One-Step Synthesis of MoS2/TiSi2 via an In Situ Photo-Assisted Reduction Method for Enhanced Photocatalytic H2 Evolution under Simulated Sunlight Illumination
Abstract
:1. Introduction
2. Results and Discussion
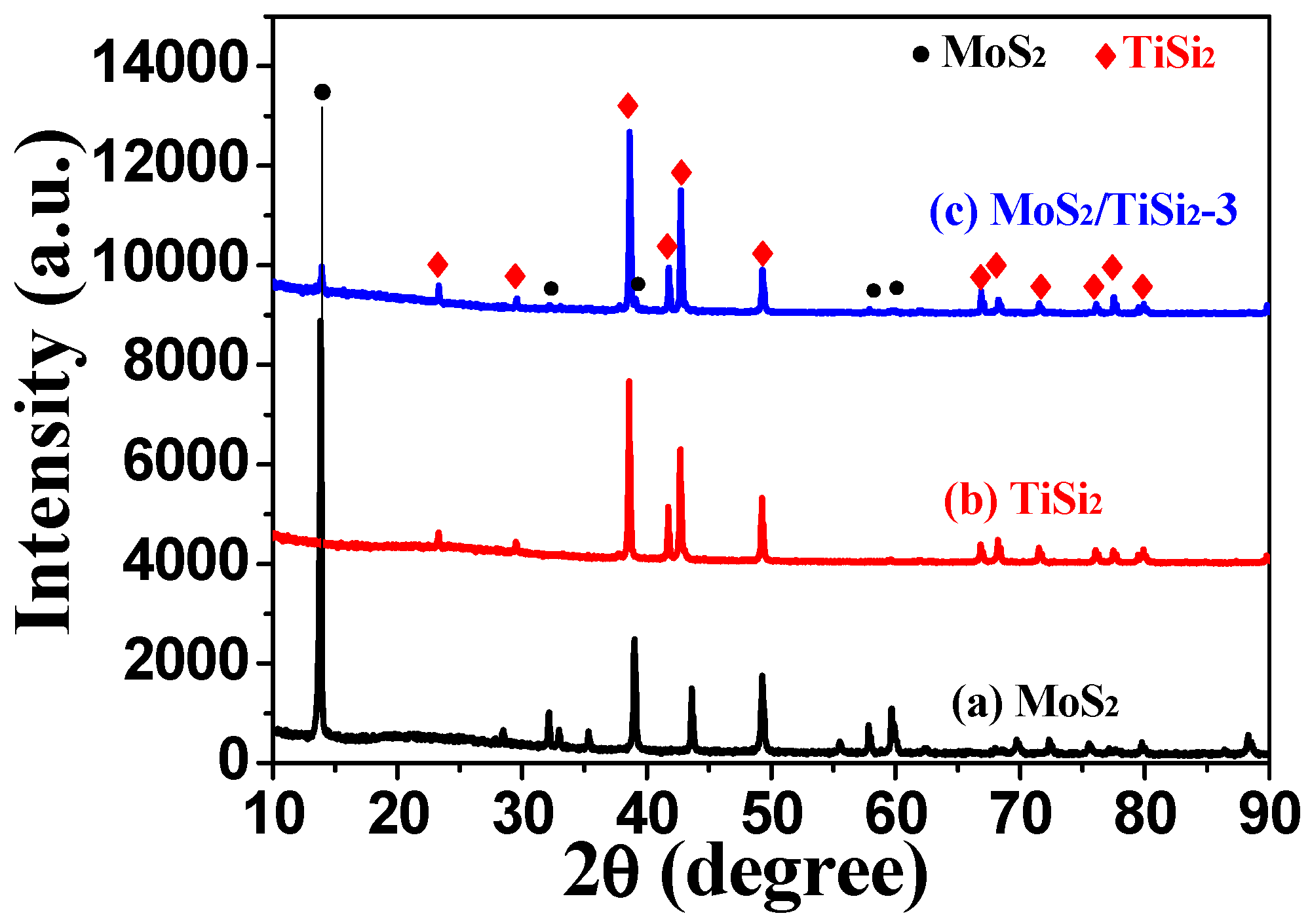
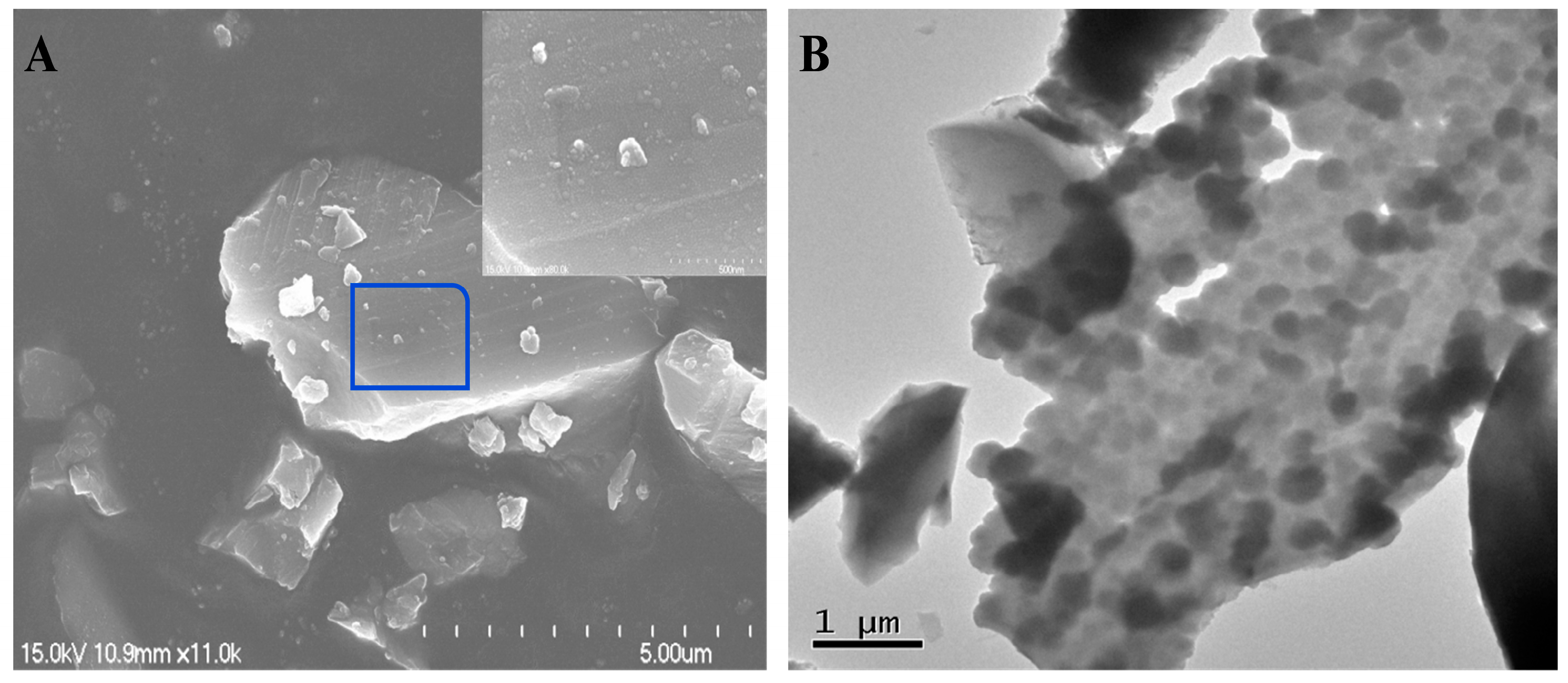
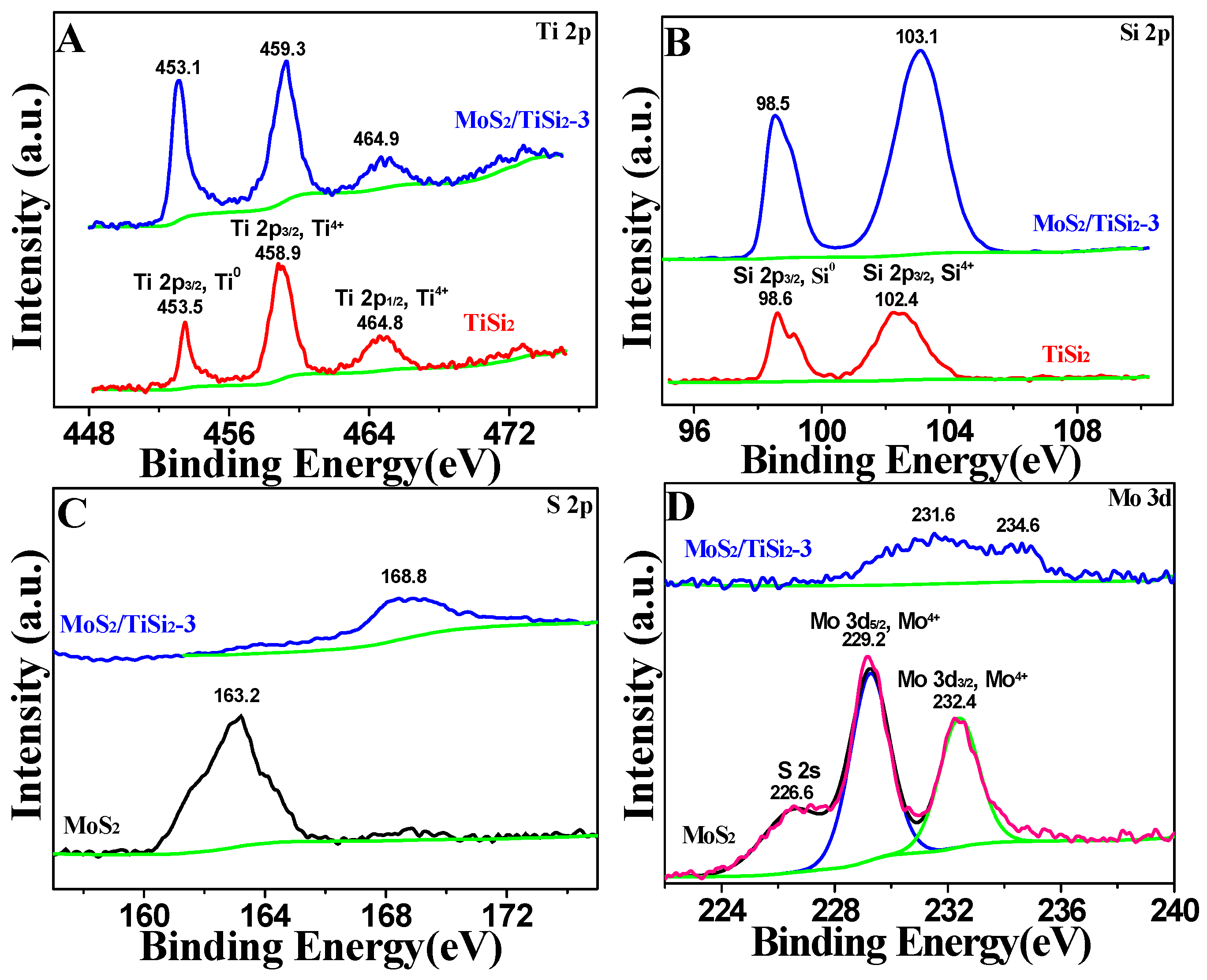
2.1. Morphology and Structure
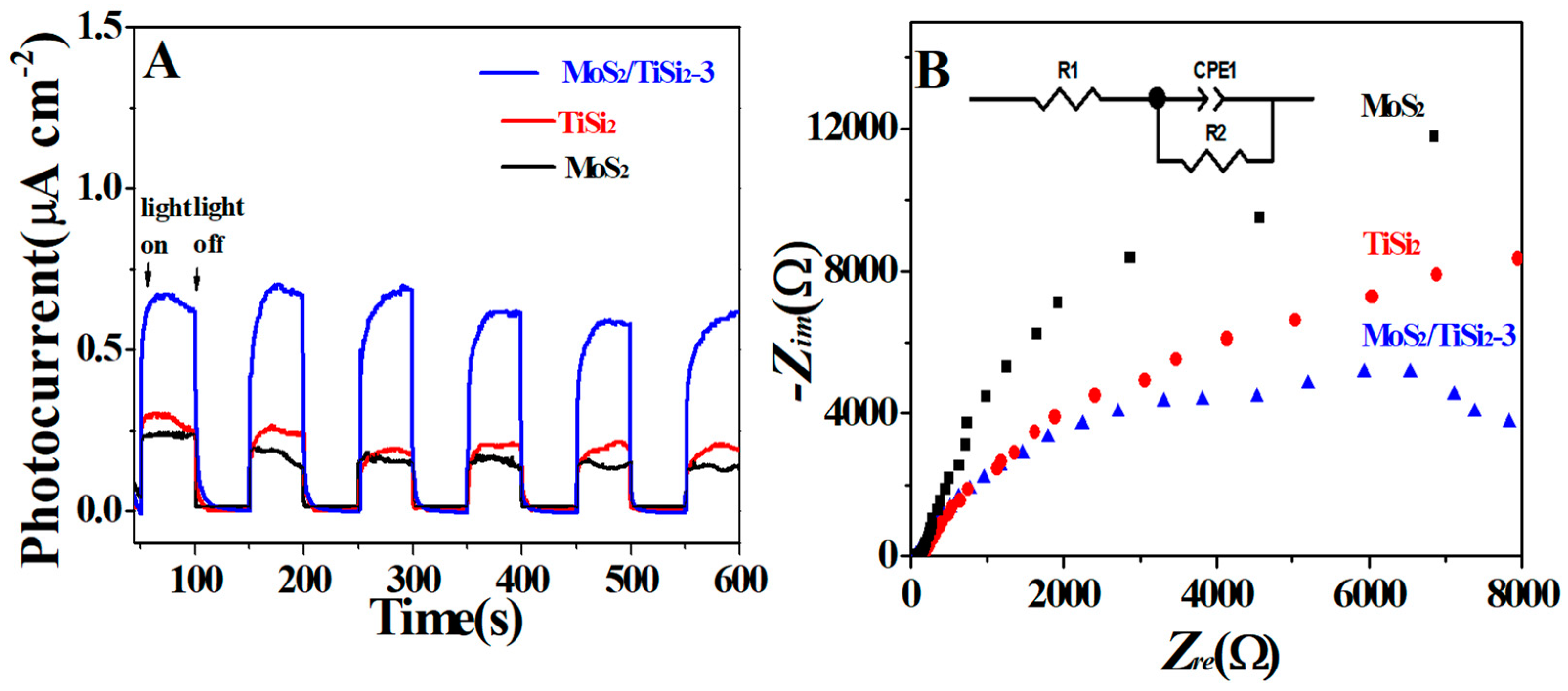
2.2. Optical and Photoelectrochemical Properties
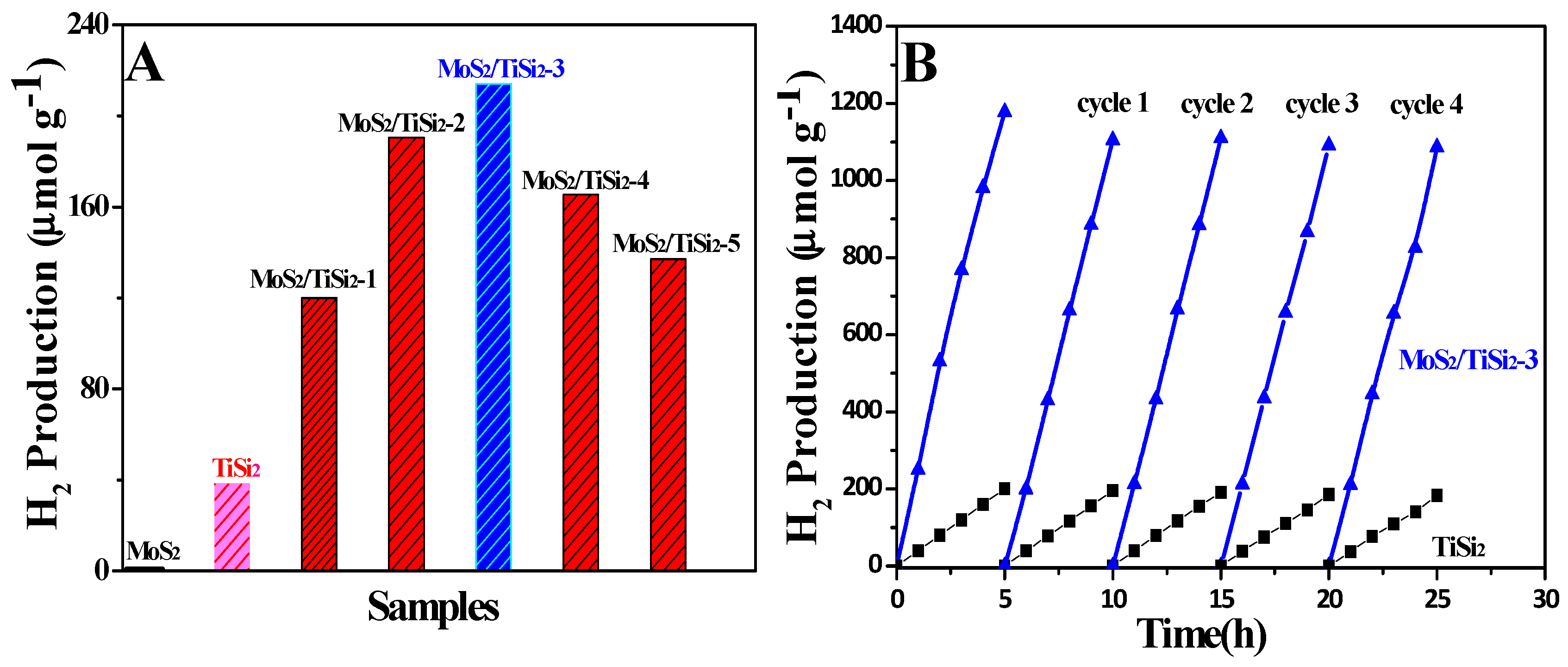
2.3. Photocatalytic Activity
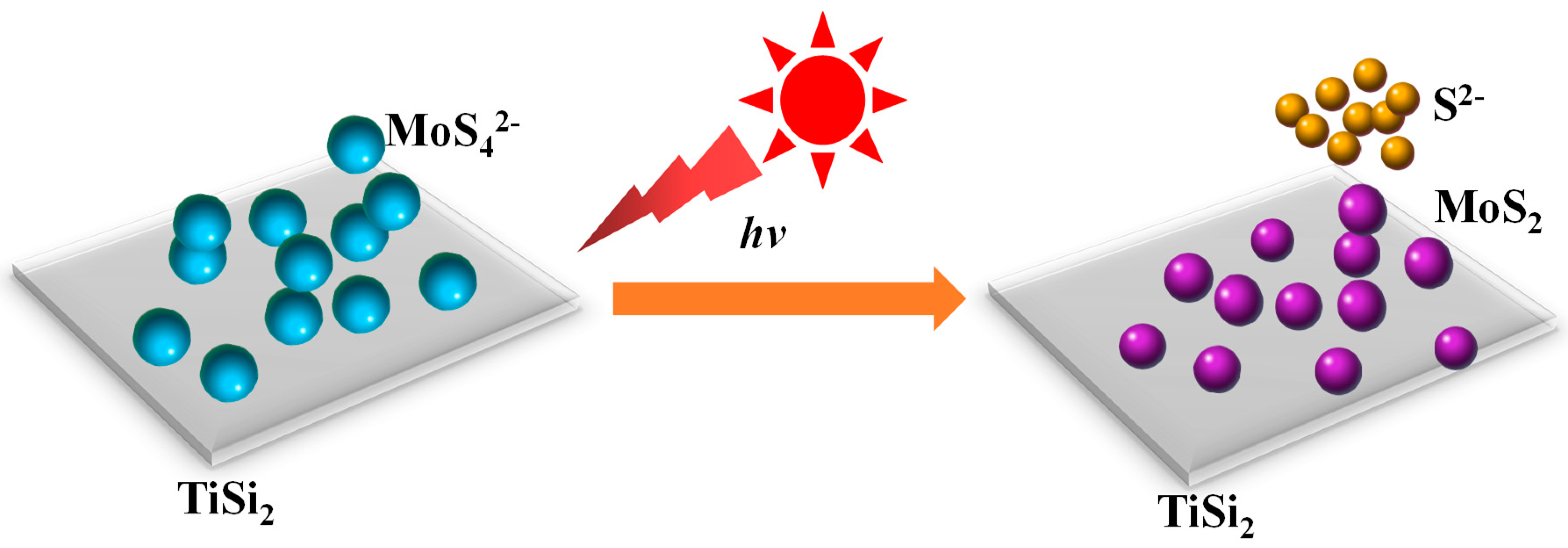
2.4. Formation Mechanism of the MoS2/TiSi2 Photocatalyst
3. Experimental
3.1. Materials
3.2. Synthesis
3.3. Characterization
3.4. Photoelectrochemical Measurements
3.5. Photocatalytic Reaction for Hydrogen Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chu, D.; Zhang, C.; Yang, P.; Du, Y.; Lu, C. WS2 as an Effective Noble-Metal Free Cocatalyst Modified TiSi2 for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts 2016, 6, 136. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, M.; Liu, J.; Deng, Z.; Li, Y.; Su, B.-L. Synergistic promotion of solar-driven H2 generation by three-dimensionally ordered macroporous structured TiO2-Au-CdS ternary photocatalyst. Appl. Catal. B Environ. 2016, 184, 182–190. [Google Scholar] [CrossRef]
- Wen, J.; Li, X.; Li, H.; Ma, S.; He, K.; Xu, Y.; Fang, Y.; Liu, W.; Gao, Q. Enhanced visible-light H2 evolution of g-C3N4 photocatalysts via the synergetic effect of amorphous NiS and cheap metal-free carbon black nanoparticles as co-catalysts. Appl. Surf. Sci. 2015, 358 Pt A, 204–212. [Google Scholar] [CrossRef]
- Rajamanickam, D.; Dhatshanamurthi, P.; Shanthi, M. Enhanced photocatalytic efficiency of NiS/TiO2 composite catalysts using sunset yellow, an azo dye under day light illumination. Mater. Res. Bull. 2015, 61, 439–447. [Google Scholar] [CrossRef]
- Lin, Y.; Zhou, S.; Liu, X.; Sheehan, S.; Wang, D. TiO2/TiSi2 Heterostructures for High-Efficiency Photoelectrochemical H2O Splitting. J. Am. Chem. Soc. 2009, 131, 2772–2773. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mohapatra, S.K.; Misra, M. Water Photooxidation by TiSi2–TiO2 Nanotubes. J. Phys. Chem. C 2011, 115, 12643–12649. [Google Scholar] [CrossRef]
- Pelleg, J.; Sade, G. TiB2/TiSi2 bilayer fabrication by various techniques: Structure and contact properties. Phys. B Condens. Matter 2006, 371, 20–34. [Google Scholar] [CrossRef]
- Ritterskamp, P.; Kuklya, A.; Stkamp, M.-A.; Kerpen, K.; Weidenthaler, C.; Demuth, M. A Titanium Disilicide Derived Semiconducting Catalyst for Water Splitting under Solar Radiation—Reversible Storage of Oxygen and Hydrogen. Angew. Chem. Int. Ed. 2007, 46, 7770–7774. [Google Scholar] [CrossRef]
- Shon, I.-J.; Park, H.-K.; Kim, H.-C.; Yoon, J.-K.; Hong, K.-T.; Ko, I.-Y. One-step synthesis and densification of nanostructured TiSi2–SiC composite from mechanically activated (TiC + 3Si) powders by high-frequency-induced heated combustion. Scr. Mater. 2007, 56, 665–668. [Google Scholar] [CrossRef]
- Liu, J.; Bai, Y.; Chen, P.; Cui, N.; Yin, H. Reaction synthesis of TiSi2 and Ti5Si3 by ball-milling and shock loading and their photocatalytic activities. J. Alloys Compd. 2013, 555, 375–380. [Google Scholar] [CrossRef]
- Mou, Z.; Yin, S.; Zhu, M.; Du, Y.; Wang, X.; Yang, P.; Zheng, J.; Lu, C. RuO2/TiSi2/graphene composite for enhanced photocatalytic hydrogen generation under visible light irradiation. Phys. Chem. Chem. Phys. 2013, 15, 2793–2799. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.-X.; Zhang, W.-D. MoS2/CdS Heterojunction with High Photoelectrochemical Activity for H2 Evolution under Visible Light: The Role of MoS2. J. Phys. Chem. C 2013, 117, 12949–12957. [Google Scholar] [CrossRef]
- Chang, K.; Chen, W. In situ synthesis of MoS2/graphene nanosheet composites with extraordinarily high electrochemical performance for lithium ion batteries. Chem. Commun. 2011, 47, 4252–4254. [Google Scholar] [CrossRef]
- Nguyen, M.; Tran, P.D.; Pramana, S.S.; Lee, R.L.; Batabyal, S.K.; Mathews, N.; Wong, L.H.; Graetzel, M. In situ photo-assisted deposition of MoS2 electrocatalyst onto zinc cadmium sulphide nanoparticle surfaces to construct an efficient photocatalyst for hydrogen generation. Nanoscale 2013, 5, 1479–1482. [Google Scholar] [CrossRef]
- Lu, X.; Jin, Y.; Zhang, X.; Xu, G.; Wang, D.; Lv, J.; Zheng, Z.; Wu, Y. Controllable synthesis of graphitic C3N4/ultrathin MoS2 nanosheet hybrid nanostructures with enhanced photocatalytic performance. Dalton Trans. 2016, 45, 15406–15414. [Google Scholar] [CrossRef]
- Larciprete, R.; Danailov, M.; Barinov, A.; Gregoratti, L.; Kiskinova, M. Thermal and pulsed laser induced surface reactions in Ti/Si(001) interfaces studied by spectromicroscopy with synchrotron radiation. J. Appl. Phys. 2001, 90, 4361–4369. [Google Scholar] [CrossRef]
- Fouad, O.A.; Yamazato, M.; Era, M.; Nagano, M.; Hirai, T.; Usui, I. Preparation and properties of TiSi2 thin films from TiCl4/H2 by plasma enhanced chemical vapor deposition. J. Cryst. Growth 2002, 234, 440–446. [Google Scholar] [CrossRef]
- Wee, A.T.S.; Huan, A.C.H.; Osipowicz, T.; Lee, K.K.; Thian, W.H.; Tan, K.L.; Hogan, R. Surface and interface studies of titanium silicide formation. Thin Solid Films 1996, 283, 130–134. [Google Scholar] [CrossRef]
- Zong, X.; Yan, H.; Wu, G.; Ma, G.; Wen, F.; Wang, L.; Li, C. Enhancement of Photocatalytic H2 Evolution on CdS by Loading MoS2 as Cocatalyst under Visible Light Irradiation. J. Am. Chem. Soc. 2008, 130, 7176–7177. [Google Scholar] [CrossRef]
- Yue, Z.; Chu, D.; Huang, H.; Huang, J.; Yang, P.; Du, Y.; Zhu, M.; Lu, C. A novel heterogeneous hybrid by incorporation of Nb2O5 microspheres and reduced graphene oxide for photocatalytic H2 evolution under visible light irradiation. RSC Adv. 2015, 5, 47117–47124. [Google Scholar] [CrossRef]
- Franceschini, E.A.; Gomez, M.J.; Lacconi, G.I. One step synthesis of high efficiency nickel/mesoporous TiO2 hybrid catalyst for hydrogen evolution reaction. J. Energy Chem. 2019, 29, 79–87. [Google Scholar] [CrossRef]
- Bahrami, A.; Kazeminezhad, I.; Abdi, Y. Pt-Ni/rGO counter electrode: Electrocatalytic activity for dye-sensitized solar cell. Superlattices Microstruct. 2019, 125, 125–137. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.-X.; Liu, F.-T.; Dong, X.-F.; Li, X.; Wang, C.-W. New design of oriented NiS nanoflower arrays as platinum-free counter electrode for high-efficient dye-sensitized solar cells. Superlattices Microstruct. 2019, 125, 66–71. [Google Scholar] [CrossRef]
- Vishnu, N.; Badhulika, S. Single step grown MoS2 on pencil graphite as an electrochemical sensor for guanine and adenine: A novel and low cost electrode for DNA studies. Biosens. Bioelectron. 2019, 124, 122–128. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Liu, A.; Li, K.; Du, Y.; Yang, P. One-Step Synthesis of MoS2/TiSi2 via an In Situ Photo-Assisted Reduction Method for Enhanced Photocatalytic H2 Evolution under Simulated Sunlight Illumination. Catalysts 2019, 9, 299. https://doi.org/10.3390/catal9030299
Zhang C, Liu A, Li K, Du Y, Yang P. One-Step Synthesis of MoS2/TiSi2 via an In Situ Photo-Assisted Reduction Method for Enhanced Photocatalytic H2 Evolution under Simulated Sunlight Illumination. Catalysts. 2019; 9(3):299. https://doi.org/10.3390/catal9030299
Chicago/Turabian StyleZhang, Chunyong, Aijuan Liu, Kezhen Li, Yukou Du, and Ping Yang. 2019. "One-Step Synthesis of MoS2/TiSi2 via an In Situ Photo-Assisted Reduction Method for Enhanced Photocatalytic H2 Evolution under Simulated Sunlight Illumination" Catalysts 9, no. 3: 299. https://doi.org/10.3390/catal9030299
APA StyleZhang, C., Liu, A., Li, K., Du, Y., & Yang, P. (2019). One-Step Synthesis of MoS2/TiSi2 via an In Situ Photo-Assisted Reduction Method for Enhanced Photocatalytic H2 Evolution under Simulated Sunlight Illumination. Catalysts, 9(3), 299. https://doi.org/10.3390/catal9030299




