2/delaminated montmorillonite as solar photocatalysts. Materials 2017, 10, 960. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qian, H.; Hu, Y.; Dai, W.; Zhong, Y.; Chen, J.; Hu, X. Facile one-pot synthesis of uniform TiO2–Ag hybrid hollow spheres with enhanced photocatalytic activity. Dalton Trans. 2013, 42, 1122–1128. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, M.; Wang, L.; Wang, F.; Yang, L.; Li, X.; Wang, C. Plasmonic Ag-TiO2−x nanocomposites for the photocatalytic removal of NO under visible light with high selectivity: The role of oxygen vacancies. Appl. Catal. B-Environ. 2017, 204, 67–77. [Google Scholar] [CrossRef]
- Lei, T.; Xie, Y.; Wang, X.; Miao, S.; Xiong, J.; Yan, C. TiO2 feather duster as effective polysulfides restrictor for enhanced electrochemical kinetics in lithium–sulfur batteries. Small 2017, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2017, 6, 3007–3014. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. H-doped black titania with very high solar absorption and excellent photocatalysis enhanced by localized surface plasmon resonance. Adv. Funct. Mater. 2013, 23, 5444–5450. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, S.; Li, C.; Liu, J.; He, S.; Zhao, Y.; Yan, H.; Wei, M.; Evans, D.G.; Duan, X. Catalytic behavior of supported Ru nanoparticles on the (101) and (001) facets of anatase TiO2. RSC Adv. 2014, 4, 10834–10840. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Ghosh, S.; Naskar, M.K. Aqueous-based synthesis of mesoporous TiO2 and Ag–TiO2 nanopowders for efficient photodegradation of methylene blue. Ceram. Int. 2016, 42, 2488–2496. [Google Scholar] [CrossRef]
- Chen, L.; Hua, H.; Yang, Q.; Hu, C. Visible-light photocatalytic activity of Ag2O coated Bi2WO6 hierarchical microspheres assembled by nanosheets. Appl. Surf. Sci. 2015, 327, 62–67. [Google Scholar] [CrossRef]
- Rokade, A.A.; Patil, M.P.; Yoo, S.I.; Lee, W.K.; Park, S.S. Pure green chemical approach for synthesis of Ag2O nanoparticles. Green Chem. Lett. Rev. 2016, 9, 216–222. [Google Scholar] [CrossRef]
- Li, M.Y.; Mao, Y.Q.; Yang, S.K.; Dai, T.T.; Yang, H.; Feng, F.; Wu, T.; Chen, M.; Xu, G.Q.; Wu, J.H. Out-of-Substrate Ag−Ag2O nanoplates: Surfactantless photochemical synthesis, structural evolution, and mechanistic study. ACS Omega 2016, 1, 696–705. [Google Scholar] [CrossRef]
- Dilla, M.; Mateblowski, A.; Ristig, S.; Strunk, J. Photocatalytic CO2 reduction under continuous flow high-purity conditions: Influence of light intensity and H2O concentration. ChemCatChem 2017, 9, 4345–4352. [Google Scholar] [CrossRef]
- Nasirian, M.; Mehrvar, M. Modification of TiO2 to enhance photocatalytic degradation of organics in aqueous solutions. J. Environ. Chem. Eng. 2016, 4, 4072–4082. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.-M. Influence of metallic silver and of platinum-silver bimetallic deposits on the photocatalytic activity of titania (anatase and rutile) in organic and aqueous media. J. Photochem. Photobiol. A 1998, 113, 181–188. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; John, T.; Yates, J. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Rtimi, S.; Baghriche, O.; Sanjines, R.; Pulgarin, C.; Bensimon, M.; Kiwi, J. TiON and TiON-Ag sputtered surfaces leading to bacterial inactivation under indoor actinic light. J. Photochem. Photobiol. A 2013, 256, 52–63. [Google Scholar] [CrossRef] [Green Version]
- Busser, G.W.; Schuhmann, W.; Mei, B.; Pougin, A.; Willinger, M.-G.; Strunk, J.; Schlögl, R.; Gutkowski, R.; Muhler, M. Photodeposition of copper and chromia on gallium oxide: The role of co-catalysts in photocatalytic water splitting. ChemSusChem 2014, 7, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Phivilay, S.P.; Roberts, C.A.; Puretzky, A.A.; Domen, K.; Wachs, I.E. Fundamental bulk/surface structure−photoactivity relationships of supported (Rh2−yCryO3)/GaN photocatalysts. J. Phys. Chem. Lett. 2013, 4, 3719–3724. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Saito, N.; Inoue, Y.; Domen, K. Roles of Rh/Cr2O3 (core/shell) nanoparticles photodeposited on visible-light-responsive (Ga1−xZnx)(N1−xOx) solid solutions in photocatalytic overall water splitting. J. Phys. Chem. C 2007, 111, 7554–7560. [Google Scholar] [CrossRef]
- Domen, K.; Kudo, A.; Onishi, T. Mechanism of photocatalytic decomposition of water into H2 and O2 over NiO-SrTiO3. J. Catal. 1986, 102, 92–98. [Google Scholar] [CrossRef]
- Yoshida, M.; Takanabe, K.; Maeda, K.; Ishikawa, A.; Kubota, J.; Sakata, Y.; Ikezawa, Y.; Domen, K. Role and function of noble-metal/Cr-layer core/shell structure cocatalysts for photocatalytic overall water splitting studied by model electrodes. J. Phys. Chem. C 2009, 113, 10151–10157. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Handoko, C.T.; Moustakas, N.G.; Peppel, T.; Springer, A.; Oropeza, F.E.; Huda, A.; Bustan, M.D.; Yudono, B.; Gulo, F.; Strunk, J. Characterization and Effect of Ag(0) vs. Ag(I) Species and Their Localized Plasmon Resonance on Photochemically Inactive TiO2. Catalysts 2019, 9, 323. https://doi.org/10.3390/catal9040323
Handoko CT, Moustakas NG, Peppel T, Springer A, Oropeza FE, Huda A, Bustan MD, Yudono B, Gulo F, Strunk J. Characterization and Effect of Ag(0) vs. Ag(I) Species and Their Localized Plasmon Resonance on Photochemically Inactive TiO2. Catalysts. 2019; 9(4):323. https://doi.org/10.3390/catal9040323
Chicago/Turabian StyleHandoko, Chanel Tri, Nikolaos G. Moustakas, Tim Peppel, Armin Springer, Freddy E. Oropeza, Adri Huda, Muhammad Djoni Bustan, Bambang Yudono, Fakhili Gulo, and Jennifer Strunk. 2019. "Characterization and Effect of Ag(0) vs. Ag(I) Species and Their Localized Plasmon Resonance on Photochemically Inactive TiO2" Catalysts 9, no. 4: 323. https://doi.org/10.3390/catal9040323




