Direct Synthesis of Ti-Containing CFI-Type Extra-Large-Pore Zeolites in the Presence of Fluorides
Abstract
:1. Introduction
2. Results and Discussion
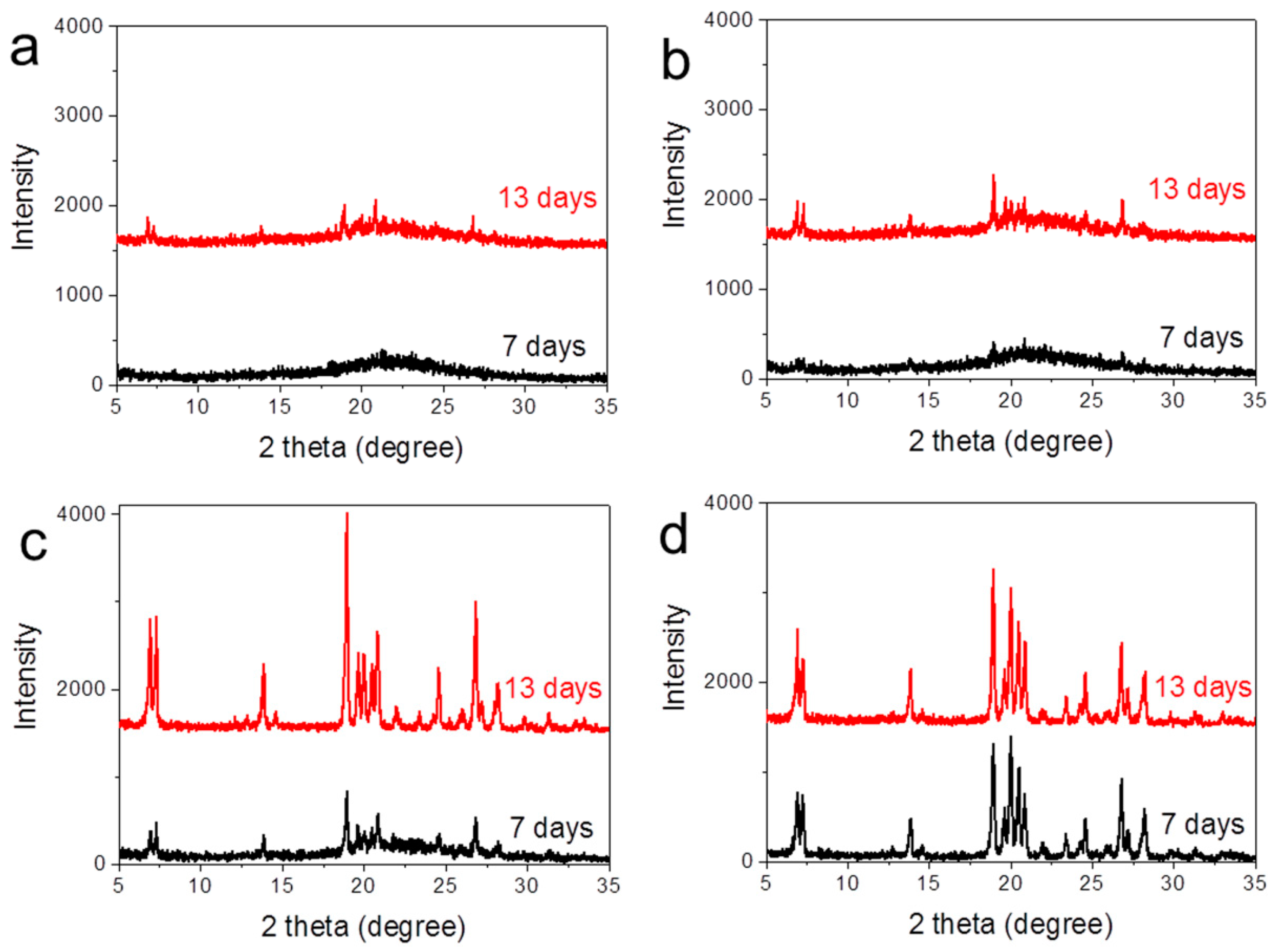
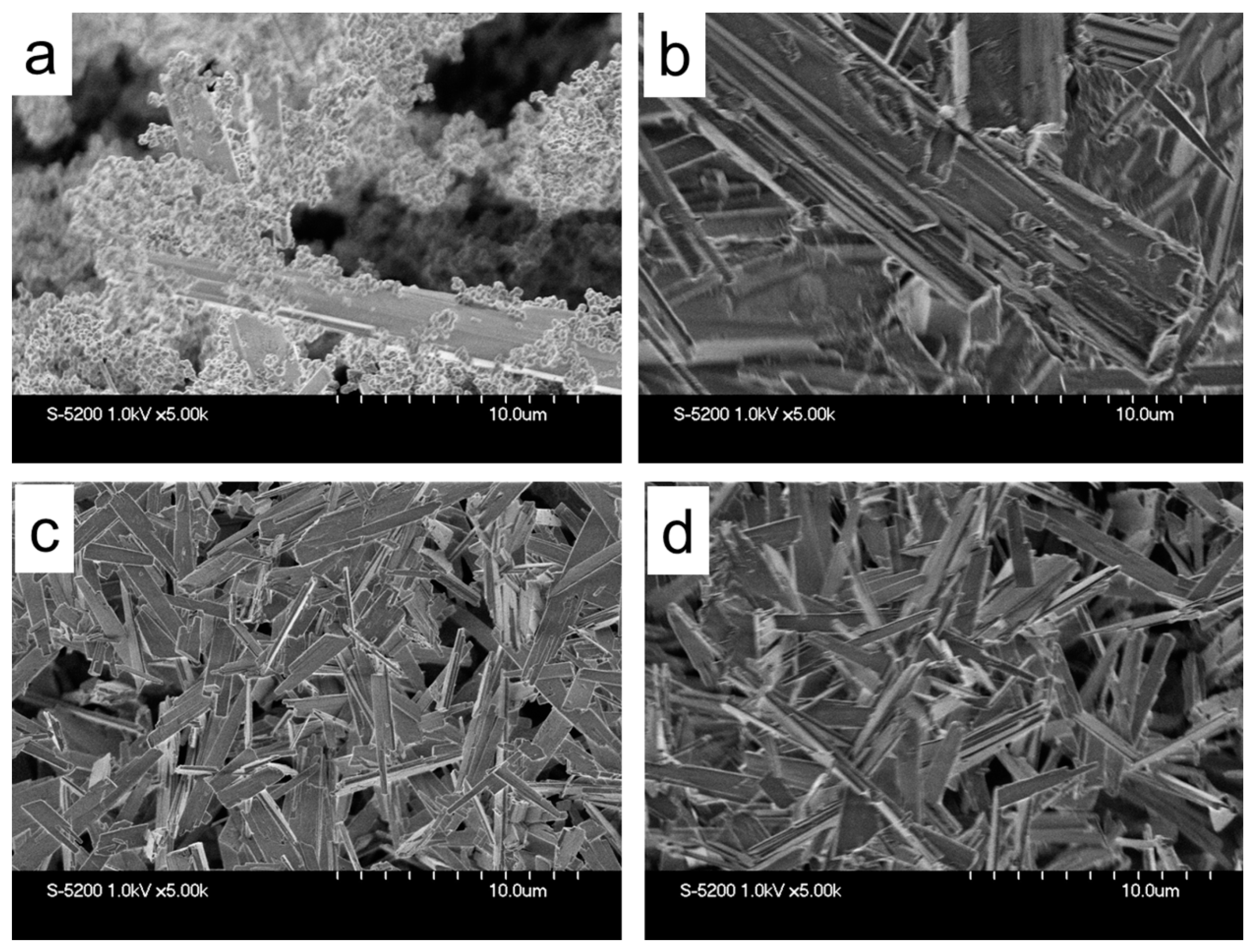
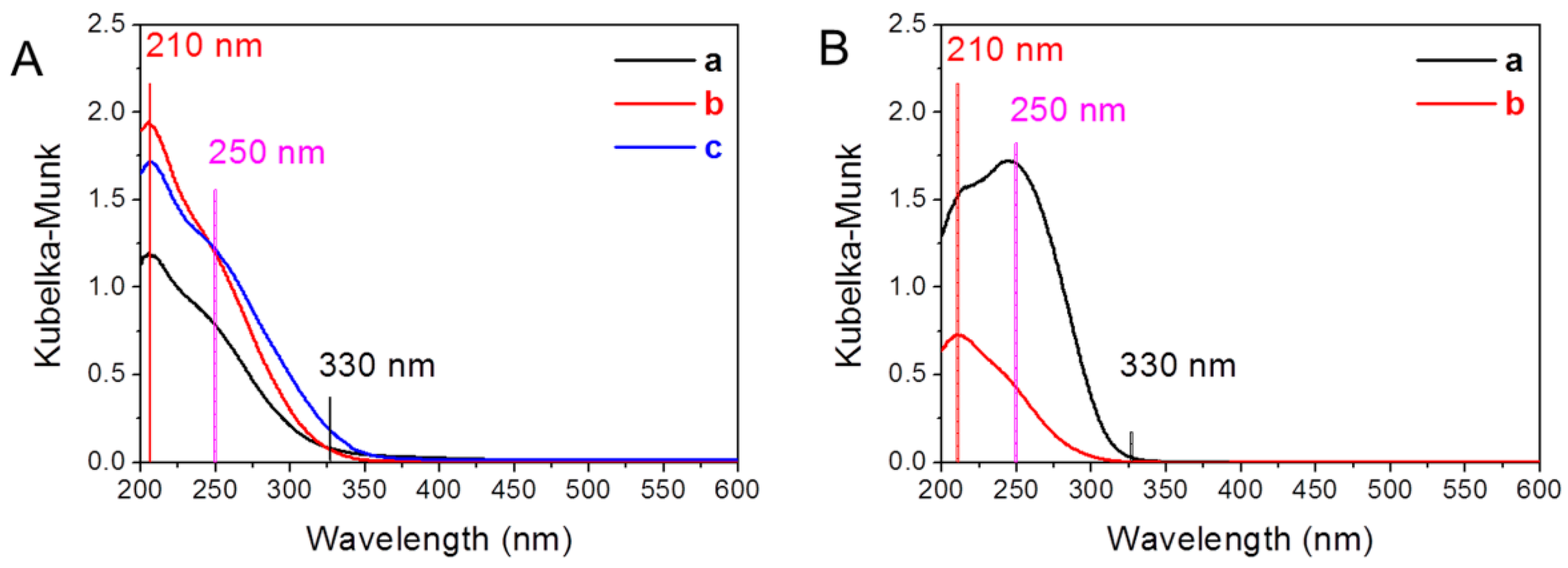
2.1. Zeolite Characterization
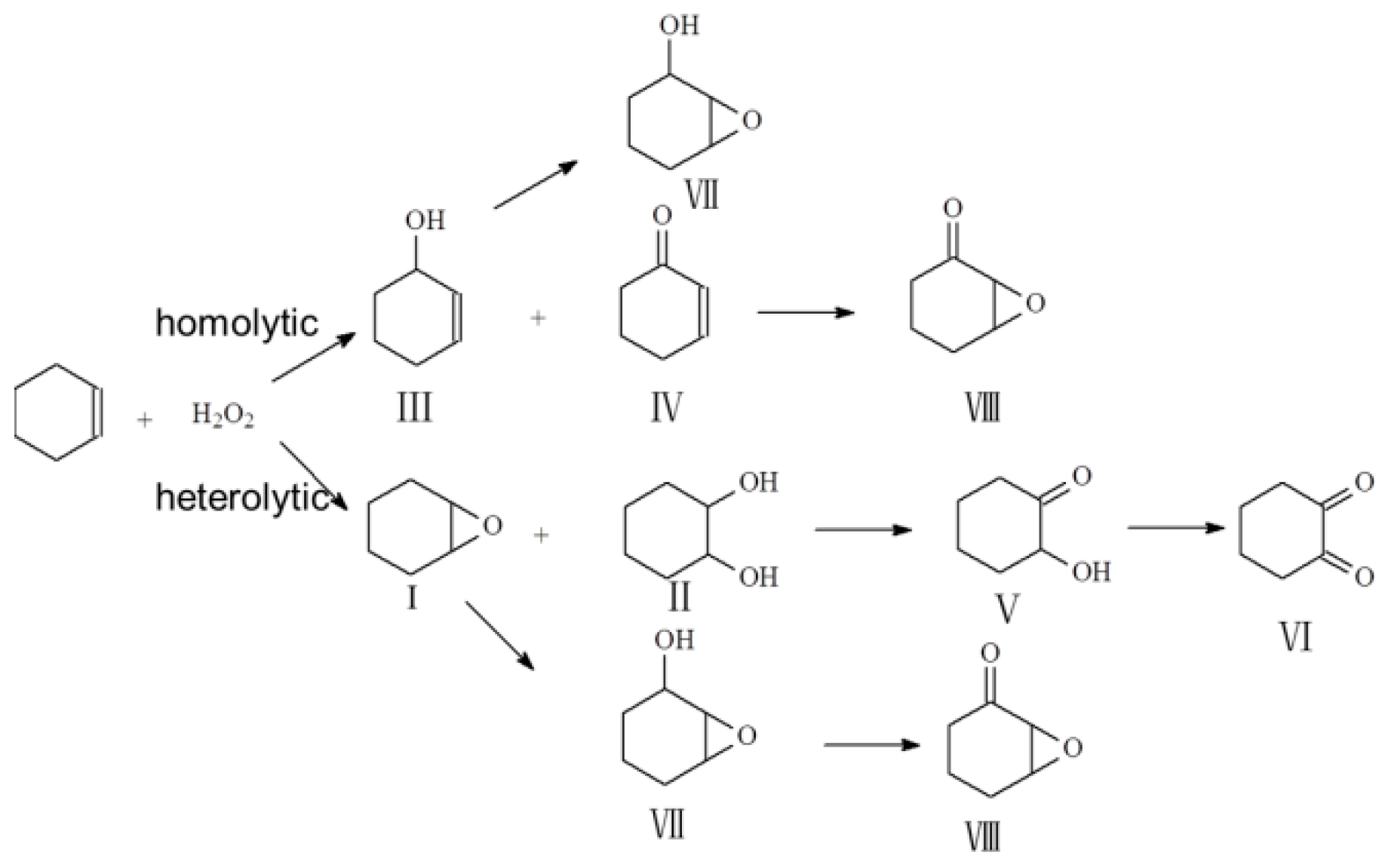
2.2. Catalytic Tests
3. Materials and Methods
3.1. Synthesis of Titanium-Containing CFI-Type Extra-Large-Pore Zeolites in the Fluoride System
3.2. Synthesis of Titanium-Containing CFI-Type Extra-Large-Pore Zeolites in the LiOH System
3.3. Catalytic Reaction
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Martinez, C.; Corma, A. Inorganic molecular sieves: Preparation, modification and industrial application in catalytic processes. Coord. Chem. Rev. 2011, 255, 1558–1580. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, D.; Xu, D.; Asahina, S.; Cychosz, K.A.; Agrawal, K.V.; Al Wahedi, Y.; Bhan, A.; Al Hashimi, S.; Terasaki, O.; et al. Synthesis of self-pillared zeolite nanosheets by repetitive branching. Science 2012, 336, 41. [Google Scholar] [CrossRef]
- Ding, K.; Corma, A.; Macia-Agullo, J.A.; Hu, J.G.; Kramer, S.; Stair, P.C.; Stucky, G.D. Constructing Hierarchical Porous Zeolites via Kinetic Regulation. J. Am. Chem. Soc. 2015, 137, 11238–11241. [Google Scholar] [CrossRef]
- Feng, G.; Cheng, P.; Yan, W.; Boronat, M.; Li, X.; Su, J.H.; Wang, J.; Li, Y.; Corma, A.; Xu, R.; et al. Accelerated crystallization of zeolites via hydroxyl free radicals. Science 2016, 351, 1188–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prech, J.; Pizarro, P.; Serrano, D.P.; Cejka, J. From 3D to 2D zeolite catalytic materials. Chem. Soc. Rev. 2018, 47, 8263–8306. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, M.; Perego, G.; Notari, B. Preparation of porous crystalline synthetic material comprised of silicon and titanium oxides. U.S. Patent 4,410,501, 18 October 1983. [Google Scholar]
- Lin, M.; Xia, C.; Zhu, B.; Li, H.; Shu, X. Green and efficient epoxidation of propylene with hydrogen peroxide (HPPO process) catalyzed by hollow TS-1 zeolite: A 1.0 kt/a pilot-scale study. Chem. Eng. J. 2016, 295, 370–375. [Google Scholar] [CrossRef]
- Wang, J.G.; Wang, Y.B.; Tatsumi, T.; Zhao, Y.L. Anionic polymer as a quasi-neutral medium for low-cost synthesis of titanosilicate molecular sieves in the presence of high-concentration alkali metal ions. J. Catal. 2016, 338, 321–328. [Google Scholar] [CrossRef]
- Zhao, H.; Yokoi, T.; Kondo, J.N.; Tatsumi, T. Hydrophobicity enhancement of Ti-MWW catalyst and its improvement in oxidation activity. Appl. Catal. A-Gen. 2015, 503, 156–164. [Google Scholar] [CrossRef]
- Zhao, S.; Xie, W.; Liu, Y.; Wu, P. Methyl Ethyl Ketone Ammoximation over Ti-MWW in a Continuous Slurry Reactor. Chinese J. Catal. 2011, 32, 179–183. [Google Scholar] [CrossRef]
- Reddy, J.S.; Kumar, R.; Ratnasamy, P. ChemInform Abstract: Titanium Silicalite-2: Synthesis, Characterization, and Catalytic Properties. Appl. Catal. 1990, 58, L1–L4. [Google Scholar] [CrossRef]
- Serrano, D.P.; Li, H.X.; Davis, M.E. Synthesis of titanium-containing ZSM-48. J. Chem. Soc. Chem. Commun. 1992, 10, 745–747. [Google Scholar] [CrossRef]
- Wu, P.; Tatsumi, T.; Komatsu, T.; Yashima, T. A novel titanosilicate with MWW structure. I. Hydrothermal synthesis, elimination of extraframework titanium, and characterizations. J. Phys. Chem. B 2001, 105, 2897–2905. [Google Scholar] [CrossRef]
- Camblor, M.A.; Corma, A.; Martínez, A.; Pérez-Pariente, J. Synthesis of a titaniumsilicoaluminate isomorphous to zeolite beta and its application as a catalyst for the selective oxidation of large organic molecules. J. Chem. Soc. Chem. Commun. 1992, 589–590. [Google Scholar] [CrossRef]
- Blasco, T.; Camblor, M.; Corma, A.; Esteve, P.; Guil, J.; Martinez, A.; Perdigon-Melon, J.; Valencia, S. Direct synthesis and characterization of hydrophobic Aluminum-free Ti−beta zeolite. J. Phys. Chem. B 1998, 102, 75–88. [Google Scholar] [CrossRef]
- Van der Waal, J.; Kooyman, P.; Jansen, J.; Van Bekkum, H. Synthesis and characterization of aluminum-free zeolite titanium beta using di (cyclohexylmethyl) dimethylammonium as a new and selective template. Micropor. Mesopor. Mater. 1998, 25, 43–57. [Google Scholar] [CrossRef]
- Tuel, A. Synthesis, characterization, and catalytic properties of the new TiZSM-12 zeolite. Zeolites 1995, 15, 236–242. [Google Scholar] [CrossRef]
- Wu, P.; Komatsu, T.; Yashima, T. Characterization of titanium species incorporated into dealuminated mordenites by means of IR spectroscopy and 18O-exchange technique. J. Phys. Chem. 1996, 100, 10316–10322. [Google Scholar] [CrossRef]
- Díaz-Cabañas, M.-J.; Villaescusa, L.A.; Camblor, M.A. Synthesis and catalytic activity of Ti-ITQ-7: a new oxidation catalyst with a three-dimensional system of large pore channels. Chem. Commun. 2000, 761–762. [Google Scholar] [CrossRef] [Green Version]
- Fan, W.; Wu, P.; Namba, S.; Tatsumi, T. A titanosilicate that is structurally analogous to an MWW-type lamellar precursor. Angew. Chem. Int. Ed. 2004, 43, 236–240. [Google Scholar] [CrossRef]
- Tamura, M.; Chaikittisilp, W.; Yokoi, T.; Okubo, T. Incorporation process of Ti species into the framework of MFI type zeolite. Microporous Mesoporous Mater. 2008, 112, 202–210. [Google Scholar] [CrossRef]
- Wang, J.G.; Yokoi, T.; Kondo, J.N.; Tatsumi, T.; Zhao, Y.L. Titanium(IV) in the Organic-Structure-Directing-Agent-Free Synthesis of Hydrophobic and Large-Pore Molecular Sieves as Redox Catalysts. ChemSuschem 2015, 8, 2476–2480. [Google Scholar] [CrossRef]
- Luna, F.J.; Ukawa, S.E.; Wallau, M.; Schuchardt, U. Cyclohexane oxidation using transition metal-containing aluminophosphates (MAPO-VFI). J. Mol. Catal. A-Chem. 1997, 117, 405–411. [Google Scholar] [CrossRef]
- Prech, J.; Cejka, J. UTL titanosilicate: An extra-large pore epoxidation catalyst with tunable textural properties. Catal. Today 2016, 277, 2–8. [Google Scholar] [CrossRef]
- Balkus, K., Jr.; Gabrielov, A.; Zones, S. The synthesis of UTD-1, Ti-UTD-1 and Ti-UTD-8 using CP* 2CoOH as a structure directing agent. Stud. Surf. Sci. Catal. 1995, 97, 519–525. [Google Scholar]
- Přech, J.; Kubů, M.; Čejka, J. Synthesis and catalytic properties of titanium containing extra-large pore zeolite CIT-5. Catal. Today 2014, 227, 80–86. [Google Scholar] [CrossRef]
- Sever, R.R.; Alcala, R.; Dumesic, J.A.; Root, T.W. Vapor-phase silylation of MCM-41 and Ti-MCM-41. Micropor. Mesopor. Mater. 2003, 66, 53–67. [Google Scholar] [CrossRef]
- Barrett, P.A.; Díaz-Cabañas, M.J.; Camblor, M.A.; Jones, R.H. Synthesis in fluoride and hydroxide media and structure of the extra-large pore pure silica zeolite CIT-5. J. Chem. Soc. Faraday Trans. 1998, 94, 2475–2481. [Google Scholar] [CrossRef]
| Samples | Yields (%) | Si/Ti (mol/mol) | SBET 1 (m2∙g−1) | Pore Volume (m3∙g−1) | Sext. 2 (m2∙g−1) |
|---|---|---|---|---|---|
| Ti-CFI[HF]-seed-7d | ~90 | 247 | 395 | 0.14 | 33 |
| Ti-CFI[HF]-seed-13d | ~90 | 189 | 413 | 0.15 | 36 |
| Ti-CFI[NH4F]-13d | ~92 | 220 | 385 | 0.14 | 22 |
| Ti-CFI[LiOH] | ~85 | 46 | 280 | 0.08 | 84 |
| Ti-CFI[LiOH]-post | - | 183 | 393 | 0.11 | 101 |
| Samples | Si/Ti (mol/mol) | Conversion (%) | Selectivity (%) | TON (mol/mol -Ti) | H2O2 Efficiency 1 (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | Others | |||||
| Ti-CFI[HF]-seed-7d | 247 | 8.2 | 17.0 | 68.9 | 4.2 | 6.1 | 3.8 | 49 | >99 |
| Ti-CFI[HF]-seed-13d | 189 | 9.0 | 14.7 | 69.9 | 4.6 | 4.5 | 11.9 | 41 | >99 |
| Ti-CFI[NH4F]-13d | 220 | 5.3 | 8.7 | 74.8 | 4.3 | 6.9 | 5.3 | 28 | >99 |
| Ti-CFI[LiOH] | 46 | 2.0 | 73.9 | 14.9 | 4.2 | 4.3 | 2.7 | 2 | 12 |
| Ti-CFI[LiOH]-post | 183 | 4.8 | 14.1 | 62.3 | 11.9 | 4.5 | 7.2 | 21 | 34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, H.; Shao, Y.; Li, T.; Tatsumi, T.; Wang, J.-G. Direct Synthesis of Ti-Containing CFI-Type Extra-Large-Pore Zeolites in the Presence of Fluorides. Catalysts 2019, 9, 257. https://doi.org/10.3390/catal9030257
Wang Y, Wang H, Shao Y, Li T, Tatsumi T, Wang J-G. Direct Synthesis of Ti-Containing CFI-Type Extra-Large-Pore Zeolites in the Presence of Fluorides. Catalysts. 2019; 9(3):257. https://doi.org/10.3390/catal9030257
Chicago/Turabian StyleWang, Yichen, Hongjuan Wang, Yuanchao Shao, Tianduo Li, Takashi Tatsumi, and Jin-Gui Wang. 2019. "Direct Synthesis of Ti-Containing CFI-Type Extra-Large-Pore Zeolites in the Presence of Fluorides" Catalysts 9, no. 3: 257. https://doi.org/10.3390/catal9030257
APA StyleWang, Y., Wang, H., Shao, Y., Li, T., Tatsumi, T., & Wang, J.-G. (2019). Direct Synthesis of Ti-Containing CFI-Type Extra-Large-Pore Zeolites in the Presence of Fluorides. Catalysts, 9(3), 257. https://doi.org/10.3390/catal9030257





