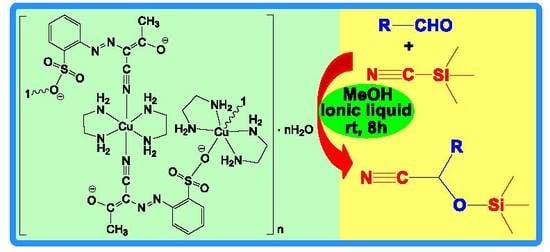
Cyanosilylation of Aldehydes Catalyzed by Ag(I)- and Cu(II)-Arylhydrazone Coordination Polymers in Conventional and in Ionic Liquid Media
Abstract
1. Introduction
2. Results and Discussion
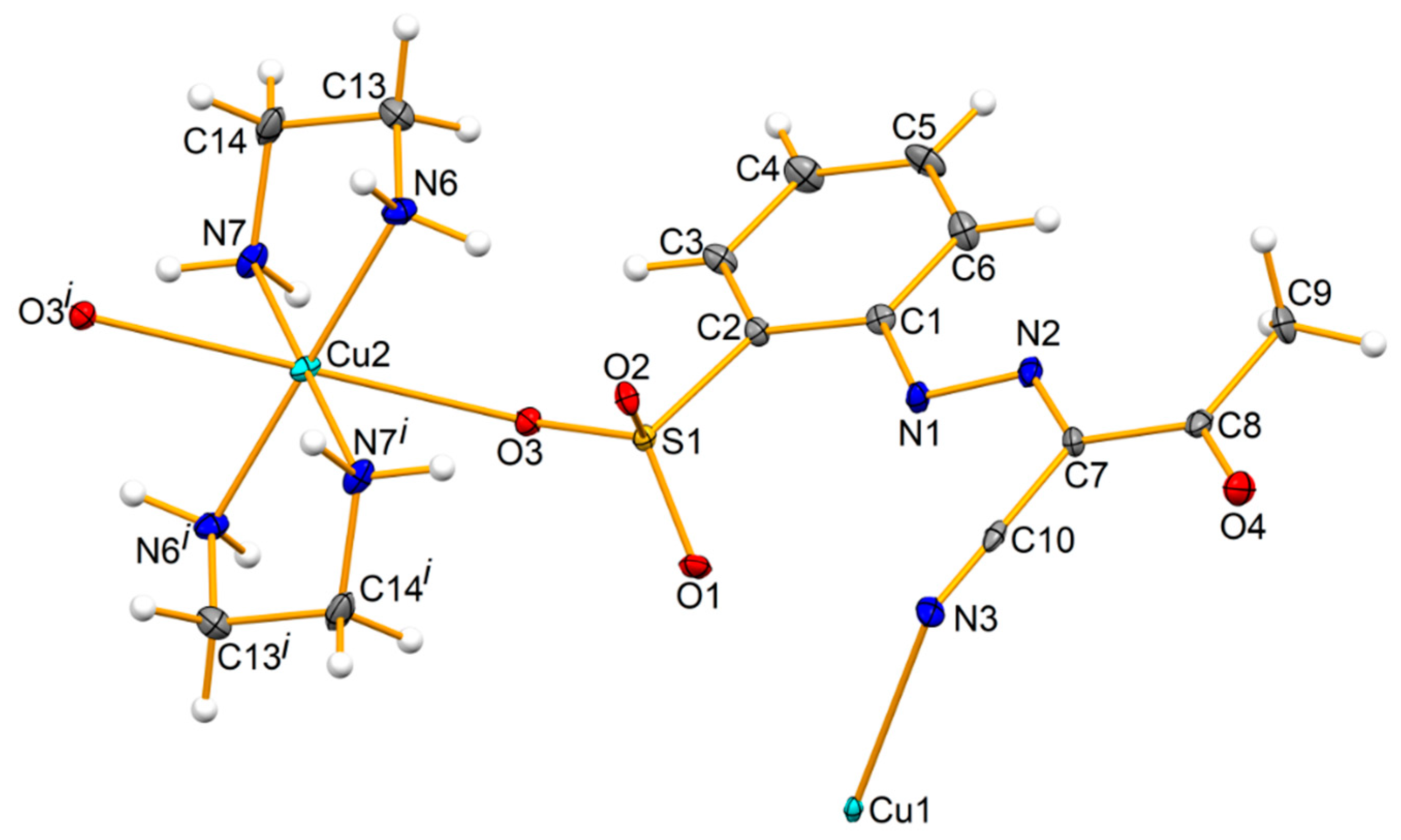
2.1. Synthesis and Characterization of 1–3
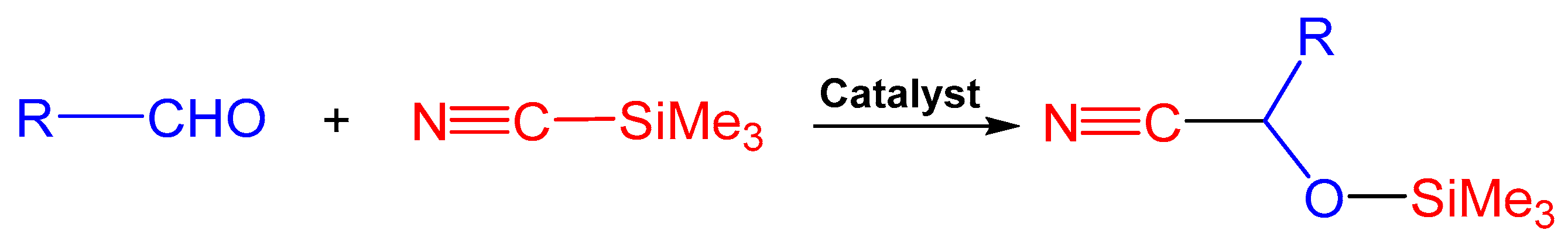
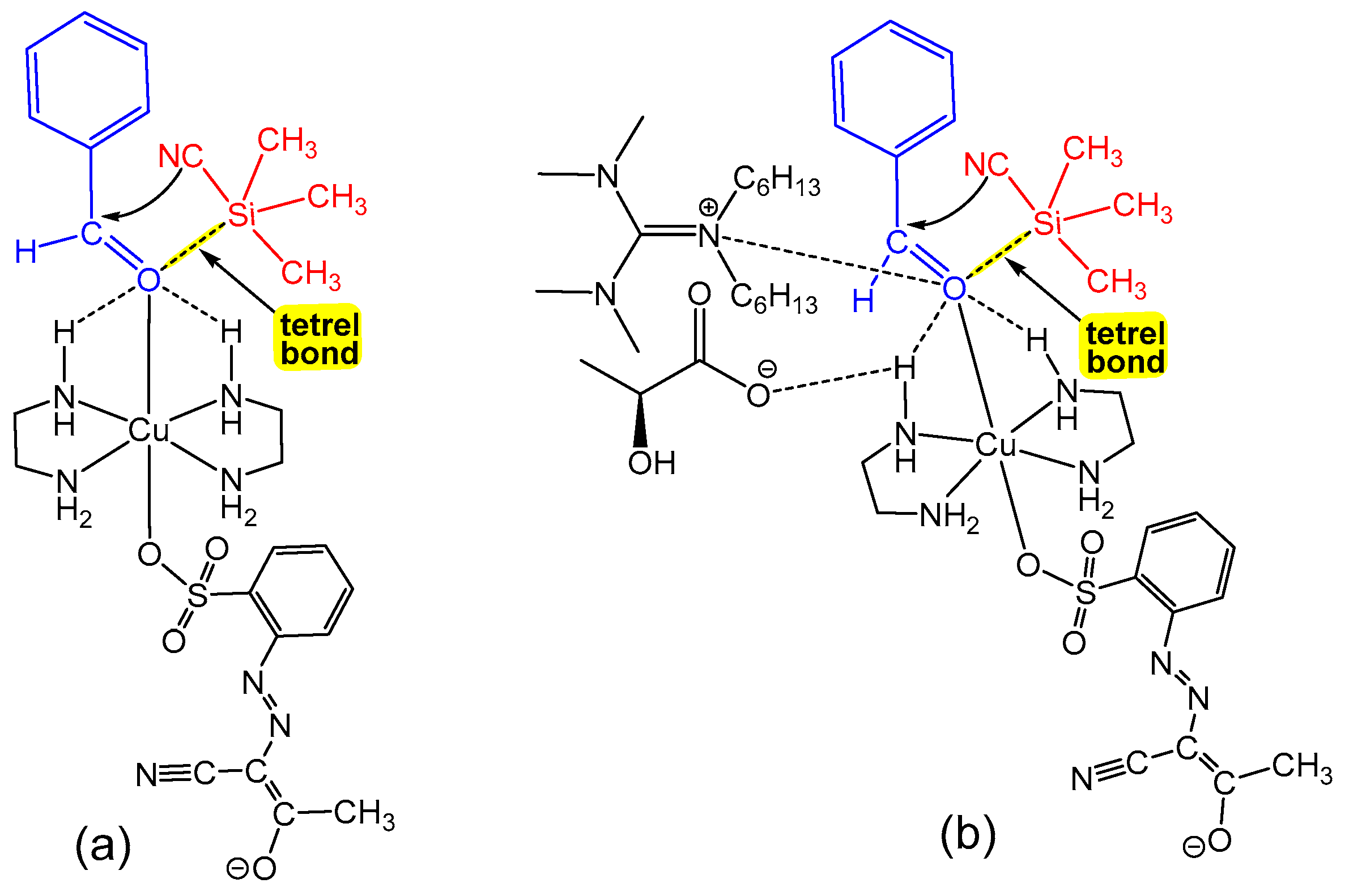
2.2. Catalytic Activity of 1–3 in Cyanosilylation Reaction
3. Experimental
3.1. Materials and Instrumentation
3.2. Synthesis
3.2.1. Synthesis of 1
3.2.2. Synthesis of 2
3.3. Crystal Structure Determination
3.4. Synthesis of ILs
3.4.1. Synthesis of 1,1,3,3-tetramethylguanidine acetate [TMGH][OAc]
3.4.2. Synthesis of trihexyl(tetradecyl)phosphonium L-prolinate [P6,6,6,14][L-Prolinate]
3.5. General Procedure for Catalytic Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kurono, N.; Ohkuma, T. Catalytic Asymmetric Cyanation Reactions. ACS Catal. 2016, 6, 989–1023. [Google Scholar] [CrossRef]
- Kantam, M.L.; Mahendar, K.; Sreedhar, B.; Kumar, K.V.; Choudary, B.M. Cyanosilylation of Aldehydes and Ketones Catalyzed by Nanocrystalline Magnesium Oxide. Synth. Commun. 2008, 38, 3919–3936. [Google Scholar] [CrossRef]
- Strappaveccia, G.; Lanari, D.; Gelman, D.; Pizzo, F.; Rosati, O.; Curinib, M.; Vaccaro, L. Efficient synthesis of cyanohydrin trimethylsilyl ethers via 1,2-chemoselective cyanosilylation of carbonyls. Green Chem. 2013, 15, 199–204. [Google Scholar] [CrossRef]
- North, M.; Omedes-Pujol, M.; Young, C. Kinetics and mechanism of the racemic addition of trimethylsilyl cyanide to aldehydes catalysed by Lewis bases. Org. Biomol. Chem. 2012, 10, 4289–4298. [Google Scholar] [CrossRef] [PubMed]
- North, M.; Omedes-Pujol, M. Kinetics and mechanism of vanadium catalysed asymmetric cyanohydrin synthesis in propylene carbonate. Beilstein J. Org. Chem. 2010, 6, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Huang, X.; Huang, J.; Xiong, Y.; Qin, B.; Feng, X. Asymmetric cyanosilylation of aldehydes catalyzed by novel organo-catalysts. Synlett 2005, 16, 2445–2448. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, J.; Bao, Z.; Chang, G.; Xing, H.; Ren, Q. Insight into the catalytic properties and applications of metal-organic frameworks in the cyanosilylation of aldehydes. RSC Adv. 2015, 5, 79355–79360. [Google Scholar] [CrossRef]
- Saravanan, P.; Anand, R.V.; Singh, V.R. Cu(OTf)2 catalyzed trimethylsilyl cyanide addition to carbonyl compounds. Tetrahedron Lett. 1998, 39, 3823–3824. [Google Scholar] [CrossRef]
- Curini, M.; Epifano, F.; Marcotullio, M.C.; Rosati, O.; Rossi, M. Potassium exchanged zirconium hydrogen phosphate as heterogeneous catalyst in cyanosilylation of carbonyl compounds. Synlett 1999, 315–316. [Google Scholar] [CrossRef]
- Surya, K.D.; Richard, A.G. Vanadyl triflate as an efficient and recyclable catalyst for trimethylsilyl cyanide addition to carbonyl compounds. J. Mol. Catal. A Chem. 2005, 232, 123–125. [Google Scholar] [CrossRef]
- Tian, S.-K.; Deng, L. A highly enantioselective chiral Lewis base-catalyzed asymmetric cyanation of ketones. J. Am. Chem. Soc. 2001, 123, 6195–6196. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.-K.; Hong, R.; Deng, L. Catalytic asymmetric cyanosilylation of ketones with chiral Lewis base. J. Am. Chem. Soc. 2003, 125, 9900–9901. [Google Scholar] [CrossRef] [PubMed]
- Denmark, S.E.; Chung, W. Lewis base catalyzed addition of trimethylsilyl cyanide to aldehydes. J. Org. Chem. 2006, 71, 4002–4005. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.H.; Corey, E.J. Highly enantioselective cyanosilylation of aldehydes catalyzed by a chiral oxazaborolidinium ion. J. Am. Chem. Soc. 2004, 126, 8106–8107. [Google Scholar] [CrossRef]
- Ryu, D.H.; Corey, E.J. Enantioselective cyanosilylation of ketones catalyzed by a chiral oxazaborolidinium ion. J. Am. Chem. Soc. 2005, 127, 5384–5387. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, D.E.; Jacobsen, E.N. Thiourea-catalyzed enantioselective cyanosilylation of ketones. J. Am. Chem. Soc. 2005, 127, 8964–8965. [Google Scholar] [CrossRef] [PubMed]
- Zuend, S.J.; Jacobsen, E.N. Cooperative catalysis by tertiary amino-thioureas: Mechanism and basis for enantioselectivity of ketone cyanosilylation. J. Am. Chem. Soc. 2007, 129, 15872–15883. [Google Scholar] [CrossRef] [PubMed]
- Kurono, N.; Yamaguchi, M.; Suzuki, K.; Ohkuma, T. Lithium chloride: An active and simple catalyst for cyanosilylation of aldehydes and ketones. J. Org. Chem. 2005, 70, 6530–6532. [Google Scholar] [CrossRef] [PubMed]
- Amurrio, I.; Cordoba, R.; Csaky, A.G.; Plumet, J. Tetrabutylammonium cyanide catalyzed diasteroselective cyanosliylation of chiral alpha-hydroxyketones. Tetrahedron 2004, 60, 10521–10524. [Google Scholar] [CrossRef]
- Liu, X.; Qin, B.; Zhou, X.; He, B.; Feng, X. Catalytic asymmetric cyanosilylation of ketones by a chiral amino acid salt. J. Am. Chem. Soc. 2005, 127, 12224–12225. [Google Scholar] [CrossRef] [PubMed]
- Raj, I.V.P.; Suryavanshi, G.; Sudalai, A. Organocatalytic activation of TMSCN by basic ammonium salts for efficient cyanation of aldehydes and imines. Tetrahedron Lett. 2007, 48, 7211–7214. [Google Scholar] [CrossRef]
- Wang, X.; Tian, S.-K. Catalytic cyanosilylation of ketones with simple phosphonium salt. Tetrahedron Lett. 2007, 48, 6010–6013. [Google Scholar] [CrossRef]
- Song, J.J.; Gallou, F.; Reeves, J.T.; Tan, Z.; Yee, N.K.; Senanayake, C.H. Activation of TMSCN by N-heterocyclic carbenes for facile cyanosilylation of carbonyl compounds. J. Org. Chem. 2006, 71, 1273–1276. [Google Scholar] [CrossRef]
- Suzuki, Y.; Bakar, A.; Muramatsu, M.D.K.; Sato, M. Cyanosilylation of aldehydes catalyzed by N-heterocyclic carbenes. Tetrahedron 2006, 62, 4227–4231. [Google Scholar] [CrossRef]
- Fetterly, B.M.; Verkade, J.G. P(RNCH2CH2)N: Efficient catalysts for the cyanosilylation of aldehydes and ketones. Tetrahedron Lett. 2005, 46, 8061–8066. [Google Scholar] [CrossRef]
- Wang, Z.; Fetterly, B.M.; Verkade, J.G. P(MeNMCH2CH2)3N: An effective catalyst for trimethylsilycyanation of aldehydes and ketones. J. Org. Met. Chem. 2002, 646, 161–166. [Google Scholar] [CrossRef]
- Hamashima, Y.; Kanai, M.; Shibasaki, M. Catalytic enantioselective cyanosilylation of ketones. J. Am. Chem. Soc. 2000, 122, 7412–7413. [Google Scholar] [CrossRef]
- Hamashima, Y.; Sawada, D.; Nogami, H.; Kanai, M.; Shibasaki, M. Highly enantioselective cyanosilylation of aldehydes catalyzed by a Lewis acid-Lewis base bifunctional catalyst. Tetrahedron 2001, 57, 805–814. [Google Scholar] [CrossRef]
- Mita, T.; Sasaki, K.; Kanai, M.; Shibasaki, M. Catalytic enantioselective conjugate addition of cyanide to alpha, beta-unsaturated N-acylpyrroles. J. Am. Chem. Soc. 2005, 127, 514–515. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Huang, X.; Gou, S.; Huang, J.; Wen, Y.; Feng, X. Enantioselective cyanosilylation of ketones catalyzed by a nitrogen-containing bifunctional catalyst. Adv. Synth. Catal. 2006, 348, 538–544. [Google Scholar] [CrossRef]
- Qin, B.; Liu, X.; Shi, J.; Zheng, K.; Zhao, H.; Feng, X. Enantioselective cyanosilylation of alpha, alpha-dialkoxy ketones catalyzed by proline-derived in-situ-prepared N-oxide as bifunctional organocatalyst. J. Org. Chem. 2007, 72, 2374–2378. [Google Scholar] [CrossRef]
- Shen, K.; Liu, X.; Li, Q.; Feng, X. Highly enantio selective cyanosilylation of ketones catalyzed by a bifunctional Ti(IV) complex. Tetrahedron 2008, 64, 147–153. [Google Scholar] [CrossRef]
- Baleizao, C.; Gigante, B.; Garcia, H.; Corma, A. Ionic liquids as green solvents for the asymmetric synthesis of cyanohydrins catalysed by VO(salen) complexes. Green Chem. 2002, 4, 272–274. [Google Scholar] [CrossRef]
- Baleizao, C.; Gigante, B.; Garcia, H.; Corma, A. Vanadyl salen complexes covalently anchored to an imidazolium ion as catalysts for the cyanosilylation of aldehydes in ionic liquids. Tetrahedron Lett. 2003, 44, 6813–6816. [Google Scholar] [CrossRef]
- Kim, S.S.; Song, D.H. Asymmetric cyanohydrin synthesis catalyzed by Al(salen)/triphenylphosphane oxide. Eur. J. Org. Chem. 2005, 1777–1780. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, S.H.; Kwak, J.M. Enantioselective cyanosilylation of ketones catalyzed by Mn(salen)/Ph3PO. Tetrahedron Asymmetry 2006, 17, 1165–1169. [Google Scholar] [CrossRef]
- Brunel, J.-M.; Legrand, O.; Buono, G. Enantioselective trimethylsilylcyanation of aromatic aldehydes catalyzed by titanium alkoxide-chiral o-hydroxyarylphosphine oxides complexes. Tetrahedron Asymmetry 1999, 10, 1979–1984. [Google Scholar] [CrossRef]
- Kim, S.S.; Kwak, J.M.; Rajagopal, G. Asymmetric cyanosilylation of aldehydes by chiral Ti-TADDOL complex. Bull. Korean Chem. Soc. 2006, 27, 1638–1640. [Google Scholar] [CrossRef]
- Gurbanov, A.V.; Maharramov, A.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Trinuclear and polymeric cobalt(II or II/III) complexes with an arylhydrazone of acetoacetanilide and their application in cyanosilylation of aldehydes. Inorg. Chim. Acta 2017, 466, 632–637. [Google Scholar] [CrossRef]
- Ma, Z.; Gurbanov, A.V.; Sutradhar, M.; Kopylovich, M.N.; Mahmudov, K.T.; Maharramov, A.M.; Guseinov, F.I.; Zubkov, F.I.; Pombeiro, A.J.L. Effective cyanosilylation of aldehydes with copper(II)-based polymeric catalysts. Mol. Catal. 2017, 428, 17–23. [Google Scholar] [CrossRef]
- Karmakar, A.; Hazra, S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Synthesis, structure and catalytic application of lead(II) complexes in cyanosilylation reactions. Dalton Trans. 2015, 44, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Xu, M.-C.; Zhang, L.-J.; Yao, R.-X.; Zhang, X.-M. Solvent-free heterogeneous catalysis for cyanosilylation in a dynamic cobalt-MOF. Dalton Trans. 2015, 44, 12711–12716. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Díaz, L.M.; Iglesias, M.; Snejko, N.; Gutiérrez-Puebla, E.; Monge, M.Á. Indium metal-organic frameworks as catalysts in solvent-free cyanosilylation reaction. CrystEngComm 2013, 15, 9562–9571. [Google Scholar] [CrossRef]
- Karmakar, A.; Paul, A.; Rubio, G.M.D.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Zinc(II) and Copper(II) Metal-Organic Frameworks Constructed from a Terphenyl-4,4’-dicarboxylic Acid Derivative: Synthesis, Structure, and Catalytic Application in the Cyanosilylation of Aldehydes. Eur. J. Inorg. Chem. 2016, 5557–5567. [Google Scholar] [CrossRef]
- Batten, S.R.; Neville, S.M.; Turner, D.R. Coordination Polymers: Design, Analysis and Application; The Royal Society of Chemistry: Cambridge, UK, 2009. [Google Scholar]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, F.; Sun, B.; Fu, R.; He, H.; Matyjaszewski, K. Design and Preparation of Porous Polymers. Chem. Rev. 2012, 112, 3959–4015. [Google Scholar] [CrossRef] [PubMed]
- Mahmudov, K.T.; Kopylovich, M.N.; Pombeiro, A.J.L. Coordination chemistry of arylhydrazones of methylene active compounds. Coord. Chem. Rev. 2013, 257, 1244–1281. [Google Scholar] [CrossRef]
- Pombeiro, A.J.L.; Guedes da Silva, M.F.C. Alkane Functionalization; Pombeiro, A.J.L., Guedes da Silva, M.F.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Kopylovich, M.N.; Ribeiro, A.P.C.; Alegria, E.C.B.A.; Martins, N.M.R.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Catalytic oxidation of alcohols: Recent advances. Adv. Organomet. Chem. 2015, 63, 91–174. [Google Scholar] [CrossRef]
- Olivier-Bourbigou, H.; Magna, L.; Morvan, D. Ionic liquids and catalysis: Recent progress from knowledge to applications. Appl. Catal. A Gen. 2010, 373, 1–56. [Google Scholar] [CrossRef]
- Shen, Z.-L.; Jib, S.-J.; Loh, T.-P. Ionic liquid [omim][PF6] as an efficient and recyclable reaction media for the cyanosilylation of aldehydes without Lewis acid or any special activation. Tetrahedron Lett. 2005, 46, 3137–3139. [Google Scholar] [CrossRef]
- Ullah, B.; Chen, J.; Zhang, Z.; Xing, H.; Yang, Q.; Bao, Z.; Ren, Q. 1-Ethyl-3-methylimidazolium acetate as a highly efficient organocatalyst for cyanosilylation of carbonyl compounds with trimethylsilyl cyanide. Sci. Rep. 2017, 7, 42699. [Google Scholar] [CrossRef] [PubMed]
- Jlassi, R.; Ribeiro, A.P.C.; Guedes da Silva, M.F.C.; Mahmudov, K.T.; Kopylovich, M.N.; Anisimova, T.B.; Naïli, H.; Tiago, G.A.O.; Pombeiro, A.J.L. Polynuclear Copper(II) Complexes as Catalysts for the Peroxidative Oxidation of Cyclohexane in Room Temperature Ionic Liquid Medium. Eur. J. Inorg. Chem. 2014, 4541–4550. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Ribeiro, A.P.C.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Branco, L.C.; Pombeiro, A.J.L. Copper(II) Complexes of Arylhydrazone of 1H-Indene-1,3(2H)-dione as Catalysts for the Oxidation of Cyclohexane in Ionic Liquids. Catalysts 2018, 8, 636. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Ribeiro, A.P.C.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Branco, L.C.; Pombeiro, A.J.L. Mononuclear Copper(II) Complexes of Arylhydrazone of 1H-Indene-1,3(2H)-dione as Catalysts for the Oxidation of 1-Phenylethanol in Ionic Liquid Medium. RSC Adv. 2016, 6, 83412–83420. [Google Scholar] [CrossRef]
- Gurbanov, A.V.; Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Sutradhar, M.; Guseinov, F.I.; Zubkov, F.I.; Maharramov, A.M.; Pombeiro, A.J.L. Molecular switching through cooperative ionic interactions and charge assisted hydrogen bonding. Dyes Pigment. 2017, 138, 107–111. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, tau(4). Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.; Gibbs, G.V.; Ribbe, P.H. Quadratic Elongation: A Quantitative Measure of Distortion in Coordination Polyhedra. Science 1971, 172, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond: Outline of a Comprehensive Hydrogen Bond Theory; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Neogi, S.; Sharma, M.K.; Bharadwaj, P.K. Knoevenagel condensation and cyanosilylation reactions catalyzed by a MOF containing Coordinatively unsaturated Zn(II) centres. J. Mol. Catal. A Chem. 2009, 299, 1–4. [Google Scholar] [CrossRef]
- Lestari, W.W.; Lönnecke, P.; Streit, H.C.; Schleife, F.; Wickleder, C.; Hey-Hawkins, E. A chiral two-dimensional coordination polymer based on Cu-II and (S)-4,4’ bis(4-carboxyphenyl)-2,2’ bis(diphenylphosphinoyl)-1,1’ binaphthyl: Synthesis, structure, and magnetic and optical properties. Inorg. Chim. Acta 2014, 421, 392–398. [Google Scholar] [CrossRef]
- Shen, Z.-L.; Ji, S.-J. Alkali Salt of L-Proline as an Efficient and Practical Catalyst for the Cyanosilylation of a Wide Variety of Carbonyl Compounds Under Solvent-Free Conditions. Synth. Commun. 2009, 39, 775–791. [Google Scholar] [CrossRef]
- Hamashima, Y.; Sawada, D.; Kanai, M.; Shibasaki, M. A new bifunctional asymmetric catalysis: An efficient catalytic asymmetric cyanosilylation of aldehydes. J. Am. Chem. Soc. 1999, 121, 2641–2642. [Google Scholar] [CrossRef]
- Kim, S.S. Asymmetric cyanohydrin synthesis from aldehydes and ketones using chiral metal (salen) complex as catalyst. Pure Appl. Chem. 2006, 78, 977–983. [Google Scholar] [CrossRef]
- Chen, F.-X.; Zhou, H.; Liu, X.; Qin, B.; Feng, X.; Zhang, G.; Jiang, Y. Enantioselective cyanosilylation of ketones by a catalytic double-activation method with an aluminium complex and an N-oxide. Chem. Eur. J. 2004, 10, 4790–4797. [Google Scholar] [CrossRef] [PubMed]
- Mahmudov, K.T.; Gurbanov, A.V.; Guseinov, F.I.; Guedes da Silva, M.F.C. Noncovalent interactions in metal complex catalysis. Coord. Chem. Rev. 2019, 387, 32–46. [Google Scholar] [CrossRef]
- Bruker. APEX2; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction; University of Gottingen: Gottingen, Germany, 2000. [Google Scholar]
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Cryst. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Cryst. 2009, 65, 148–155. [Google Scholar] [CrossRef]
- Gouveia, A.S.L.; Tomé, L.C.; Marrucho, I.M. Density, Viscosity, and Refractive Index of Ionic Liquid Mixtures Containing Cyano and Amino Acid-Based Anions. J. Chem. Eng. Data 2016, 61, 83–93. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, T.; Gao, H.; Han, B.; Liu, Z.; Wu, W.; Chang, Y.; Zhao, G. Pd Nanoparticles Immobilized on Molecular Sieves by Ionic Liquids: Heterogeneous Catalysts for Solvent-Free Hydrogenation. Angew. Chem. 2004, 116, 1421–1423. [Google Scholar] [CrossRef]


| 1 | 2 | 3 | |
|---|---|---|---|
Involving the hydrazone skeleton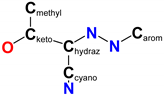 | |||
| NN | 1.294(9) | 1.277(10) | 1.312(3) |
| C−Nhydraz | 1.310(10) | 1.359(11) | 1.303(3) |
| Cketo−Chydraz | 1.449(11) | 1.432(12) | 1.479(3) |
| Cketo−O | 1.237(10) | 1.246(11) | 1.218(4) |
| C≡N | 1.142(10) | 1.148(12) | 1.139(4) |
| Involving the metal centre | |||
| M−Ncyano | 2.249(7) | 2.458(7) | - |
| M−Namine | - | 1.994(7) to 2.027(7) | 2.002(2) 2.020(2) |
| M−Osulfonate | 2.364(6) 2.402(6) 2.580(6) | 2.635(3) | - |
| C≡N−M | 165.2(7) | 126.5(6) | - |
| Intramolecular M⋅⋅⋅M | 3.871(1) {Ag2O2} 5.861(1) {Ag2O4S2} | 7.6836(4) | - |
| Intermolecular M⋅⋅⋅M | >10 | 6.0884(4) | 6.9798(4) |
| Entry | Catalyst | Solvent | Yield (%) b |
|---|---|---|---|
| 1 | 1 | THF | 15.1 |
| 2 | CH2Cl2 | 15.9 | |
| 3 | MeOH | 26.8 | |
| 4 | 2 | THF | 26.1 |
| 5 | CH2Cl2 | 28.7 | |
| 6 | MeOH | 79.9 | |
| 7 | 3 | THF | 25.9 |
| 8 | CH2Cl2 | 28.3 | |
| 9 | MeOH | 75.3 | |
| 10 | NaHL | THF | 22.0 |
| 11 | CH2Cl2 | 25.2 | |
| 12 | MeOH | 28.3 | |
| 13 | AgNO3 | MeOH | 30.0 |
| 14 | Cu(NO3)2·2.5H2O | MeOH | 32.9 |
| 15 c | - | - | 20.0 |
| 16 c | - | THF | 14.0 |
| 17 c | - | CH2Cl2 | 14.4 |
| 18 c | - | MeOH | 24.7 |
| Entry | Time (h) | Amount (mol %) of Catalyst | T (°C) | Yield b (%) |
|---|---|---|---|---|
| 1 | 1 | 5 | 25 | 42.6 |
| 2 | 4 | 5 | 25 | 65.4 |
| 3 | 6 | 5 | 25 | 73.5 |
| 4 | 8 | 5 | 25 | 79.0 |
| 5 | 12 | 5 | 25 | 78.9 |
| 6 | 24 | 5 | 25 | 79.9 |
| 7 | 8 | 1 | 25 | 44.9 |
| 8 | 8 | 3 | 25 | 72.9 |
| 9 | 8 | 5 | 25 | 79.0 |
| 10 | 8 | 7 | 25 | 78.9 |
| 11 | 8 | 9 | 25 | 79.0 |
| 12 | 8 | 5 | 15 | 70.3 |
| 13 | 8 | 5 | 35 | 79.2 |
| 14 | 8 | 5 | 45 | 80.8 |
| 15 | 8 | 5 | 55 | 82.5 |
| Entry | Ionic Liquid | IL or IL:MeOH (v/v) | Yield,% b |
|---|---|---|---|
| 1 c | 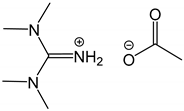 1,1,3,3-Tetramethylguanidine acetate [TMGH][OAc] | [TMGH][OAc] | 70.8 |
| 2 | [TMGH][OAc] | 76.7 | |
| 3 | 1:1 | 79.0 | |
| 4 | 1:10 | 78.3 | |
| 5 | 1:20 | 77.7 | |
| 6 c | 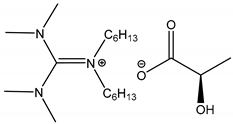 1′,1′-Dihexyl-3,3,3′,3′-tetramethylguanidine L-Lactate [DHTMG][L-Lactate] | [DHTMG][L-Lactate] | 75.2 |
| 7 | [DHTMG][L-Lactate] | 82.2 | |
| 8 | 1:1 | 86.5 | |
| 9 | 1:10 | 92.5 | |
| 10 | 1:20 | 91.3 | |
| 11 c | 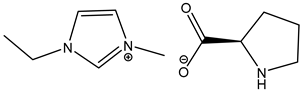 1-Ethyl-3-methylimidazolium L-Prolinate [EMIM][L-Prolinate] | [EMIM][L-Prolinate] | 70.4 |
| 12 | [EMIM][L-Prolinate] | 76.6 | |
| 13 | 1:1 | 78.6 | |
| 14 | 1:10 | 78.1 | |
| 15 | 1:20 | 77.6 | |
| 16c | 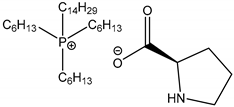 Trihexyl(tetradecyl)phosphonium L-Prolinate [P6,6,6,14][L-Prolinate] | [P6,6,6,14][L-Prolinate] | 68.7 |
| 17 | [P6,6,6,14][L-Prolinate] | 75.1 | |
| 18 | 1:1 | 78.2 | |
| 19 | 1:10 | 77.3 | |
| 20 | 1:20 | 75.9 |
| Entry | Substrate | Yield,% b | |
|---|---|---|---|
| MeOH Solvent | [DHTMG][L-Lactate]:MeOH (1:10, v/v) Medium | ||
| 1 | 4-Nitrobenzaldehyde | 83.4 | 97.8 |
| 2 | 4-Chlorobenzaldehyde | 79.7 | 94.0 |
| 3 | 4-Bromobenzaldehyde | 79.6 | 93.3 |
| 4 | Benzaldehyde | 79.0 | 92.6 |
| 5 | 4-Methylbenzaldehyde | 78.0 | 92.1 |
| 6 | 4-Methoxybenzaldehyde | 76.4 | 90.7 |
| 7 | Acetaldehyde | 87.7 | 99.0 |
| 8 | Propionaldehyde | 87.4 | 98.5 |
| 9 | Hexanal | 85.2 | 97.9 |
| 1 | 2 | |
|---|---|---|
| Empirical formula | C20H18Ag2N6O9S2 | C14H25CuN7O5S |
| fw | 766.26 | 467.01 |
| Temperature (K) | 150(2) | 150(2) |
| Cryst. Syst. | monoclinic | triclinic |
| Space group | C 2/c | P-1 |
| a (Å) | 12.982(2) | 7.2744(8) |
| b (Å) | 5.7571(10) | 11.7588(14) |
| c (Å) | 33.603(6) | 11.8024(13) |
| α, ° | 90 | 83.559(4) |
| β, ° | 98.673(6) | 75.174(3) |
| γ, ° | 90 | 84.810(4) |
| V (Å3) | 2482.7(7) | 967.78(19) |
| Z | 4 | 2 |
| ρcalc (g cm−3) | 2.050 | 1.603 |
| μ(Mo Kα) (mm−1) | 1.810 | 1.279 |
| F (000) | 1512 | 486 |
| Rint | 0.0554 | 0.0594 |
| R1 a (I ≥ 2σ) | 0.0572 | 0.0718 |
| wR2 b (I ≥ 2σ) | 0.1304 | 0.2039 |
| GOOF | 1.190 | 1.114 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiago, G.A.O.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Ribeiro, A.P.C.; Branco, L.C.; Zubkov, F.I.; Pombeiro, A.J.L. Cyanosilylation of Aldehydes Catalyzed by Ag(I)- and Cu(II)-Arylhydrazone Coordination Polymers in Conventional and in Ionic Liquid Media. Catalysts 2019, 9, 284. https://doi.org/10.3390/catal9030284
Tiago GAO, Mahmudov KT, Guedes da Silva MFC, Ribeiro APC, Branco LC, Zubkov FI, Pombeiro AJL. Cyanosilylation of Aldehydes Catalyzed by Ag(I)- and Cu(II)-Arylhydrazone Coordination Polymers in Conventional and in Ionic Liquid Media. Catalysts. 2019; 9(3):284. https://doi.org/10.3390/catal9030284
Chicago/Turabian StyleTiago, Gonçalo A. O., Kamran T. Mahmudov, M. Fátima C. Guedes da Silva, Ana P. C. Ribeiro, Luís C. Branco, Fedor I. Zubkov, and Armando J. L. Pombeiro. 2019. "Cyanosilylation of Aldehydes Catalyzed by Ag(I)- and Cu(II)-Arylhydrazone Coordination Polymers in Conventional and in Ionic Liquid Media" Catalysts 9, no. 3: 284. https://doi.org/10.3390/catal9030284
APA StyleTiago, G. A. O., Mahmudov, K. T., Guedes da Silva, M. F. C., Ribeiro, A. P. C., Branco, L. C., Zubkov, F. I., & Pombeiro, A. J. L. (2019). Cyanosilylation of Aldehydes Catalyzed by Ag(I)- and Cu(II)-Arylhydrazone Coordination Polymers in Conventional and in Ionic Liquid Media. Catalysts, 9(3), 284. https://doi.org/10.3390/catal9030284