1. Introduction
Several types of porphyrins have been used for photocatalytic oxygenations of various organic compounds. Both free-base ligands and metalloporphyrins proved to be efficient for such reactions. In earlier and more recent works, cycloalkenes were effectively functionalized in such reactions, using porphyrin complexes of various transition metal ions [
1,
2,
3,
4,
5,
6]. Also, some aromatic (and aliphatic) alcohols and aldehydes were oxidized to carboxylic acids by solar irradiation of a Sb(V)-porphyrin [
7]. Photocatalytic oxygenation of cyclohexene was carried out in the system sensitized by Sn(IV)-porphyrin adsorbed on Pt loaded TiO
2 nano-particles [
8]. In air- or oxygen-saturated solutions, free-base porphyrins generate only singlet oxygen in these photocatalytic reactions, while metalloporphyrins can be involved in other light-induced processes producing species with higher oxidation potential than that of
1O
2. Application of manganese(III) porphyrins well demonstrated this diversity. In most of these processes, the first reaction step of the (triplet) excited-state complex was an electron transfer. These reactions produce directly or indirectly Mn(IV) then Mn(V) porphyrins as intermediates playing important roles in the oxygenation processes. This has been exemplified in earlier works [
1,
9] and more recent studies [
10,
11,
12,
13], regarding the photocatalytic oxygenation of cyclohexene. In aqueous systems, photo-induced homolysis of the bond between the metal center and chloride or hydroxide axial ligands led to the formation of (P)Mn
IV=O [
9,
13,
14]. The oxidized ligand is a very reactive oxidative agent, e.g., hydroxyl radical in the latter case (Equation (1)). The Mn(II) species formed in this reaction undergoes an oxidation with the dissolved O
2 (Equation (2)).
The latter equation is a summary of several steps. The Mn(IV) complexes formed disproportionate, producing highly reactive manganese(V)-oxo species (Equation (3)) [
11,
12,
13].
Since disproportionation is significantly faster than the back reaction in this equilibrium system, especially in polar solvent, it is a nearly diffusion-controlled process [
11]. Additionally, the rate constants for epoxidation of olefins are several orders of magnitude higher for manganese(V)-oxo porphyrins than for the corresponding Mn(IV) species. Hence, (P)Mn
V=O can be considered as the major oxidant in the photocatalytic oxygenations in these systems. Nevertheless, in some cases, the (P)Mn
IV=O complexes can also result in direct hydrogen abstraction from hydrocarbon moiety [
15].
Another photochemical electron transfer in the primary reaction was observed between the excited (triplet) state of the (P)Mn
III complex and a dissolved oxygen molecule (Equation (4)) [
16].
The putative (P)MnIV superoxo complex abstracts a hydrogen atom from a saturated hydrocarbon moiety generating a hydroperoxo complex (P)MnIV-OOH. (Equation (5)). The subsequent reductive O–O bond cleavage by >–•CH produces (P)MnV=O and >CH(OH) (Equation (6)), followed by the oxidation of >CH(OH) by (P)MnV=O to yield >C═O with regeneration of (P)MnIII (Equation (7)).
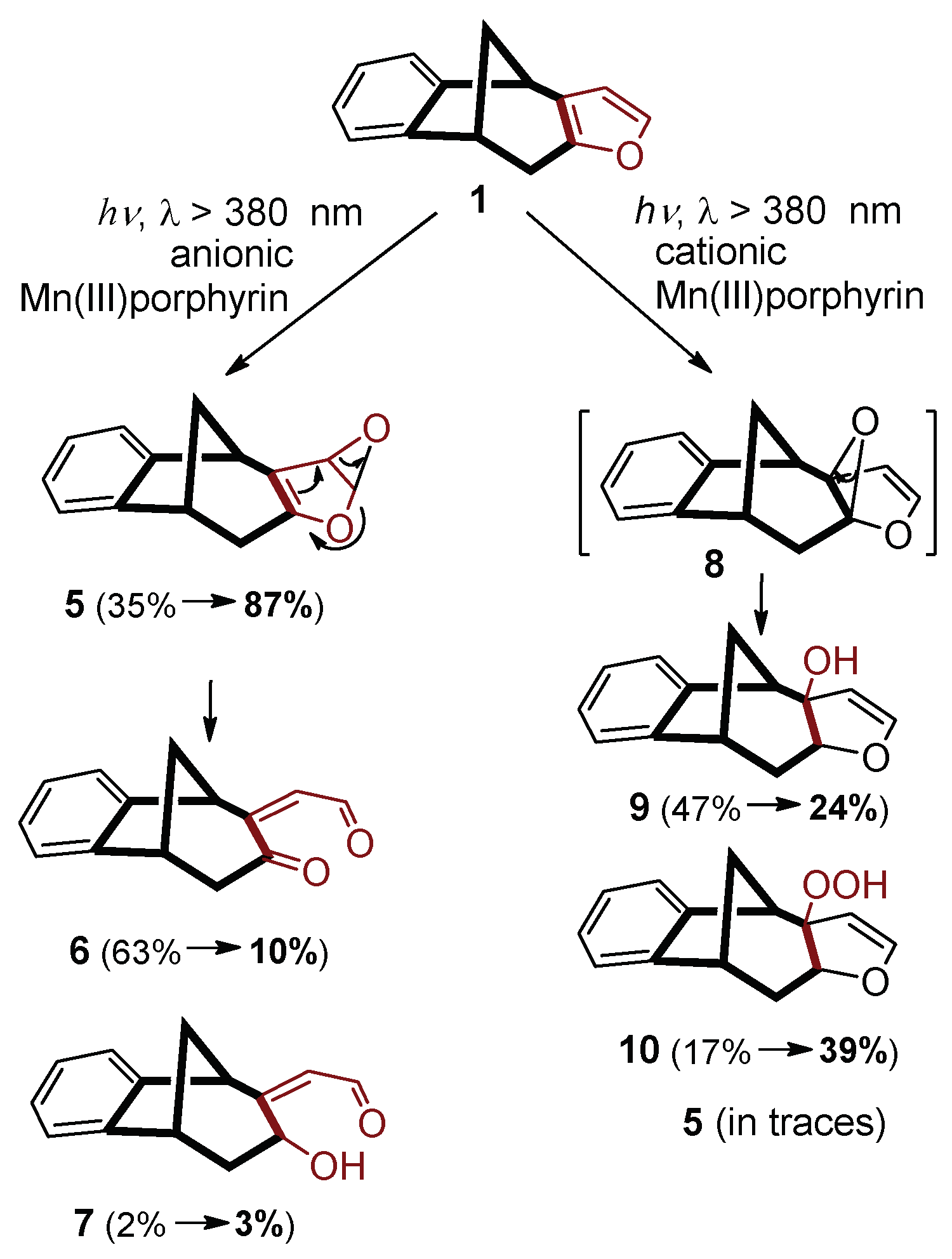
Manganese(III) porphyrins proved to be efficient photocatalysts for oxygenation reactions, i.e. functionalization, of various derivatives of the benzobicyclo[3.2.1]octadiene skeleton in aqueous systems (in water/acetone (50:50) mixture). In these experiments, Mn(III) complexes with both anionic and cationic porphyrins were utilized, namely 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrin (Mn(III)TSPP
3−) and 5,10,15,20-tetrakis(1-methyl-4-pyridinium)porphyrin (Mn(III)TMPyP
5+). Photocatalytic oxygenation of the 2-furyl derivative
1 clearly indicated that the charge of the porphyrin ligand may considerably determine the reaction pathways [
17]. As
Scheme 1 demonstrates, the cationic catalyst favored the electrophilic attack on the inner double bond of the furan ring, while the negatively charged complex reacted on the outer C=C bond, due to both electronic and steric effects. The more electrophilic positively charged complex, forming (P)Mn
V=O (as in Equation (3)) can more effectively attack a sterically less accessible atom than the corresponding anionic catalyst does. However, if the accessibility of the outer C=C bond became hindered by annulation of a benzene to the outer side of the furan ring, even the negatively charged catalyst could promote oxygenation at the inner positions [
18].
The significant effects of the charge of these photocatalysts were also observed in the photocatalytic oxygenation of different phenyl derivatives of the same skeleton [
19]. In that case the substituents on the phenyl ring could also influence the types of the end-products
More interestingly, changes of the position and the type of the heteroatom in the derivatives similar to
1 resulted in considerably (sometimes strikingly) deviating reaction routes under the same experimental conditions [
20]. For instance, in the case of a thienyl derivative (
3, see later in Scheme 4), even the cleavage of the basic skeleton took place. This was the consequence of the higher stability of the thiophene ring, compared to that of the furan ring, due to the significantly stronger aromacity of the previous one.
Since earlier results with the derivative 1 suggested that a change of pH may considerably affect the ratio (or even the type) of the end-products, and the previous experiments with the other furyl and thienyl derivatives were carried out only in neutral systems (pH = 7), in this work, photocatalytic oxygenation reactions of these derivatives of benzobicyclo[3.2.1]octadiene skeleton were studied in basic media (at pH = 10), by application of both anionic and cationic manganese(III) porphyrins, i.e., (Mn(III)TSPP3−) and (Mn(III)TMPyP5+), in aerated and oxygen-saturated systems. As our new results indicate, this change of pH, combining with the appropriate selection of the charge of the photocatalyst and the starting substrate, offers a suitable possibility for the improvement of the selectivity of these functionalization reactions. For instance, this pH increase dramatically enhanced the yield of the hydroxy-furyl product (from 18–28% to 70%) with both photocatalysts.
2. Results and Discussion
In this section, the presentation of the experimental results along with their interpretation is organically connected to the corresponding introductory part. Hence, it begins with the photocatalytic oxygenation of another, 3-furyl derivative (
2) for an easy comparison with the behavior of
1 (see
Scheme 1), a constitutional isomer of
2. In the continuation, the results obtained with the analogous 2- and 3-thienyl derivatives (
3 and
4) are discussed. Blank experiments were also carried out for all the three starting bicyclo structures
2–
4. No permanent change was observed in the dark (in oxygen-saturated solutions containing both substrate and anionic/cationic catalyst), neither upon irradiation in the absence of porphyrin. These observations clearly indicated that the substrate does not undergo any thermal reaction even in the presence of ground-state porphyrins.
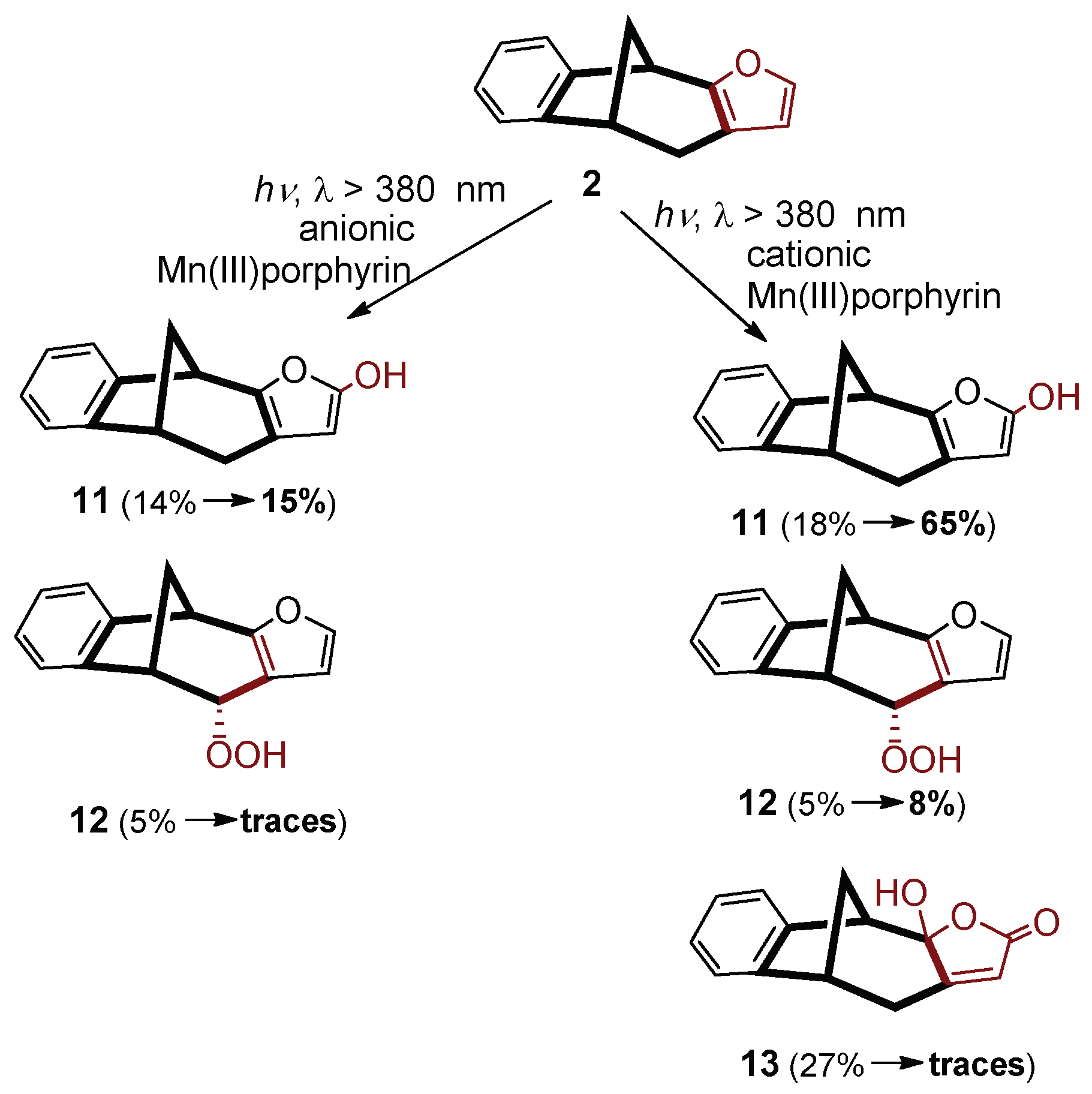
In the case of the air-saturated system of
2, the efficiency for the formation of the hydroxy derivative (
11) was just very slightly affected by the increase of pH from 7 to 10 in the presence of the anionic photocatalyst (
Scheme 2). This observation suggests that hydroxylation at an outer position of the furan ring is not promoted by an increase of the HO
− concentration. This observation indicates that an enhanced basicity of the solution hardly decreased the electrophile character of the oxidative agent formed from the anionic catalyst. Regarding the ratio of the peroxo derivative (
12), although rather moderately changed upon this pH increase, it decreased from 5% to “traces”.
In the presence of the cationic photocatalyst, the pH effects observed for the formation of derivatives 11 and 12 strongly deviate from those with the anionic porphyrin. While pH increase hardly enhanced the ratio of 11 in the latter case, it enormously increased (from 18% to 65%) with the cationic one. This difference suggests that the more basic medium favored the formation of the oxidative agent resulting in the hydroxylation at that outer position. This oxidant is presumably hydroxyl radical because its photoinduced production, according to Equation (1), is promoted by the increased HO− concentration, due to the more efficient formation of the (P)MnIIIOH complex, which is also favored by the highly positive charge of the porphyrin ligand.
For the peroxo derivative (
12), even the direction of the change in the product yield was just the opposite of that with the anionic catalyst. Instead of an obvious decrease, a modest increase (from 5% to 8%) was observed, in accordance with the result regarding
11. While in both products the substitution took place at an outer position, in the presence of the cationic catalyst, a third derivative (
13) with an inner hydroxy group (and a carbonyl in the ring) was also formed. Its yield significantly decreased upon the pH increase (from 27% to traces). These results suggest that the Mn
V=O species plays an important role in the formation of
13, where the higher electrophilicity of the cationic complex is crucial, promoting the attack even at a carbon atom of inner position. The increased concentration of HO
−, however, effectively shielded the cationic Mn
V=O complex in such a reaction. These results are in accordance with recent observations regarding photogenerated (P)Mn
V=O complexes, the reactivity of which was significantly decreased by anionic axial ligands (on the opposite side of the coordinated O atom), due to the diminished electrophilicity [
21]. Additionally, the (P)Mn
IV-O
2•− complex could also promote the formation of the keto group at the outer position, according to Equations (4)–(7). Its reactivity, however, was also dramatically decreased by the axial coordination of a hydroxo ligand.
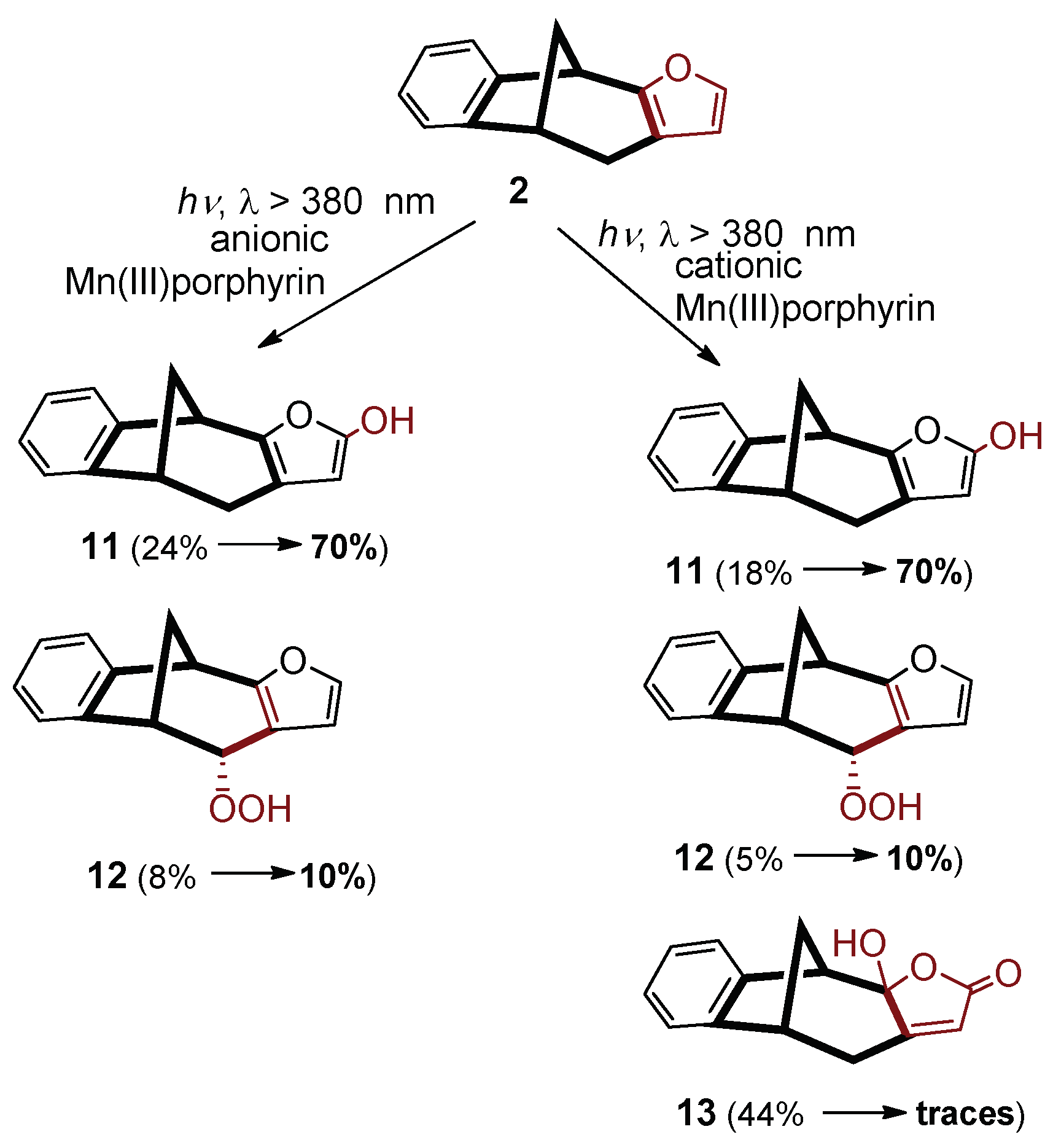
An increase in the concentration of dissolved O
2, by application of oxygen saturation, caused dramatic enhancements in the product ratios with the anionic catalyst, compared to the cases of air saturation (
Scheme 3). Changing the pH from 7 to 10, the ratio of the hydroxy derivative (
11) increased by 46%, while that of the peroxo product (
12) displayed a slight increase, contrary to the significant diminution in air-saturated system. These results suggest that an increase of the HO
− concentration resulted in opposite effects, the resultant of which depends on further conditions. In this case, the oxygenation processes depending on the O
2 concentration were much more facilitated than the interactions impeding these substitutions. Hence, also the Mn
V=O species plays some, even if minor, role in the production of
11, too, because its formation is promoted by higher O
2 concentration.
In the case of the cationic catalyst, the tendency of the changes in the product ratios upon this pH increase remained the same at higher oxygen concentration, but with moderately higher enhancements (to 70% for 11 and to 10% for 12). These results confirm the concept of the resultant of opposite effects. This is valid for the change in the ratio of product 13. In that case, in accordance with the air saturated system, higher pH caused a dramatic decrease of the ratio to trace level, but from a 44% (due to the higher O2 concentration). This observation indicates that the hampering, presumably shielding (of the positively charged complex) effect of the increased alkalinity is far predominant regarding the formation of 13.
The changes caused by an increase in the HO
- concentration in the case of the thienyl derivatives considerably deviated from those observed for the corresponding furan compounds. For instance, the results obtained in aerated systems practically agreed with those observed in the oxygen-saturated solutions (see
Scheme 4 and
Scheme 5). Besides, the products formed from the thienyl derivative
5 significantly differed from those originated from the corresponding furyl compound (
1), due to the more stable aromatic system in the previous one. Hence, in this case more oxidation reactions took place on carbon atoms out of the thiophene ring. Accordingly, in the case of the furan derivative
1 the products were functionalized exclusively at the carbon atoms originally belonging to the furan ring (via both substitution and ring cleavage), those formed from the corresponding thienyl compound possess the oxygen-containing groups predominantly on the saturated hydrocarbon skeleton. However, the types of the two products of the other analogous thionyl (
4) and furyl (
2) isomers fully agreed.
The pH increase in the system with anionic photocatalyst led to the disappearance of the hydroxy derivative
14, which is similar to the observation with the cationic one (
Scheme 4). These results indicate that the Mn
V=O species played the determining role in the production of this compound. However, the ratios of the products with higher oxygen content (peroxo and skeleton-cleaved keto-formyl derivatives,
15 and
16, respectively) perceptively increased. These results are quite the opposite of those obtained with the cationic manganese(III) porphyrin, where none of these derivatives could be detected at pH = 10, while ratios of 35–36% were obtained at pH = 7. These phenomena suggest that the role of the Mn
V=O species is crucial with the cationic complex, while hydroxyl radicals may be important with the anionic catalyst.
Like the 3-furyl derivative
2, the photocatalytic oxygenation of the corresponding thienyl compound
4 at pH = 10 resulted in a product (
18) that is hydroxylated at the outer carbon atom adjacent to the heteroatom of the aromatic ring (
Scheme 5). However, the yield of this product increased from about 18–28% to 70% in the case of the furan derivative, while the corresponding thiophene product appeared only at the higher pH (=10). This phenomenon confirms that an increase of pH promotes this type of hydroxylation of both compounds with both catalysts, but the reactivity of the thienyl derivative is significantly less than that of the furyl type. Consequently, in accordance with the corresponding furyl derivative (
2), hydroxyl radical is the main oxidative agent in this reaction, due to its favored production at higher pH.
Interestingly, compared to the furan compound 2, the ratio of product 17 just moderately decreased upon increasing pH (from 7 to 10), while that of the corresponding hydroxy furanon product (13, formed only with the cationic catalyst) diminished to “traces”. These decreases confirm the determining role of the MnV=O and partly the (P)MnIV-O2•− species in the formation of this type of product. However, the significantly higher product ratios for the thiophene product needs further considerations.
The yields and turnover numbers of the products obtained in the photocatalytic oxygenations of
2,
3, and
4 under various conditions are summarized in
Table 1.
The structures of all of the obtained compounds
11–
18 were confirmed by complete characterizations utilizing modern spectroscopic methods. The spectra were in accordance with those of the structures previously synthesized under different reaction conditions (including pH) [
17,
18,
20]. All of these compounds have a specific pattern of aliphatic protons which is characteristic for the benzobicyclo[3.2.1]octadiene skeleton with different functionalities gained in the photooxygenation reactions. Functionalization of the 3-thienyl substituted benzobicyclo[3.2.1]octadiene
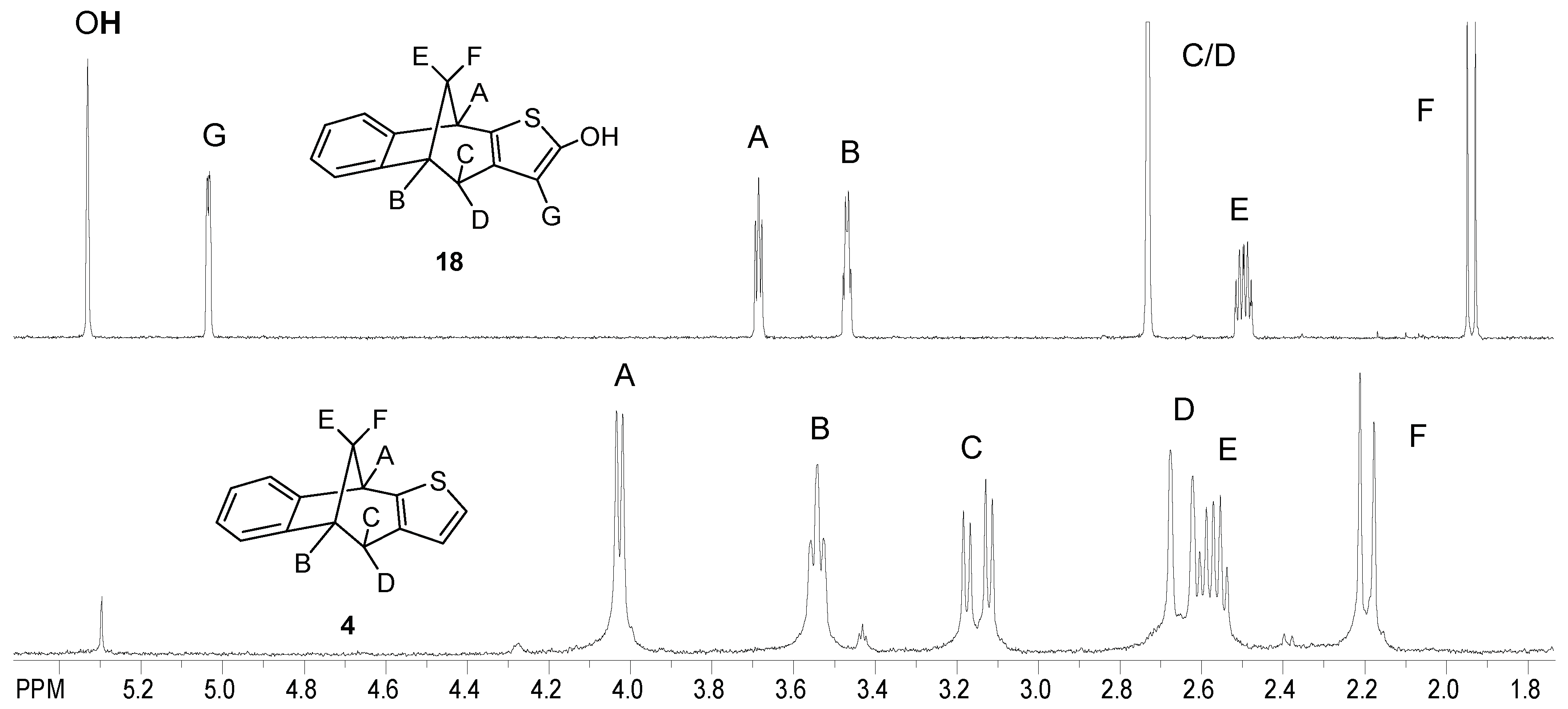
4 gave a new product,
18, that is hydroxylated at position 5 of the furan ring
. It has a pattern of protons in
1H NMR that are distinguishable and differ from the pattern of the starting compound
4 (
Figure 1). Here, the protons H
A-H
G and the proton of the OH group are situated in the region between 5.3 and 1.9 ppm. The synthesis of this kind of hydroxylated products is very difficult in thermal reactions and there is no procedure in the literature regarding the introduction of OH group onto the heteroaromatic (furan) ring. According to these facts, both hydroxylated products (
11 and
18) are the most valuable ones as intermediates for further functionalization, the long-term goal of which is to increase the lipophilicity of all the new structures and to get better physico-chemical properties.
Comparing the products and the changes of their yields obtained for the furan and thiophene derivatives oxygenated under various conditions, it is clearly seen that the products of the furan compound (
1) and the analogous thiophene derivative (
3) are totally different, due to the dramatic effect of the heteroatom on the ways of oxygenation. Accordingly, the effects of the pH increase cannot be interpreted by an overall scheme. However, the types of the main products in the case of substrates
2 and
3 well agree, hence, they properly demonstrate the effects of the pH change on their photocatalytically oxygenated derivatives. As shown in
Scheme 6, in the oxygenation leading to the analogous products
11 and
18 the main oxidative agent is hydroxyl radical, the formation of which is promoted by an increase of the HO
− concentration. Its reactions with the substrates cannot be affected by the charge of the photocatalyst. In the case of products
13 and
17, however, the pH increase significantly diminished the yields obtained with the cationic catalyst, due to the axial coordination of a hydroxide ion, decreasing the electrophylicity of (P)Mn
V=O, the main agent in these oxygenation reactions.
Utilization of this type of photocatalytic oxygenation can also be applied for synthesis of other novel benzobicyclo[3.2.1]octadiene derivatives with oxygen or other functional groups, even more similar to natural structures, by their subsequent transformations as presented in
Scheme 7.
 3-thiatetracyclo[6.6.1.02,⁶.0⁹,1⁴]pentadeca-2(6),4,9,11,13-pentaen-7-ol (18): 25%, Rf 0.75 (dichloromethane/diethylether = 9:1); colourless oil; 1H NMR (CDCl3, 600 MHz) δ 7.19 (d, J = 7.3 Hz, 1H), 7.10–7.16 (m, 3H), 5.32 (s, –OH, 1H), 5.03 (d, JG-OH = 2.5 Hz, 1H), 3.77 (t, 1H, J = 5.3 Hz, H-A), 3.46 (dd, J = 8.3; 4.1 Hz, 1H, H-B), 2.70–2.74 (m, 2H, H-C, H-D), 2.45–2.51 (m, 1H, H-E), 1.93 (d, 1H, J = 11.9 Hz, H-F); 13C NMR (CDCl3, 150 MHz) δ 167.9 (s), 144.8 (s), 139.7 (s), 127.9 (d), 127.4 (d), 125.0 (d), 122.6 (d), 118.0 (d), 82.8 (d), 46.4 (d), 41.8 (d), 40.7 (d), 35.4 (t); MS m/z: 228 (M+, 100%), 211 (60%); HRMS (Q-TOF LC/MS) for C14H12OS: (M + H)+ calcd 229.0635, (M + H)+ measured 229.0677.
3-thiatetracyclo[6.6.1.02,⁶.0⁹,1⁴]pentadeca-2(6),4,9,11,13-pentaen-7-ol (18): 25%, Rf 0.75 (dichloromethane/diethylether = 9:1); colourless oil; 1H NMR (CDCl3, 600 MHz) δ 7.19 (d, J = 7.3 Hz, 1H), 7.10–7.16 (m, 3H), 5.32 (s, –OH, 1H), 5.03 (d, JG-OH = 2.5 Hz, 1H), 3.77 (t, 1H, J = 5.3 Hz, H-A), 3.46 (dd, J = 8.3; 4.1 Hz, 1H, H-B), 2.70–2.74 (m, 2H, H-C, H-D), 2.45–2.51 (m, 1H, H-E), 1.93 (d, 1H, J = 11.9 Hz, H-F); 13C NMR (CDCl3, 150 MHz) δ 167.9 (s), 144.8 (s), 139.7 (s), 127.9 (d), 127.4 (d), 125.0 (d), 122.6 (d), 118.0 (d), 82.8 (d), 46.4 (d), 41.8 (d), 40.7 (d), 35.4 (t); MS m/z: 228 (M+, 100%), 211 (60%); HRMS (Q-TOF LC/MS) for C14H12OS: (M + H)+ calcd 229.0635, (M + H)+ measured 229.0677.